Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Sebastien Couillard-Despres.
Amongst the consequences of spinal cord injury (SCI) on the central nervous system, the loss of inhibition is a common finding, albeit not always observed, and it is likely to fluctuate over time. Changes in cortical excitability involve a plethora of mechanisms, which individual effects may combine in complex and variable outcomes on the process of functional and structural recovery.
- cortical inhibition
- spinal cord injury
- neocortex
- disinhibition
- interneuron
1. Introduction: SCI Harms the Brain
Traumatic spinal cord injury (SCI) is a sudden and unpredictable incident that destroys portions of the spinal cord, leading to motor and sensory deficits, as well as dysfunctions of the somatic and autonomic nervous systems [1]. Beyond the loss of movement control, typical deficits include the loss of bladder and bowel control, declined sexual functions and chronic pain, among others [1,2][1][2]. SCI can occur at any age, and the damage is irreversible. However, constant improvements in healthcare and treatment, as well as increased awareness about the needs of patients over the last century, have significantly ameliorated the quality of life and lifespan following SCI [3,4,5,6,7,8,9][3][4][5][6][7][8][9]. Thus, it is even more pressing to identify interventions enabling the recovery of functions lost after SCI. The recovery of muscle control is a crucial element to improve the quality of life and the autonomy of SCI patients. Accordingly, rehabilitation and active lifestyle have been recognized as crucial processes that help to regain independence and to reduce health complications resulting from prolonged inactivity [10,11,12,13,14][10][11][12][13][14]. Nevertheless, the timely implementation of efficient strategies remains often neglected, affecting motor recovery and, together with accompanying morbidities, decreasing the likelihood of returning to a fully independent life routine [15,16][15][16]. Furthermore, various therapies addressing the symptoms of SCI are being developed with promising outcomes for management and reduction in secondary damage, increased neuroprotection and improved neuroregeneration [5,17][5][17]. However, despite constant improvements, an effective cure, leading to major functional recovery based on the regeneration of neuronal connectivity across the lesion, is still missing [17,18,19,20][17][18][19][20].
Many of the neurons that become disconnected following SCI reside outside the spinal cord, such as the motor neurons of the primary motor cortex, which are crucial for the control of voluntary movements. These disconnected neurons are a resource for the long-term regeneration and functional recovery of the central nervous system. However, the axotomy resulting from SCI has an impact on the physiology of the cortical and corticospinal network [21[21][22][23],22,23], which can complicate or even hinder the recovery process. Several attempts to address the clinical symptoms of SCI have therefore explored the possibility to retune neuronal activity in the corticospinal network [24,25][24][25]. Finding ways to reconnect cortical motor neurons to their original targets constitutes a daunting task. In addition, early assessments of the severity of SCI, especially in an acute situation, are difficult and inherently inaccurate [26]. This lack of knowledge is a major hurdle for the design of an effective and patient-specific treatment. Therefore, the assessment of cortical activity in SCI patients, for example, using electro-encephalography (EEG), transcranial magnetic stimulation (TMS), etc., has been extremely convenient, as it relies on non-invasive techniques [27,28][27][28]. In addition, animal models are available to resolve the molecular mechanisms of brain dysfunction after SCI.
2. Possible Causes for the Loss of Inhibition
Pre-clinical research helps to define mechanisms that converge to an altered ratio of excitation and inhibition following SCI and to refine hypotheses about the reasons for phenotypic heterogeneity, as reported in clinical findings. Focusing on molecular “bottlenecks” of inhibitory neurotransmission, the likely mechanisms of cortical disinhibition after SCI are presented in the following section (Figure 1).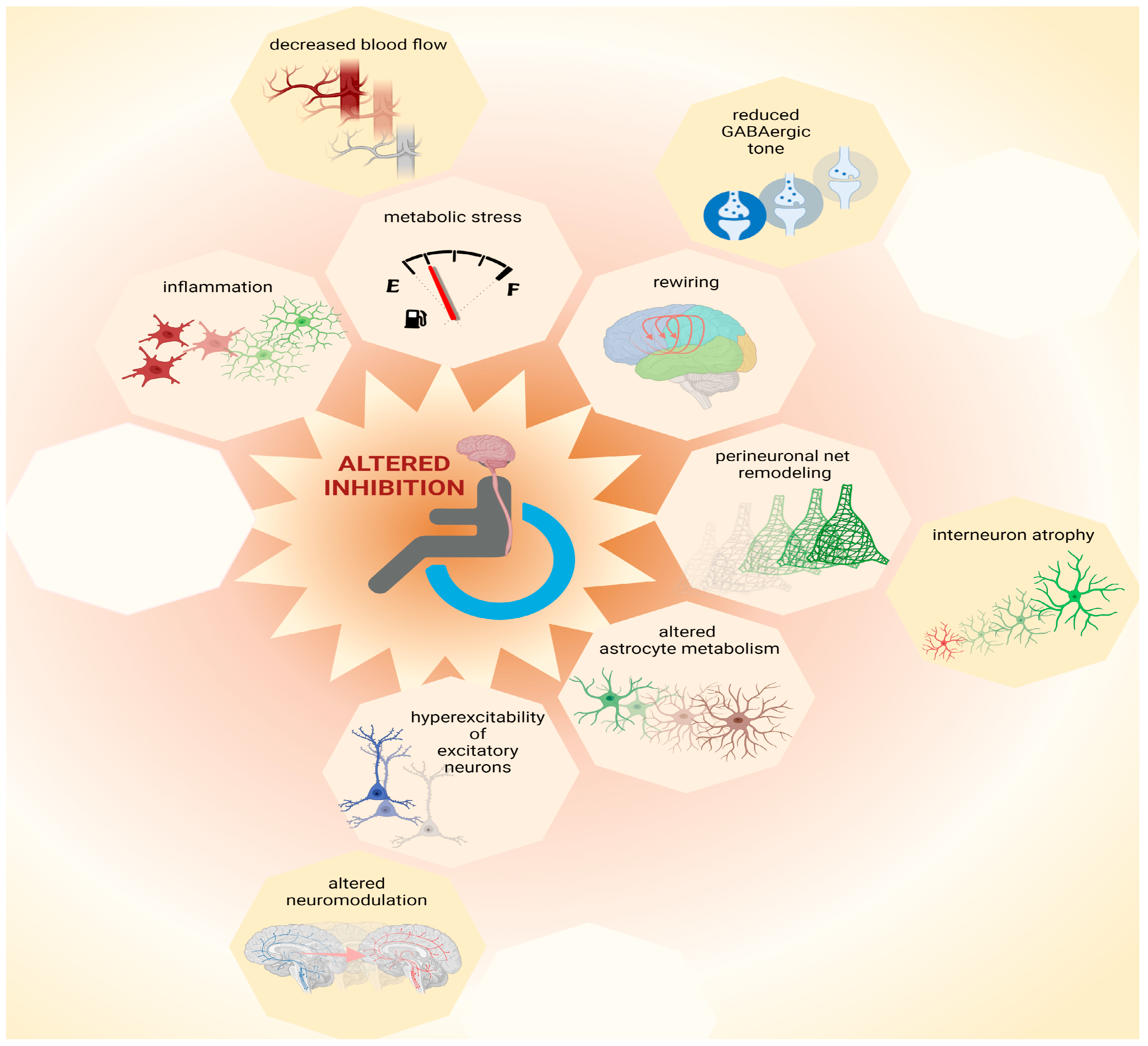
Figure 1. Possible causes for loss of inhibition. Multiple mechanisms contribute to network plasticity and altered inhibition in cortical and subcortical areas after SCI. These include metabolic stress, which can be exacerbated by inflammation and by decreased blood flow, as well as altered astrocytic metabolism. Furthermore, increased excitability of principal (excitatory) neurons may be exacerbated by altered neuromodulation, contributing to increased output volume in corticospinal circuits. Moreover, disinhibition is associated with decreased GABAergic tone that contributes to plasticity during network rewiring. Additionally, remodeling of perineuronal nets, which involves atrophy of interneurons, can contribute to complex patterns of altered inhibition in the central nervous system after SCI. Thus, multiple mechanisms, some of which are yet to be identified (represented by empty octagons) may coexist and combine heterogeneously amongst each other and/or to other pathophysiological components, increasing inter-patient variability. This figure was created with biorender.com.
2.1. Metabolic Stress
The synthesis of neurotransmitters, such as glutamate and GABA, demand considerable energy consumption [93][29]. Of these, GABAergic neurotransmission has the highest demand. For this reason, inhibitory neurons such as parvalbumin-positive and axo-axonic interneurons are vulnerable and respond to stress with decreased GABAergic neurotransmission [94][30]. After SCI, the axotomy of corticospinal neurons may cause stress, spreading from the spinal cord to cortical areas [21,22,23][21][22][23]. Additional sources of stress may be inflammation [95][31] and impaired cerebral blood flow [96][32]. Furthermore, stress may coincide with high metabolic demand due to increased cortical excitability [22]. Hence, multiple stressors may affect interneurons, as also suggested by the direct observation of their atrophy after SCI [32][33]. The alteration in brain network performance as a consequence of dysfunctional interneurons may impact cognition negatively [97[34][35],98], such as occasionally documented in SCI patients [99][36].2.2. Inflammation
Microglia activation plays a pivotal role in SCI. Furthermore, chronic inflammation seems to affect supraspinal regions as well [100][37]. This is not surprising, given the axotomy of several cortical and subcortical neurons [21,22,23][21][22][23] and the continuity of the spinal cord and brain. Regrettably, although the wave of inflammation after SCI has been well time-resolved near the lesion site [101[38][39][40],102,103], little is known about the spatial distribution of immune cell activation across the CNS following various types of injury and treatment. Thus, it is hard to define which neurons are most exposed to inflammatory processes and which are spared and exclusively undergo plasticity processes independent of inflammation. Among several disinhibitory effects [104][41], activated microglia affect the activity of proteins that transport chloride across the neuronal cell membrane and cause the dissipation of chloride trans-membrane gradients with consequent reductions in the GABAergic inhibitory drive [105][42]. Decreased GABAergic drive causes the neurotransmitter GABA to be intrinsically less effective in hyperpolarizing the neuronal membrane to mediate inhibition. The dissipation of chloride gradients upon microglia activation after SCI may therefore contribute to disinhibition as well, as documented in other pathologies [105,106][42][43].2.3. Remodeling of Perineuronal Nets
The extracellular elements of proteoglycans, known as perineuronal nets, mediate stability in network architecture as well as protection from stress [107][44]. Dismantling perineuronal nets promotes plasticity and network remodeling [108][45], which are pertinent for recovery after SCI [109,110][46][47]. Furthermore, perineuronal nets are closely associated with specific categories of cortical interneurons [111,112][48][49]. Thereby, these extracellular elements can control neuronal synaptic connectivity and intrinsic firing properties [112,113][49][50]. Thus, the dismantling of perineuronal nets can affect the activity and connectivity of interneurons, determining altered cortical inhibition after SCI. Strikingly, the advantage of increased plasticity derived from the dismantling of perineuronal nets comes along with the challenge presented by increased metabolic stress. Indeed, perineuronal nets limit excitotoxicity by sheltering synaptic contacts and reducing oxidative stress to which interneurons are most vulnerable [94,114,115][30][51][52]. Since neocortical perineuronal nets undergo remodeling after SCI that is associated with the atrophy of interneurons [32][33], these extracellular elements may be involved in both beneficial and detrimental aspects of cortical disinhibition.2.4. Altered Astrocyte Metabolism and Physiology
Axotomy and neuronal trauma perturb the physiology of astrocytes [116][53]. Trauma suffered by cortical principal neurons upon axotomy [21,22,23][21][22][23] may therefore alter signaling from astrocytes to surrounding neurons. Astrocytes can release neuromodulators controlling neuronal activity by gliotransmission [117,118,119][54][55][56]. Moreover, astrocytes are crucial for the metabolism of neurotransmitters [120,121][57][58]. Thus, under pathological conditions, altered astrocytic activity may affect neurotransmission, and in particular GABAergic inhibition, via altered metabolic support as well [122][59]. The involvement of astrocytes in SCI-derived pathology has been widely studied [123][60], albeit not in cerebral regions.2.5. Rewiring of Cortical Circuits
A physiological process closely tied to disinhibitory mechanisms is the rewiring of cortical circuits after SCI. Like other injuries involving deafferentation of the central nervous system, SCI causes the rewiring of cortical and subcortical areas, as well as the shift of somatotopic representations and changes in competence of motor areas, which relay on significant events of network plasticity [124][61]. Decreases in GABAergic inhibition appear crucial for network remodeling [125,126][62][63] because plasticity and learning are tightly related to changes in cortical GABA [127][64]. For instance, reduced GABAergic inhibition causes qualitative and quantitative changes in the inducibility of long term potentiation in the neocortex [128,129][65][66]. Furthermore, reduced GABAergic tone, i.e., diminished concentrations of extrasynaptic GABA, supports motor plasticity and motor recovery in various types of pathology [126,130][63][67]. Thus, several mechanisms that contribute to reduced GABAergic inhibition in the brain after SCI may also partake into the process of cortical rewiring and recovery.2.6. Hyperexcitability of Excitatory Neurons
Some causes of hyperexcitability may be independent from disinhibition. Nevertheless, solving pathological hyperexcitability may necessitate retuning inhibitory components. For instance, axotomy results in the depolarization and hyperexcitability of cortical principal neurons after SCI [22]. The retuning of hyperexcitable neurons may benefit from increased inhibition, for instance, through GABAB receptor signaling, which controls dendritic excitability via intracellular calcium signaling [131][68]. In contrast, disinhibition has additive exacerbating effects on the intrinsic hyperexcitability of principal neurons. Furthermore, other types of neuromodulation that exert inhibitory control on CNS neurons, e.g., the ones mediated by serotonin, are impaired after SCI [132][69]. The activation of serotonin receptors has been directly shown to control the excitability of cortical neurons [133][70]. Additionally, the enrichment of serotonin receptors at the axonal hillock of layer V pyramidal neurons implies a crucial role in the control of cortical functional output [134][71]. Thus, non-GABAergic neuromodulation can directly control the excitability of principal neurons as well as the excitability of interneurons [135,136][72][73]. Since altered serotoninergic neuromodulation can affect directly and indirectly the volume of corticospinal output, serotonin neuromodulation may be a key component in controlling cortical output after SCI. Strikingly, controlling serotoninergic neuromodulation has already proven to support better recovery after SCI [137,138,139][74][75][76].References
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. 2017, 3, 17018.
- Masri, R.; Keller, A. Chronic Pain Following Spinal Cord Injury. Adv. Exp. Med. Biol. 2012, 760, 74–88.
- Lifshutz, J.; Colohan, A. A brief history of therapy for traumatic spinal cord injury. Neurosurg. Focus 2004, 16, 1–8.
- Hulsebosch, C.E. Recent advances in pathophysiology and treatment of spinal cord injury. Am. J. Physiol. Adv. Physiol. Educ. 2002, 26, 238–255.
- Cadotte, D.W.; Fehlings, M.G. Spinal cord injury: A systematic review of current treatment options. Clin. Orthop. Relat. Res. 2011, 469, 732–741.
- Venkatesh, K. Spinal cord injury: Pathophysiology, treatment strategies, associated challengeenges. Cell Tissue Res. 2019, 377, 125–151.
- Clayton, K.S.; Chubon, R.A. Factors associated with the quality of life of long-term spinal cord injured persons. Arch. Phys. Med. Rehabil. 1994, 75, 633–638.
- Krause, J.; Saunders, L. Risk of mortality and life expectancy after spinal cord injury: The role of health behaviors and participation. Top. Spinal Cord Inj. Rehabil. 2010, 16, 53–60.
- Anneken, V.; Hanssen-Doose, A.; Hirschfeld, S.; Scheuer, T.; Thietje, R. Influence of physical exercise on quality of life in individuals with spinal cord injury. Spinal Cord 2010, 48, 393–399.
- Van Der Woude, L.H.V.; De Groot, S.; Postema, K.; Bussmann, J.B.J.; Janssen, T.W.J.; Post, M.W.M. Active LifestyLe Rehabilitation Interventions in aging Spinal Cord injury (ALLRISC): A multicentre research program. Disabil. Rehabil. 2013, 35, 1097–1103.
- Van den Akker, L.E.; Holla, J.F.M.; Dadema, T.; Visser, B.; Valent, L.J.; de Groot, S.; Dallinga, J.M.; Deutekom, M. Determinants of physical activity in wheelchair users with spinal cord injury or lower limb amputation: Perspectives of rehabilitation professionals and wheelchair users. Disabil. Rehabil. 2020, 42, 1934–1941.
- Nas, K.; Yazmalar, L.; Şah, V.; Aydin, A.; Öneş, K. Rehabilitation of spinal cord injuries. World J. Orthop. 2015, 6, 8–16.
- Behrman, A.L.; Harkema, S.J. Physical Rehabilitation as an Agent for Recovery After Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 183–202.
- Spooren, A.I.F.; Janssen-Potten, Y.J.M.; Kerckhofs, E.; Seelen, H.A.M. Outcome of motor training programmes on arm and hand functioning in patients with cervical spinal cord injury according to different levels of the ICF: A systematic review. J. Rehabil. Med. 2009, 41, 497–505.
- Montesinos-Magraner, L.; Serra-Ano, P.; García-Masso, X.; Ramírez-Garcerán, L.; González, L.M.; González-Viejo, M. Comorbidity and physical activity in people with paraplegia: A descriptive cross-sectional study. Spinal Cord 2018, 56, 52–56.
- Floríndez, L.I.; Carlson, M.E.; Pyatak, E.; Blanchard, J.; Cogan, A.M.; Sleight, A.G.; Hill, V.; Diaz, J.; Blanche, E.; Garber, S.L.; et al. A qualitative analysis of pressure injury development among medically underserved adults with spinal cord injury. Disabil. Rehabil. 2020, 42, 2093–2099.
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505.
- Huang, H.; Sharma, H.S.; Chen, L.; Otom, A.; Al Zoubi, Z.M.; Saberi, H.; Muresanu, D.F.; He, X. Review of clinical neurorestorative strategies for spinal cord injury: Exploring history and latest progresses. J. Neurorestoratology 2018, 1, 171–178.
- Shah, M.; Peterson, C.; Yilmaz, E.; Halalmeh, D.R.; Moisi, M. Current advancements in the management of spinal cord injury: A comprehensive review of literature. Surg. Neurol. Int. 2020, 11, 2.
- Hutson, T.H.; Di Giovanni, S. The translational landscape in spinal cord injury: Focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019, 15, 732–745.
- Hains, B.C.; Black, J.; Waxman, S.G. Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J. Comp. Neurol. 2003, 462, 328–341.
- Nagendran, T.; Larsen, R.S.; Bigler, R.L.; Frost, S.B.; Philpot, B.D.; Nudo, R.J.; Taylor, A.M. Distal axotomy enhances retrograde presynaptic excitability onto injured pyramidal neurons via trans-synaptic signaling. Nat. Commun. 2017, 8, 625.
- Beaud, M.; Schmidlin, E.; Wannier, T.; Freund, P.; Bloch, J.; Mir, A.; Schwab, M.E.; Rouiller, E.M. Anti-Nogo-A antibody treatment does not prevent cell body shrinkage in the motor cortex in adult monkeys subjected to unilateral cervical cord lesion. BMC Neurosci. 2008, 9, 5.
- Onifer, S.M.; Smith, G.M.; Fouad, K. Plasticity After Spinal Cord Injury: Relevance to Recovery and Approaches to Facilitate It. Neurotherapeutics 2011, 8, 283–293.
- Dietz, V.; Fouad, K. Restoration of sensorimotor functions after spinal cord injury. Brain 2014, 137, 654–667.
- Van Middendorp, J.J.; Goss, B.; Urquhart, S.; Atresh, S.; Williams, R.P.; Schuetz, M. Diagnosis and Prognosis of Traumatic Spinal Cord Injury. Glob. Spine J. 2011, 1, 001–007.
- Nardone, R.; Höller, Y.; Brigo, F.; Orioli, A.; Tezzon, F.; Schwenker, K.; Christova, M.; Golaszewski, S.; Trinka, E. Descending motor pathways and cortical physiology after spinal cord injury assessed by transcranial magnetic stimulation: A systematic review. Brain Res. 2015, 1619, 139–154.
- Nardone, R.; Langthaler, P.B.; Höller, Y.; Bathke, A.; Frey, V.N.; Brigo, F.; Trinka, E. Modulation of non-painful phantom sensation in subjects with spinal cord injury by means of rTMS. Brain Res. Bull. 2015, 118, 82–86.
- Rowley, N.M.; Madsen, K.K.; Schousboe, A.; Steve White, H. Glutamate and GABA synthesis, release, transport and metabolism as targets for seizure control. Neurochem. Int. 2012, 61, 546–558.
- Kann, O. The interneuron energy hypothesis: Implications for brain disease. Neurobiol. Dis. 2016, 90, 75–85.
- Azbill, R.D.; Mu, X.; Bruce-Keller, A.J.; Mattson, M.P.; Springer, J.E. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997, 765, 283–290.
- Phillips, A.A.; Ainslie, P.N.; Krassioukov, A.V.; Warburton, D.E.R. Regulation of cerebral blood flow after spinal cord injury. J. Neurotrauma 2013, 30, 1551–1563.
- Orlando, C.; Raineteau, O. Integrity of cortical perineuronal nets influences corticospinal tract plasticity after spinal cord injury. Brain Struct. Funct. 2015, 220, 1077–1091.
- Melloni, L.; Molina, C.; Pena, M.; Torres, D.; Singer, W.; Rodriguez, E. Synchronization of neural activity across cortical areas correlates with conscious perception. J. Neurosci. 2007, 27, 2858–2865.
- Uhlhaas, P.J.; Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010, 11, 100–113.
- Craig, A.; Guest, R.; Tran, Y.; Middleton, J. Cognitive Impairment and Mood States after Spinal Cord Injury. J. Neurotrauma 2017, 34, 1156–1163.
- Wu, J.; Zhao, Z.; Sabirzhanov, B.; Stoica, B.A.; Kumar, A.; Luo, T.; Skovira, J.; Faden, A.I. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: Role of cell cycle pathways. J. Neurosci. 2014, 34, 10989–11006.
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11.
- Ren, Y.; Young, W. Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plast. 2013, 2013, 945034.
- Pineau, I.; Steve, L. Proinflammatory Cytokine Synthesis in the Injured Mouse Spinal Cord: Multiphasic Expression Pattern and Identification of the Cell Types Involved. J. Comp. Neurol. 2007, 346, 339–346.
- Zeilhofer, H.U. Loss of glycinergic and GABAergic inhibition in chronic pain-contributions of inflammation and microglia. Int. Immunopharmacol. 2008, 8, 182–187.
- Ferrini, F.; De Koninck, Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013, 2013, 429815.
- Pozzi, D.; Rasile, M.; Corradini, I.; Matteoli, M. Environmental regulation of the chloride transporter KCC2: Switching inflammation off to switch the GABA on? Transl. Psychiatry 2020, 10, 349.
- Reichelt, A.C.; Hare, D.J.; Bussey, T.J.; Saksida, L.M. Perineuronal Nets: Plasticity, Protection, and Therapeutic Potential. Trends Neurosci. 2019, 42, 458–470.
- Carstens, K.E.; Phillips, M.L.; Pozzo-Miller, L.; Weinberg, R.J.; Dudek, S.M. Perineuronal nets suppress plasticity of excitatory synapses on CA2 pyramidal neurons. J. Neurosci. 2016, 36, 6312–6320.
- Kwok, J.C.F.; Dick, G.; Wang, D.; Fawcett, J.W. Extracellular matrix and perineuronal nets in CNS repair. Dev. Neurobiol. 2011, 71, 1073–1089.
- Casha, S.; Zygun, D.; McGowan, M.D.; Bains, I.; Yong, V.W.; John Hurlbert, R. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012, 135, 1224–1236.
- Rossier, J.; Bernard, A.; Cabungcal, J.H.; Perrenoud, Q.; Savoye, A.; Gallopin, T.; Hawrylycz, M.; Cuénod, M.; Do, K.; Urban, A.; et al. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol. Psychiatry 2015, 20, 154–161.
- Härtig, W.; Derouiche, A.; Welt, K.; Brauer, K.; Grosche, J.; Mäder, M.; Reichenbach, A.; Brückner, G. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999, 842, 15–29.
- Lorenzo Bozzelli, P.; Alaiyed, S.; Kim, E.; Villapol, S.; Conant, K. Proteolytic Remodeling of Perineuronal Nets: Effects on Synaptic Plasticity and Neuronal Population Dynamics. Neural Plast. 2018, 2018, 5735789.
- Cabungcal, J.H.; Steullet, P.; Morishita, H.; Kraftsik, R.; Cuenod, M.; Hensch, T.K.; Do, K.Q. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 9130–9135.
- Morishita, H.; Cabungcal, J.H.; Chen, Y.; Do, K.Q.; Hensch, T.K. Prolonged Period of Cortical Plasticity upon Redox Dysregulation in Fast-Spiking Interneurons. Biol. Psychiatry 2015, 78, 396–402.
- Agarwala, S.; Kalil, R.E. Axotomy-induced neuronal death and reactive astrogliosis in the lateral geniculate nucleus following a lesion of the visual cortex in the rat. J. Comp. Neurol. 1998, 392, 252–263.
- Halassa, M.M.; Fellin, T.; Haydon, P.G. The tripartite synapse: Roles for gliotransmission in health and disease. Trends Mol. Med. 2007, 13, 54–63.
- Le Meur, K.; Mendizabal-Zubiaga, J.; Grandes, P.; Audinat, E. GABA release by hippocampal astrocytes. Front. Comput. Neurosci. 2012, 6, 59.
- Benedetti, B.; Matyash, V.; Kettenmann, H. Astrocytes control GABAergic inhibition of neurons in the mouse barrel cortex. J. Physiol. 2011, 589, 1159–1172.
- Waagepetersen, H.S.; Sonnewald, U.; Schousboe, A. Compartmentation of glutamine, glutamate, and GABA metabolism in neurons and astrocytes: Functional implications. Neuroscientist 2003, 9, 398–403.
- Walls, A.B.; Waagepetersen, H.S.; Bak, L.K.; Schousboe, A.; Sonnewald, U. The Glutamine–Glutamate/GABA Cycle: Function, Regional Differences in Glutamate and GABA Production and Effects of Interference with GABA Metabolism. Neurochem. Res. 2015, 40, 402–409.
- MacVicar, B.A.; Choi, H.B. Astrocytes Provide Metabolic Support for Neuronal Synaptic Function in Response to Extracellular K+. Neurochem. Res. 2017, 42, 2588–2594.
- Hassanzadeh, S.; Jalessi, M.; Jameie, S.B.; Khanmohammadi, M.; Bagher, Z.; Namjoo, Z.; Davachi, S.M. More attention on glial cells to have better recovery after spinal cord injury. Biochem. Biophys. Rep. 2021, 25, 100905.
- Leemhuis, E.; Giuffrida, V.; De Martino, M.L.; Forte, G.; Pecchinenda, A.; De Gennaro, L.; Giannini, A.M.; Pazzaglia, M. Rethinking the Body in the Brain after Spinal Cord Injury. J. Clin. Med. 2022, 11, 388.
- Spolidoro, M.; Sale, A.; Berardi, N.; Maffei, L. Plasticity in the adult brain: Lessons from the visual system. Exp. Brain Res. 2009, 192, 335–341.
- Bachtiar, V.; Stagg, C.J. The role of inhibition in human motor cortical plasticity. Neuroscience 2014, 278, 93–104.
- Kolasinski, J.; Hinson, E.L.; Divanbeighi Zand, A.P.; Rizov, A.; Emir, U.E.; Stagg, C.J. The dynamics of cortical GABA in human motor learning. J. Physiol. 2019, 597, 271–282.
- Castro-Alamancos, M.; Donoghue, J.P.; Connors, B.W. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J. Neurosci. 1995, 15, 5324–5333.
- Trepel, C.; Racine, R.J. GABAergic Modulation of Neocortical Long-Term Potentiation in the Freely Moving Rat. Synapse 2000, 35, 120–128.
- Clarkson, A.N.; Huang, B.S.; Macisaac, S.E.; Mody, I.; Carmichael, S.T. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 2010, 468, 305–309.
- Murphy, S.C.; Palmer, L.M.; Nyffeler, T.; Müri, R.M.; Larkum, M.E. Transcranial magnetic stimulation (TMS) inhibits cortical dendrites. eLife 2016, 5, e13598.
- Nardone, R.; Höller, Y.; Thomschewski, A.; Höller, P.; Lochner, P.; Golaszewski, S.; Brigo, F.; Trinka, E. Serotonergic transmission after spinal cord injury. J. Neural Transm. 2014, 122, 279–295.
- Araneda, R.; Andrade, R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 1991, 40, 399–412.
- Azmitia, E.C.; Gannon, P.J.; Kheck, N.M.; Whitaker-Azmitia, P.M. Cellular localization of the 5-HT(1A) receptor in primate brain neurons and glial cells. Neuropsychopharmacology 1996, 14, 35–46.
- Sheldon, P.W.; Aghajanian, G.K. Excitatory responses to serotonin (5-HT) in neurons of the rat piriform cortex: Evidence for mediation by 5-HT1C receptors in pyramidal cells and 5-HT2 receptors in interneurons. Synapse 1991, 9, 208–218.
- Sheldon, P.W.; Aghajanian, G.K. Serotonin (5-HT) induces IPSPs in pyramidal layer cells of rat piriform cortex: Evidence for the involvement of a 5-HT2 -activated interneuron. Brain Res. 1990, 506, 62–69.
- Fouad, K.; Rank, M.M.; Vavrek, R.; Murray, K.C.; Sanelli, L.; Bennett, D.J. Locomotion after spinal cord injury depends on constitutive activity in serotonin receptors. J. Neurophysiol. 2010, 104, 2975–2984.
- Giménez Y Ribotta, M.; Provencher, J.; Feraboli-Lohnherr, D.; Rossignol, S.; Privát, A.; Orsal, D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J. Neurosci. 2000, 20, 5144–5152.
- Guertin, P.A. Role of NMDA receptor activation in serotonin agonist-induced air-stepping in paraplegic mice. Spinal Cord 2004, 42, 185–190.
More
