Lung cancer is a highly aggressive neoplasm that is now a leading cause of cancer death worldwide. One of the major approaches for killing cancer cells is related with activation of apoptotic cell death with anti-cancer drugs. Regarding lung-cancer therapy, graphene was already considered as an attractive material for screening (biosensing) or direct anti-cancer treatment.
- flake graphene
- graphene oxide
- ferroptosis
- non-small lung cancer
- nanomaterials
1. Non-Small Lung Cancer
2. Ferroptosis
Reactive oxygen species (ROS) [13][14][15,16] along with excessive accumulation of iron is one of the ferroptosis pivotal mechanisms. It is worth underlining that ferroptosis is a newly defined programmed cell death process. It is recognized as an efficient mechanism to eliminate malignant cells. It plays an essential role in the inhibition of oncogenic processes by removing cells that are not able to keep nutrients in the environment or cells damaged by infection or stress [9]. Moreover, ferroptosis occurs together with defeated of repair system that are responsible for the removal of lipid peroxidation products in physiological homeostatic conditions. Contrary to healthy cells, cancer cells in majority lack repair systems, which means that they are more vulnerable to oxidative damage and, thus, ferroptosis. Nevertheless, the natural function of ferroptosis in physiological contexts is still not clear. There are some ideas that ferroptosis may serve as a normal tumor suppressive function, including the observation that tumor suppressors p53, fumarase, and BAP1 can drive ferroptosis under specific conditions, and that some negative regulators of ferroptosis, such as SLC7A11, GPX4, and NRF2 are overexpressed or activated in a diversity of tumors [15][16][17,18]. It is worth mentioning that ferroptosis can be responsible for a failure of different crucial organs, including, i.e., lungs, heart, or brain (Figure 1). For instance, one can find scientific articles describing ferroptosis-dependent neural diseases, including the following: cerebral stroke, Parkinson’s, or Alzheimer’s disease. Having this in mind, ferrotosis can be considered as an unwanted and dangerous factor affecting human health. However, if properly designed and controlled (using nanomaterial-based approach), it can be also be a useful tool for targeted anticancer remedies. It is well known that tumor cell death is the most desirable effect of any cancer therapy. However, similarly to what was mentioned earlier, available anticancer drugs directed to kill cancer cells through apoptosis have limited effects since acquired resistance to apoptosis occurs [17][19]. Therefore, the main goal of ferroptosis-based therapy in cancer is to prevent treatment resistance. Unlike other forms of cell death, ferroptosis is iron- and ROS-dependent. To date, several mechanisms on which ferroptosis can act in tumor cells have been indicated. Researchers described in great detail how the resistance to targeted cancer therapy could be reversed by inducing ferroptosis through iron and lipid metabolism pathways, as well as other signaling pathways. A perfect example of new ferroptosis-based therapeutic strategy in NSCLC is the overcoming of cisplatin resistance within NF-E2 related factor 2 (Nrf2)/light chain of System xc−(xCT) pathway given by Yu Li et al. [18][20]. Moreover, it has been recently presented that the inhibition of ferroptosis is engaged in cancer immunotherapy by anti-programmed cell death 1 (PD-1)/programmed death-ligand 1 (PD-L1) treatment resistance [9].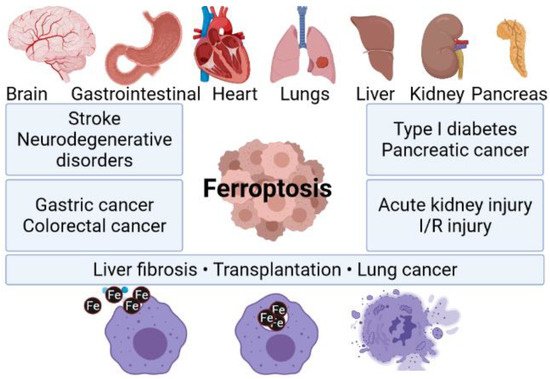
3. Nanomaterial Based Ferroptosis Inducers in NSCLC
A literature analysis of ferroptosis inducers (including small molecules and nanomaterials) is presented to define their design, action mechanisms, and anticancer applications; however, there are still many questions about mechanisms governing the killing activity of cancer cells through ferroptosis and its implications at molecular levels. The mechanisms of action of apoptosis or necroptosis are reliant on caspases or can be inhibited by their activity. It should be mentioned that ferroptosis appears to have advanced independent and very little known yet direct molecular cross-talk to other pathways of regulated cell death [32][34]. Specially designed nanomaterials (NMs) are known to be able to penetrate the human body through respiratory systems, oral ingestion, or a skin. Furthermore, NMs are capable to cross the plasma membrane in order to start cell death processes. Nanoparticles are considered as a novel ferroptosis inducer with possible therapeutic effects in a wide range of cancer types, including NSCLC. It is widely known that the distraction of cell-death homeostasis is related to various diseases, including neurodegenerative diseases, immune disorders, diabetes, and cancer. Available data indicate that iron- or iron-oxide-based NMs can be considered as a good approach to study ferroptosis and its implications in cancer. Mechanisms of iron-based NPs and non-iron NPs for ferroptosis-based cancer therapy are similar. Both types of NPs can be incorporated into cells through endocytosis and release iron in lysosomes. Furthermore, they can be involved in the Fenton reaction to produce reactive oxygen species (ROS) and, as a consequence, induce ferroptosis. Moreover, the drugs carried by nanoparticles can facilitate the production of ROS, which is caused by excess iron. β-lapachone (β-lap), a novel anticancer drug, has shown significant cancer specificity by increasing (ROS) stress in cancer cells. A 10-fold increase in ROS stress was detected in β-lap-exposed cells pretreated with iron oxide nanoparticle over cells treated with β-lap alone in A549 cells, which also correlates with significantly increased cell death [33][35]. Regardless of the nanomaterials’ size and origin, the overriding conclusion drawn by the authors of the manuscripts provided in Table 1 was that this theranostic approach is very promising. The results of their work clearly showed that nanomaterials can be implemented as an efficient tool for anticancer therapy. Interestingly, different types of cancer models were investigated, including inter alia breast, lung, colorectal, cervical, pancreatic, colon, and leukemia. All of the cited publications underlined that the utilization of nanoparticles enabled the triggering of ferroptosis. Hence, it can be hypothesized that nanomaterials can be considered as a future cancer remedy. However, a lot of work still has to be performed in order to fully understand and describe underlying ferroptosis mechanisms activated by nanomaterials.Nanoparticle Type | Nanoparticle Size | Cancer Model | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
AIEgen/vermiculite nanohybrid | 300 nm diameter, 1.1 nm thickness | MC38 tumor model |
[34] |
[36] |
|||||||
polyvinyl pyrrolidone dispersed nanoscale metal-organic framework of Fe-TCPP (TCPP = tetrakis (4-carboxyphenyl) porphyrin) loaded with hypoxia-activable prodrug tirapazamine and coated by the cancer cell membrane | 201 nm diameter of the whole system | Breast cancer cells (MDA-MB-231), human liver cancer cells (Huh7, HepG2), human colon cancer cells (HCT116), human pancreatic cancer cells (PATU8988), cervical cancer cells (HeLa) |
[35] |
[37] |
|||||||
Glycyrrhetinic acid loaded PLGA nanoparticles | 133 nm in diameter | Leukemia cells: Kasumi-1, U937, MV4–11, NB4, and colorectal cancer cells: HT29, Caco-2, SW480 |
[36] |
[38] |
|||||||
Gallic acid-ferrous (GA-Fe(II)) | average particle size of 3.1 ± 1.2 nm and hydrodynamic diameter of 4.7 ± 1.1 nm | breast cancer cell (MCF-7/ADR) |
[37] |
[39] |
|||||||
Zinc oxide nanospheres | 120 nm in diameter | CT26 and HCT116 colorectal cancer cells |
[38] |
[40] |
|||||||
piperlongumine (PL) loaded metal–organic framework (MOF) coated with transferrin decorated pH sensitive lipid layer | hydrodynamic radius of 185 ± 5.7 nm | 4T1 brest cancer cells |
[39] |
[41] |
|||||||
silver coated zero-valent-iron nanoparticles (ZVI@Ag) and carboxymethylcellulose coated zero-valent-iron nanoparticles (ZVI@CMC) | mean physical diameters of ZVI@Ag NPs and ZVI@CMC NPs were 81.08 ± 14.29 nm and 70.17 ± 14.4 nm | Human lung cancer cell lines H1299, H460, A549, mouse Lewis lung carcinoma (LLC) |
[40] |
[42] |
|||||||
Fe(II) and Tannic Acid-Cloaked MOF | 125–225 nm | MDA-MB-231 epithelial, human breast cancer cell line |
[41] |
[43] |
|||||||
Manganese doped silica nanoparticle (MnMSN) and folate modified long-circulating MnMSN (FaPEG-MnMSN) | MnMSN—diameter of 101.40 ± 0.36 nm, FaPEG-MnMSN diameter of 122.67 ± 2.98 nm | human hepatic carcinoma cells (HepG2), human non-small lung cancer cells (A549) and mouse breast cancer cells (4T1) |
[42] |
[44] |
|||||||
Superparamagnetic iron oxide nanoparticles (SPION) | 99–115 nm of hydrodynamic dimeter | Mouse mammary breast tumor cell line (4T1), human breast cancer cell line (MDA-MB-231) and human breast cancer cell line (MCF-7) |
[43] |
[45] |
