Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Thorsten Steinberg.
Oral diseases such as gingivitis, periodontitis, and oral cancer affect millions of people worldwide. Much research has been conducted to understand the pathogenetic mechanisms of these diseases and translate this knowledge into therapeutics.
- carcinogenesis
- cell transformation
- tissue homeostasis
- mechanotransduction
1. Introduction
Diseases of the oral cavity affect millions of people worldwide and lead to considerable morbidity and mortality [1,2][1][2]. From a clinicopathological perspective, these diseases can be classified as infectious/inflammatory (e.g., stomatitis, pulpitis, periodontitis), autoimmune/dysimmune (e.g., oral manifestations of Systemic Lupus Erythematosus or Sjögren syndrome), neoplastic (e.g., squamous dysplasia of the oral cavity or oral squamous cell carcinoma (OSCC)), traumatic, or congenital (e.g., odontogenic cysts) [3,4,5,6,7,8][3][4][5][6][7][8]. In 2020, 377,713 new cases of cancer of the lip and oral cavity and a total of 177,757 deaths of both sexes and all ages due to this disease were registered by the International Agency for Research on Cancer (IARC), World Health Organization (WHO) (https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 19 January 2022)). According to the Global Burden of Disease Study, severe periodontal disease was the 11th most prevalent health condition in the world. Herein, the prevalence of periodontal disease, comprising gingivitis and periodontitis, was reported to range from 20% to 50% around the world (The Global Burden of Diseases, Injuries, and Risk Factors Study 2017, GBD 2017) [9]. These numbers illustrate the urgent need for basic and clinical research in this field, as well as reliable and innovative treatment options for these diseases [10,11][10][11]. Primary prevention as well as secondary prevention, e.g., early detection with screening programs, is of pivotal importance to reduce the social and medical impact of these diseases. This is especially important for either highly prevalent diseases such as periodontitis (e.g., via the detection of inflammatory biomarkers in saliva), or for the detection of precancerous lesions, where the development of the actual tumor can be avoided [12,13,14,15][12][13][14][15]. In the context of OSCC’s early detection, innovative noninvasive diagnostic approaches, such as vital staining with Toluidine Blue, tissue autofluorescence, optic coherence tomography, or in vivo confocal microscopy, are promising tools since they overcome the disadvantages of classical biopsies [11,16,17,18,19][11][16][17][18][19].
For decades, the study of oral pathogenetic mechanisms has been a major goal of basic research in the field of oral biology. In addition to clinical studies on patients and animal models, in vitro cell culture systems have become precious tools to understand the molecular mechanisms underlying oral tissue morphogenesis as well as the above-mentioned oral diseases [20,21,22,23][20][21][22][23]. In almost all oral diseases, the cells of the periodontal tissues and the oral cavity are damaged by external noxae. These noxae consequently lead to alterations in the cells’ physiology and impair the function of the whole tissue [24]. While metabolic shifts are reversible during acute inflammation, cellular adaptations and tissue remodeling become permanent during chronic inflammation and tumorigenesis [25,26][25][26]. In the case of oral cancer, changes in the cells’ DNA via mutations and epigenome via epigenetic modifications severely impair cellular behavior [27]. Processes such as cell adhesion, migration, proliferation, differentiation, and apoptosis are dysregulated and finally lead to cellular invasion and metastasis [28,29,30,31][28][29][30][31]. In this context, cell culture platforms allow for, e.g., monitoring epithelial growth under physiological and pathological conditions, the molecular analysis of protein and gene expression patterns within the tissue, and the spatio-temporal manipulation of these processes [32]. The simulation of major pathogenetic factors, such as inflammatory stimuli or exposure to noxae such as ethanol, led to valuable insights into the pathogenesis of oral diseases [33,34][33][34]. Such a cell culture system will be presented here to illustrate the advantages but also the major shortcomings of this approach. In conjunction with clinical data, these findings are, nonetheless, the basis of the accurate diagnosis of disease entities as well as targeted, personalized treatment regimens in the future [35,36][35][36].
The periodontium is a complex tissue, harboring diverse cell types, including gingival keratinocytes (GKs), gingival connective tissue fibroblasts (GFs), periodontal ligament stem cells (PDLSCs), periodontal ligament fibroblasts (PDLFs), mesenchymal stem cells (MSCs), osteoblasts, and osteoclasts [37]. Although simple cell culture models form the basis of understanding in periodontal biology, they do not adequately represent the tissue architecture and are not sufficient to study tissue regeneration. This is especially important when it comes to the surgical treatment of periodontitis or OSCC, where large tissue defects need to be covered. In this context, functional tissue engineering has been an important issue in oral biology for more than a decade. In comparison with classical tissue engineering, this rather modern sub-discipline addresses both the biochemical and biophysical/biomechanical properties of a candidate biomaterial to support cell growth and tissue formation in the best possible way [38]. Compelling experimental evidence has established the concept of molecular mechanotransduction, the process by which external biophysical cues are sensed by a cell, and are subsequently transformed into intracellular biochemical signals and adaptive cellular responses [39]. Consequently, amongst others, material properties such as lack of cytotoxicity, elasticity/stiffness, and the number and density of cell adhesion points are now accepted as decisive parameters in the development of biomaterials [40,41][40][41]. The importance of environmental mechanical signals in regulating the aforementioned cell functions is evident in a paper published by the Ingber group. Herein, activation of the cellular tyrosine-kinase Sarcoma (c-Src) in response to mechanical forces is described to occur 40 times faster than that induced by the epidermal growth factor (EGF), as predicted by physical models [42,43][42][43]. This is of high relevance to the idea of the periodontium as an anatomical region with diverse tissues, which all have different biophysical properties. Thus, designing biomaterials for periodontal regeneration is extremely challenging [44,45,46,47][44][45][46][47]. A non-woven biohybrid for gingival regeneration and related work will be discussed, which illustrates the principles of biomechanics when implemented in tissue engineering and its application to oral medicine.
Regarding periodontal disease causatives, the pathogenetic role of bacteria such as porphyromonas gingivalis (PG) [48], treponema denticola (TD) [49], tannerella forsythia (TF) [50], aggregatibacter actinomycetemcomitans (AA) [51], and others is well established. Therefore, treatment strategies for, e.g., periodontitis, can also address non-host cells, i.e., the pathogens, instead of focusing on the actual host tissue by tissue engineering. Fighting the microbes is effective in mitigating the host–pathogen interaction that contributes to the destruction of the periodontium [52,53][52][53]. Non-surgical treatment of periodontitis often includes the local or systemic administration of antibiotics such as macrolides [54]. There is still a controversy regarding the proper treatment regime, since randomized, controlled trials are scarce [55,56][55][56]. Thus, alternative intervention strategies are needed. One possibility is to stimulate the proliferation of periodontal cells by, e.g., photobiomodulation, which is especially effective for soft tissue repair [57,58][57][58]. The development of synthetic antimicrobial peptides is another promising approach to support periodontitis treatment under experimental and clinical conditions. Such peptides occur naturally or are specifically designed to kill bacteria. Many antimicrobial peptides act as pore-forming toxins or pass the cell membrane of the bacteria to interfere with the function of cytosolic proteins [59,60][59][60]. The major advantage of antimicrobial peptides is their local administration, which reduces adverse effects and antibiotic resistance [61]. Thus, such peptides are a good complement to biomaterial-based oral tissue engineering to restore periodontal function. Interesting aspects of such polymers are discussed herein.
The understanding of oral tissues that arises from cell culture studies and biomaterial/polymer-based approaches is, however, only one facet of oral regenerative medicine. Diverse other technologies and techniques have been developed, which both broaden and complement the understanding of the (patho)-physiology of the oral cavity and can be used to mitigate disease conditions. Similar to other medical disciplines, cell-based treatment strategies that make use of stem cells (SCs) derived from various periodontal tissues, but also from bone marrow, adipose tissue, or induced pluripotent stem cells (iPSCs), have become important in periodontal tissue regeneration research in recent years [62]. SCs are characterized by their developmental potential, which enables them to differentiate into diverse cell types [63]. Thus, they can theoretically be used to build up tissues or whole organs from scratch [64,65][64][65]. This property renders them an interesting tool for periodontal regeneration. A further promising approach is the intentional light-responsive control of gene expression. This research area is called optogenetics (opto = light and genetics = genetic). Optogenetic gene control makes use of proteins, whose conformation changes in response to the application of light. This conformational change induces downstream effects, which can be directed towards the intentional induction or shutdown of certain transcripts. Although initially developed for neuronal cell research, this principle can be applied to tissue engineering or tissue regeneration in dental medicine and support, e.g., cell differentiation and wound healing or suppress malignant cellular phenotypes [66,67,68,69][66][67][68][69]. Consequently, the integration of SC biology and targeted gene regulation is an auspicious extension of cell culture studies and biomaterial-based tissue engineering in oral medicine and will be referred to in this article.
2. Biomaterial-Based Approaches in the Context of Oral Diseases
The biomaterial-based regeneration of oral tissues is a complex issue, since the periodontium is a highly specialized anatomic compartment with both soft and hard tissues. Material selection is, therefore, an essential first step in establishing sustainable and successful treatment options [142][70]. Important parameters include biocompatibility, biodegradability, and mechanical strength [143][71]. The choice between natural or synthetic polymers or hybrid approaches determines the physicochemical properties of the scaffolds. Next, the fabrication mode, e.g., electrospinning, 3D printing, or salt leaching, contributes to the material properties. Functionalization of the scaffold with chemically active residues (e.g., coating with ECM components) influences the material’s interaction with different cell types, which is the reason that an in-depth cell biological characterization of the target cells is crucial (see above) [142,144][70][72]. Currently, guided tissue regeneration, which prevents epithelial overgrowth in periodontitis treatment, autogenous bone substitutes, and diverse natural and synthetic polymers (e.g., polylactic acid, polycaprolactone, chitosan, alginate, collagen, silk) are in clinical use in oral medicine. The advantages and shortcoming of these approaches are discussed elsewhere [142,144][70][72]. Functional tissue engineering, however, also considers the mechanobiological aspects of the target tissue. Therefore, examples of biomaterial design for oral epithelial and soft tissue regeneration are discussed next [145][73].3. Cell-Based and Optogenetic Approaches: Perspectives in the Context of Oral Diseases
In the previous chapters, molecular alterations, and the biomaterial-based approaches derived in the context of oral diseases were discussed. This, however, does not represent the full spectrum of current and newly developed strategies to mitigate oral diseases. Against this background, this chapter deals with cell-based (Section 2.3.1) and optogenetic (Section 2.3.2) approaches, which integrate both cell biological and biomaterial-related principles. These selected possibilities offer new perspectives in regenerative oral medicine.3.1. Cell-Based Approaches
To successfully treat inflammatory oral diseases such as gingivitis and periodontitis, a profound understanding of the molecular basis of these diseases is required. In vitro monoculture cell models and interactive co-cultures can be very valuable tools, as discussed above. The inflammation-associated molecules and molecular mechanisms that are uncovered can then be translated into diagnostic and therapeutic applications. For diagnostic purposes, the immortalized gingival keratinocyte cell line HPV-16GM (=GKs) (see Section 2.3) was used to detect the molecular processes that occur in the oral cavity as part of an inflammatory response. To simulate inflammation, GKs were exposed to the pro-inflammatory cytokine IL-1β for 24 h and the changes in gene expression were detected by epithelial-specific cDNA microarray analysis. Differentially expressed genes were assigned to different functional domains of cellular biology: (i) cell stress (several heat shock proteins (HSPs)), (ii) DNA repair (topoisomerase II), (iii) cell cycle and proliferation (cyclin B, cell division cycle 2 (cdc2; a cyclin-dependent kinase)), (iv) anti-pathogen response (IL-8, IP-10 (CXCL10, chemokine ligand 10), and MIP3a (CCL20, chemokine ligand 20)), (v) extracellular matrix and turnover (tenascin, laminins a5 (LAMA5), b1 (LAMB1), a3 (LAMA3), and c2 (LAMC2), as well as the matrix metalloproteinases MMP2 and MMP10), and (vi) angiogenesis (vascular endothelial growth factor (VEGF) and the related VEGF-C). Interestingly, the differential gene expression induced by IL-1β stimulation correlated with the translocation of NF-kB to the nucleus [203][74]. These findings show that similar molecular analyses can be used to identify the new candidate genes that are involved in inflammation-related oral and periodontal diseases. Further characterization of these candidates is the basis for their potential establishment as diagnostic oral disease markers. Regarding the myriad of transcription factors, NF-kB activity is intimately linked to periodontitis. In this context, the finding of inflammation-associated NF-kB nuclear translocation was confirmed in a recent work, in which the authors exposed PDLFs to bacterial LPS as an inflammatory stimulus [204][75]. As outlined in the introduction (see Section 1), SCs from the oral cavity, which largely exhibit the features of hMSCs, are a cornerstone of cell-based regenerative approaches for oral diseases including caries, periodontitis, and oral cancers. This broad field was recently reviewed by Yang et al. [205][76]. The expression of hMSC-featuring biomarkers was exemplified in a study on human dental follicle cells (DFCs), while distinct DFC-fractions, apart from embryonic and neural SC markers, expressed MSC-specific biomarkers (Notch homolog 1 (Notch1), mesenchyme-1 (STRO-1), cell surface receptor CD44, and HLA-ABC [(MHC) class I] as well as the IgG super-family member CD90) [206][77]. A more detailed analysis of the tissue-regeneration abilities of SCs derived from different oral tissues revealed that, for example, gingival mesenchymal SCs were superior to dental pulp-derived mesenchymal SCs with respect to cell functions such as (i) proliferation, (ii) migration, and (iii) induction of angiogenesis. While proliferation and migration were only analyzed under in vitro conditions, looking at colony-forming ability and wound scratch assays, angiogenesis was also analyzed in vivo by vessel formation in nude mice, following the grafting of SC-harboring MatrigelTM constructs [207][78]. To exert their full regenerative potential, molecular interactions between SCs and their neighboring cells are indispensable. The interplay between dental MSCs and host tissue cells not only includes genuine periodontal cells, but also immune cells. This points to an SC-inherent immunomodulatory competence. This competence is exemplified by an in vitro study on PDLSCs, which were shown to modulate the immunological responses of neutrophils in a paracrine fashion. To this end, neutrophil-differentiated human promyelocytic leukemia HL-60 cells (HL60D) were treated with PDLSCs supernatants after PDLSC exposure to PG protein extracts. The response was determined by a significant increase in the HL60D recruitment rate and intracellular reactive oxygen species (ROS) compared to control conditions [208,209][79][80]. Moreover, osteogenic DPSCs were shown to inhibit the proliferation of peripheral blood mononuclear cells (PBMCs) when cocultured in vitro. Vice versa, levels of anti-inflammatory cytokines, e.g., transforming growth factor-β (TGF-β), increased [208,210][79][81]. Against this background, studies were conducted on the biophysics and interactions of SCs with other periodontal cell types. With respect to periodontal SCs, studies on the effects of the environmental biomechanical stiffness on cell behavior are very common, as exemplified by a current publication by Liu et al. In this, the authors showed that increasing substrate stiffness promotes the proliferation and osteogenic phenotype of PDLSCs. This was indicated by the increased expression of alkaline phosphatase (ALP), osteopontin (OPN), osteocalcin (OCN), bone morphogenic protein-2 (BMP-2), and Runt-related transcription factor 2 (RUNX2, also known as core-binding factor subunit alpha-1 (CBF-alpha-1)) [211][82]. Contrary to this, studies on the spacing of anchor points for SCs’ cell adhesion are rare. However, it could be shown that the micropatterns, and thus the provision of potential adhesion points for FN-biofunctionalized PDMS microcolumns, determine the adhesion and morphogenesis, as well as the proliferation and differentiation of hMSCs. Smaller column spacings of 5 µm and 7 µm favored cell attachment and cell spreading/morphogenesis, when compared to spacings of 9 µm and 11 µm. At the cell behavioral level, the hMSCs’ proliferation continuously decreased with increasing distances. Regarding differentiation, typical mesenchymal SC-associated genes were expressed at higher levels in cells grown on micropatterns with large pillar distances, preferentially 9 µm and 11 µm. This was revealed by an analysis of leukemia inhibitory factor (LIF), basic fibroblast growth factor (bFGF), nucleoside diphosphate-linked moiety X motif 6 (NUDT6), and nestin, suggesting the preservation of the SC character under these environmental conditions [212][83]. These findings show that basic SC functions, such as adhesion, morphogenesis, proliferation, and the SC character per se, also depend on biophysical cues, as summarized at the end of Section 2.2. Cell behavior in its natural environment, however, is even more complex. When in vitro-cultured SCs are transferred to the respective periodontal tissue for the purpose of tissue regeneration, the transplanted SCs come into direct or indirect contact with the surrounding cells of the host tissue and interact with them. This interaction implies that SCs influence the growth and differentiation properties of host cells and vice versa. Therefore, it is important for the SC-based regeneration of periodontal tissues to broaden the knowledge of the phenotypic or cell behavioral effects of such interactions and the molecules involved. To address this important aspect of intercellular crosstalk, interactive cocultures of hMSCs with human (h) hGFs, hPDLFs, and osteoblasts of alveolar bone (hOA), were established. The resulting hMSCs behavior was examined. The analyses revealed that these oral cell types affected hMSCs’ behavior, in that the SCs showed a reduction in both proliferation and typical SC marker gene expression (e.g., POU domain class 5 transcription factor 1 (POU5F1), transcription factor homeobox protein Nanog, and SC growth factor receptor, also known as tyrosine protein kinase kit (c-kit)) within a 2-week observation period. This proliferation-reducing and differentiation-inducing effect was most pronounced when hMSCs were cocultured in the presence of hOA and was less pronounced in the presence of hGFs and hPDLFs. hMSCs reduced the number of apoptotic cells, regardless of the cocultured oral cell type [213][84]. These findings show that hMSCs and the respective periodontal host cell entities mutually influence each other regarding cell behavioral features including proliferation, hMSC stemness, and cell survival. Thus, the results obtained from this kind of interactive coculture are form the basis of predictions regarding stem and host cell behavior in hMSC-based oral tissue regeneration. In further studies with these cocultures, it could be demonstrated that hMSCs elicited the expression of markers of osteogenic differentiation, e.g., OPN, OCN, RUNX2, and collagen 1a1 (COL-1a1), in the three cell types described above. Additionally, 3D-hMSC-periodontal cell cocultures resulted in increased bone matrix production and a higher presence of mineralization nodes compared to control cultures without hMSCs. The expression of the osteogenic phenotype in the co-culture decreased from hOAs to hGFs and hPDLFs [214][85]. These findings indicate that hMSCs are potentially capable of stimulating the osteogenic phenotype to varying degrees in oral host cells. They also show that different periodontal cells have a different degree of inherent plasticity concerning osteogenic differentiation. These insights provide valuable clues regarding the use of hMSCs for oral bone regeneration. In this context, the cell behavioral effects of hMSCs on hOAs were further analyzed. In hMSC-hOA-cocultures, hMSC-derived VEGF release induced the chemoattraction of hOAs. This mechanistic connection was confirmed by chemoattraction assays and employment of the VEGF receptor inhibitor 3-[4-(dimethylamino)benzylidenyl]indolin-2 (=SU4312), with the latter completely abolishing hOA chemoattraction towards hMSCs [215][86]. This finding shows that hMSCs may contribute to periodontal/oral bone regeneration, not only through the induction of the osteogenic phenotype in oral host cells, but also by attracting hard tissue-forming cells to sites of bone regeneration. Moreover, tresearchis study suers supports the notion that oral tissue/periodontal tissue-derived SC may exert a regenerative action on damaged oral/periodontal tissues via secreted bioactive molecules, as suggested by the observed chemo-attractive capacity of hMSC-derived VEGF. In the context of oral bone homeostasis, this suggestion is backed up by an in vitro study on murine MSCs (mMSCs), which were cocultured together with osteoclast precursor cells (RAW264). Here, the authors found that the chemokine C-C motif chemokine ligand 2 (CCL2) secreted by the mMSCs was responsible for the chemotaxis of RAW264 cells in a Boyden chamber assay [216][87]. The importance of soluble factors such as VEGF as determinants of cellular differentiation and behavior is a long-established concept. This notion is increasingly important for oral and periodontal tissues. It could be shown that the medium supernatants obtained from oral/periodontal SCs (also designated as a conditioned medium (CM)) can support periodontal tissue regeneration. The regenerative potential of CM was recently reviewed by Lin et al. [217][88], and exemplified in a study by Qiu and coworkers. Herein, collagen membranes harboring the CM of gingival mesenchymal stem cells (GMSCs) and PDLSCs were able to effectively support periodontal tissue regeneration in a rat periodontal defect model. Methodically, regeneration was detected by comparing newly formed periodontal ligament and alveolar bone in CM-treated versus non-treated animals [218][89]. In a further rat periodontal defect study, employing the CM of PDLSCs and GFs, only the PDLSC-CM was able to support periodontal tissue regeneration, as indicated by newly formed bone, whereas the GF-CM failed to do this. PDLSC-CM proteome analysis revealed a complex mixture of components, including extracellular matrix proteins, enzymes, angiogenic factors, growth factors, and cytokines. Thus, many potential candidates for this so-called secretome may be involved in the actual tissue regeneration process. In addition, the authors could show that PDLSC-CM treatment led to a decrease in periodontal inflammation, as indicated by decreased mRNA levels for tumor necrosis factor alpha (TNF-α) [219][90]. These findings underscore the SCs’ various mechanisms of action influencing and directing their neighboring cells in the context of tissue regeneration. Regarding SC-based regeneration, another interesting question arises: is periodontal regeneration also possible without the selected SC populations? To answer this question, an alveolar bone regenerative approach that is completely devoid of preselected SCs was established. Herein, mixed cell populations were created from oral tissues, namely, the alveolar bone, the PDL, and the gingival connective tissue. The tests for biomarkers of progenitor and SCs c-kit, STRO-1, and melanoma cell adhesion molecule (MCAM/CD146), an accepted marker for stem/progenitor cells in combination with STRO-1 in periodontal cells [220][91], only showed a marginal proportion of progenitor and SCs, regardless of the cell fraction. After culturing the three cell populations in a medium specialized for osteogenic differentiation, classical osteogenic biomarkers (e.g., bone gamma-carboxyglutamate protein (BGLAP/Osteocalcin), RUNX2, Osterix (OSX = transcription factor SP7) and ALP) were increased in the cell population of alveolar bone and PDL at the gene expression level, while they were downregulated in the gingival connective tissue fraction (GCTF). While the increase in biomarker expression in the bone fraction was not surprising, the results in the PDL cell fraction (PDLCF) show that the osteogenic phenotype is inducible. Moreover, a comparison of PDLCF with GCTF revealed a much higher matrix mineralization and ALP activity in PDLCF, as detected by the quantification of alizarin red stain and enzyme activity [221][92]. These data suggest that PDL cells do not require SC input to contribute to bone regeneration and may, therefore, be promising candidates in the development of prospective cell-based therapy concepts. Another strategy for prospective SC-free alveolar bone regeneration is the use of osteoblasts. These cells can be stimulated under in vitro conditions so that they are pushed as much as possible towards bone formation, i.e., they are preconditioned. Due to their growth and differentiation behavior, these preconditioned cells could be promising candidates for in vivo re-transference to efficiently support bone formation at the implantation site. To be as independent as possible from expensive growth factors and cytokines, as well as hormones or xenobiotics, during this preconditioning, a poly(methyl methacrylate) (PMMA)/polycarbonate (PC)-based microchip with an FN biofunctionalization was chosen as a platform. The latter is also suitable for 3D cell culture [222][93]. Osteoblasts inoculated into the FN-functionalized microcavities (300 µm × 300 µm × 300 µm; width × length × depth) of the chip revealed homogenous cell adhesion and morphogenesis, as well as a high vitality and growth, as indicated by scanning electron microscopy (SEM) and dye-based live/dead-staining for up to 14 days of culture. This was accompanied by significantly increased gene expression levels for osteonectin (ON), OCN, and ALP in comparison with matched conventional 2D-monolayer cultures. In addition, the formation of multilayered osteoblast aggregates of a uniform size within the microcavities was detected by Azur II staining from day 7, concomitant with the intercellular deposition of ECM bone matrix constituents, including OCN, ON and FN [223][94]. In current in vitro and in vivo bone-healing studies, FN was shown to contribute to fracture healing. Lee and coworkers reported an experimental setup in which the type III domains of FN (domains 9 and 10) were fused to elastin-like polypeptides (FN-ELPs). When using these FN-ELPs as a cell culture dish coating, the osteogenic differentiation of hMSCs is triggered. hMSCs exhibited elevated ALP and mineralization activity in conjunction with an increased gene expression of OPN and COL-1 [224][95]. Under in vivo conditions, a coating of beta-tricalcium phosphate (β-TCP) particles (size 0.25–1 mm) with an FN solution (1 g/L) significantly increased the guided formation of bone within 8 weeks in a rat calvaria critical-sized defect model [225][96]. This renders FN a suitable candidate for prospective bone fracture healing in the case of oral diseases. In a further approach using the previously described PMMA/PC-microchip, biomechanical cues, such as interstitial biomechanical fluid flow through the lacunar–canalicular system (LCS), were imitated. LCS flow is an important environmental cue in hard tissue/bone homeostasis and remodeling [226][97]. The optimization of LCS flow has been analyzed in a brand-new article published by Wang and coworkers, which demonstrates, with the help of a multiscale model, that a few load cycles with rest insertion, high strain magnitude and rate support LCS flow within the osteocyte LCS [227][98]. Concerning the importance of LCS flow for bone tissue, osteoblasts of the alveolar bone were inoculated into the microcavities of the 3D-chip and cultivated under static and fluid flow conditions in perfusion bioreactors for 7 days. An SEM-based comparison of the two culture conditions revealed that the osteoblasts differed significantly in terms of morphogenesis. The cells cultivated under fluid flow conditions were exclusively reorganized into a rotund, osteon/bone-like tissue that consisted of densely packed, multicellular, three-dimensional cell aggregates. As indicated by time-lapse microscopy, the formation of these mulberry-like cell aggregates occurred within the first 24 h. Cell aggregate formation was also accompanied by higher ECM gene expression and the expression of bone differentiation-associated genes (COL-1, OCN, ON and ALP) after 7 days of culture. Mathematical modeling of the fluid flow conditions within the microchips revealed that this ranged from 8 to 32 µm/s, which is roughly in the range of the flow velocity of 24–84 µm/s for the osteocyte process membrane of native bone tissue [228][99]. These results suggest that, on the one hand, the culture can precondition osteoblasts in the direction of bone-like structures under 3D conditions, and, on the other hand, fluid flow in 3D cultures represents a further trigger to stimulate the bone micro-tissue formation of osteoblasts in vitro.3.2. Optogenetic Strategies
Cell interactions and biomaterials can induce a specific cell-behavioral response in target cells (see Section 2.2 and Section 2.3.1). This is, however, a complex process, whose specific steps and interactions are not always well understood. Many applications and research questions in regenerative medicine make the more specific control of cellular responses desirable. Optogenetics is a subtle concept to induce the spatiotemporally controlled expression of regeneration-promoting genes in target cells. To this end, light-inducible transgene expression systems are used. A red/far-red light-triggered on/off gene switch is presented to demonstrate the possible mode of operation of such a system. This was tested in diverse mammalian cell types in vitro for its general applicability. Subsequent in vivo testing aimed for spatially controlled angiogenesis in a chicken embryo model [229][100]. “Switch-on” of the optogenetic system: The first step is the transfection of target cells, e.g., primary human umbilical vein endothelial cells (HUVEC), for the controlled expression of a target gene, e.g., VEGF. Illumination with red light (660 nm) leads to the isomerization of the chromophore phycocyanobilin B (PCB, purified from the cyanobacterium Spirulina), which is bound to the intracellularly expressed phytochromobilin (PhyB). Induced by the isomerization-dependent conformal change in PhyB, the chromoprotein interacts with a split transcription factor comprising two subunits, U1 and U2. The PhyB-U1-U2 complex represents a functional transcription factor, which binds via a distinct element (DE) to a certain operator region (CR) of a vector DNA. Complex binding to CR recruits RNA polymerase II and induces the transcription of the gene of interest (GOI) within a so-called response vector (RV). In an exemplary study, genes such as human VEGF, which is supportive of angiogenesis during wound-healing and tissue regeneration, were used. For oral tissues, VEGF derived from saliva was recently reported to support wound-healing in patients in response to tooth extraction, which qualifies this optogenetic system for potential use in the oral cavity [229][100]. “Switch-off” of the system: Upon illumination with far-red light (740 nm), the PhyB-U1-U2 complex dissociates, thereby silencing the GOI expression within the RV [230][101]. The functioning of the optogenetic system is shown in Figure 51.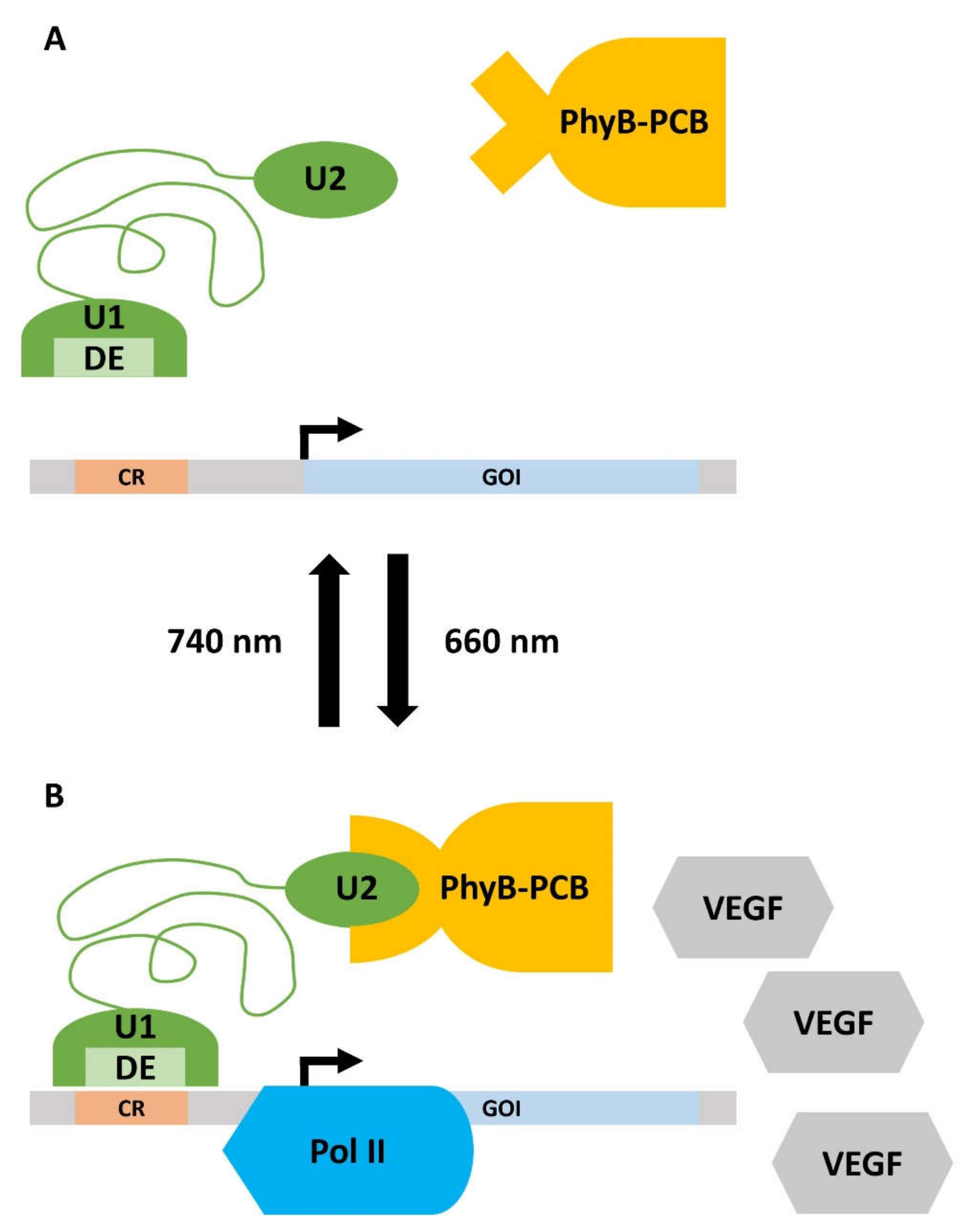
Figure 51. Working principle of the optogenetic gene expression switch. (A) Upon irradiation with far-red light (740 nm), the gene expression is turned off. The phycocyanobilin B (PCB), which is bound to phytochromobilin (PhyB), is in a closed conformation and cannot bind to unit 2 (U2) of the split transcription factor (green). Thus, the distinct element (DE) of unit 1 (U1) of the transcription factor does not interact with the certain operator region (CR) on the response vector. Consequently, there is no detectable expression of the gene of interest (GOI). (B) Illumination with red light (660 nm) leads to the isomerization of PCB, which induces a conformational change in the PhyB-PCB complex. PhyB-PCB can now recognize U2, which activates the split transcription factor. DE recognizes CR and recruits RNA Polymerase II (Pol II) to the promotor of the response vector. The GOI, in this case, vascular endothelial growth factor (VEGF), is now expressed. Details are given in the main text.
References
- Ahmad, P.; Rubbia Nawaz, M.Q.; Shaikh, G.M.; Mohamed, R.N.; Nagarajappa, A.K.; Asif, J.A.; Alam, M.K. Risk factors associated with the mortality rate of oral squamous cell carcinoma patients: A 10-year retrospective study. Medicine 2021, 100, e27127.
- Wu, L.; Zhang, S.Q.; Zhao, L.; Ren, Z.H.; Hu, C.Y. Global, regional, and national burden of periodontitis from 1990 to 2019: Results from the Global Burden of Disease study 2019. J. Periodontol. 2022.
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S219–S229.
- Muller, S.; Tilakaratne, W.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumours of the Oral Cavity and Mobile Tongue. Head Neck Pathol. 2022, 16, 54–62.
- Saccucci, M.; Di Carlo, G.; Bossù, M.; Giovarruscio, F.; Salucci, A.; Polimeni, A. Autoimmune diseases and their manifestations on oral cavity: Diagnosis and clinical management. J. Immunol. Res. 2018, 2018.
- Schmalz, G.; Garbade, J.; Kollmar, O.; Ziebolz, D. Does oral health-related quality of life of patients after solid organ transplantation indicate a response shift? Results of a systematic review. BMC Oral Health 2020, 20, 1–12.
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880.
- Wenig, B.M.; Childers, E.L.; Richardson, M.S.; Seethala, R.R.; Thompson, L.D. Non-Neoplastic Diseases of the Head and Neck; American Registry of Pathology in collaboration with the Armed Forces: Washington, DC, USA, 2017.
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80.
- Ghantous, Y.; Elnaaj, A. Global incidence and risk factors of oral cancer. Harefuah 2017, 156, 645–649.
- Romano, A.; Di Stasio, D.; Petruzzi, M.; Fiori, F.; Lajolo, C.; Santarelli, A.; Lucchese, A.; Serpico, R.; Contaldo, M. Noninvasive Imaging Methods to Improve the Diagnosis of Oral Carcinoma and Its Precursors: State of the Art and Proposal of a Three-Step Diagnostic Process. Cancers 2021, 13, 2864.
- Bouaoud, J.; Bossi, P.; Elkabets, M.; Schmitz, S.; van Kempen, L.C.; Martinez, P.; Jagadeeshan, S.; Breuskin, I.; Puppels, G.J.; Hoffmann, C. Unmet Needs and Perspectives in Oral Cancer Prevention. Cancers 2022, 14, 1815.
- Nocini, R.; Capocasale, G.; Marchioni, D.; Zotti, F. A snapshot of knowledge about oral cancer in Italy: A 505 person survey. Int. J. Environ. Res. Public Health 2020, 17, 4889.
- Räisänen, I.T.; Umeizudike, K.A.; Pärnänen, P.; Heikkilä, P.; Tervahartiala, T.; Nwhator, S.O.; Grigoriadis, A.; Sakellari, D.; Sorsa, T. Periodontal disease and targeted prevention using aMMP-8 point-of-care oral fluid analytics in the COVID-19 era. Med. Hypotheses 2020, 144, 110276.
- Schmalz, G.; Ziebolz, D. Changing the focus to the whole patient instead of one oral disease: The concept of individualized prevention. Adv. Prev. Med. 2020, 2020, 6752342.
- Algadi, H.A.H.; Abou-Bakr, A.A.-E.; Jamali, O.M.; Fathy, L.M. Toluidine blue versus frozen section for assessment of mucosal tumor margins in oral squamous cell carcinoma. BMC Cancer 2020, 20, 1147.
- Shavlokhova, V.; Sandhu, S.; Flechtenmacher, C.; Koveshazi, I.; Neumeier, F.; Padrón-Laso, V.; Jonke, Ž.; Saravi, B.; Vollmer, M.; Vollmer, A. Deep Learning on Oral Squamous Cell Carcinoma Ex Vivo Fluorescent Confocal Microscopy Data: A Feasibility Study. J. Clin. Med. 2021, 10, 5326.
- Sun, L.-F.; Wang, C.-X.; Cao, Z.-Y.; Han, W.; Guo, S.-S.; Wang, Y.-Z.; Meng, Y.; Hou, C.-X.; Zhu, Q.-H.; Tang, Y.-T. Evaluation of autofluorescence visualization system in the delineation of oral squamous cell carcinoma surgical margins. Photodiagnosis Photodyn. Ther. 2021, 36, 102487.
- Yang, Z.; Shang, J.; Liu, C.; Zhang, J.; Liang, Y. Identification of oral precancerous and cancerous tissue by swept source optical coherence tomography. Lasers Surg. Med. 2022, 54, 320–328.
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083.
- Al-Dabbagh, R.; Al-Hazmi, N.; Alhazzazi, T.Y.; Barrett, A.; Speight, P.M. Human papillomavirus and head and neck squamous cell carcinoma in a UK population: Is there an association. Indian J. Cancer 2021.
- Duan, Y.; Huang, X.; Qiao, B.; Ma, R.; Li, J. Eugenol inhibits the biological activities of an oral squamous cell carcinoma cell line SCC9 via targeting MIF. Anti-Cancer Agents Med. Chem. 2022. online ahead of print.
- Wang, W.-C.; Huang, M.-Y.; Chen, Y.-K.; Lan, W.-C.; Shieh, T.-M.; Shih, Y.-H. Salivary Exosome Proteomics and Bioinformatics Analysis in 7, 12-Dimethylbenz anthracene-Induced Oral Cancer with Radiation Therapy—A Syrian Golden Hamster Model. Diagnostics 2021, 12, 65.
- Truchard, E.; Bertolus, C.; Martinez, P.; Thomas, E.; Saintigny, P.; Foy, J.-P. Identification of a Gene-Expression-Based Surrogate of Genomic Instability during Oral Carcinogenesis. Cancers 2022, 14, 834.
- Fleetwood, A.J.; Lee, M.K.; Singleton, W.; Achuthan, A.; Lee, M.-C.; O’Brien-Simpson, N.M.; Cook, A.D.; Murphy, A.J.; Dashper, S.G.; Reynolds, E.C. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Front. Cell. Infect. Microbiol. 2017, 7, 351.
- Ram-Mohan, N.; Meyer, M.M. Comparative metatranscriptomics of periodontitis supports a common polymicrobial shift in metabolic function and identifies novel putative disease-associated ncRNAs. Front. Microbiol. 2020, 11, 482.
- Nakagaki, T.; Tamura, M.; Kobashi, K.; Koyama, R.; Fukushima, H.; Ohashi, T.; Idogawa, M.; Ogi, K.; Hiratsuka, H.; Tokino, T.; et al. Profiling cancer-related gene mutations in oral squamous cell carcinoma from Japanese patients by targeted amplicon sequencing. Oncotarget 2017, 8, 59113–59122.
- Kang, W.; Ji, X.; Zhang, X.; Tang, D.; Feng, Q. Persistent exposure to Fusobacterium nucleatum triggers chemokine/cytokine release and inhibits the proliferation and osteogenic differentiation capabilities of human gingiva-derived mesenchymal stem cells. Front. Cell. Infect. Microbiol. 2019, 9, 429.
- Keong, J.Y.; Low, L.W.; Chong, J.M.; Ong, Y.Y.; Pulikkotil, S.J.; Singh, G.; Nagendrababu, V.; Banavar, S.R.; Khoo, S.P. Effect of lipopolysaccharide on cell proliferation and vascular endothelial growth factor secretion of periodontal ligament stem cells. Saudi Dent. J. 2020, 32, 148–154.
- Listyarifah, D.; Al-Samadi, A.; Salem, A.; Syaify, A.; Salo, T.; Tervahartiala, T.; Grenier, D.; Nordström, D.C.; Sorsa, T.; Ainola, M. Infection and apoptosis associated with inflammation in periodontitis: An immunohistologic study. Oral Dis. 2017, 23, 1144–1154.
- Ramenzoni, L.L.; Russo, G.; Moccia, M.D.; Attin, T.; Schmidlin, P.R. Periodontal bacterial supernatants modify differentiation, migration and inflammatory cytokine expression in human periodontal ligament stem cells. PLoS ONE 2019, 14, e0219181.
- Mountcastle, S.E.; Cox, S.C.; Sammons, R.L.; Jabbari, S.; Shelton, R.M.; Kuehne, S.A. A review of co-culture models to study the oral microenvironment and disease. J. Oral Microbiol. 2020, 12, 1773122.
- Müssig, E.; Steinberg, T.; Kohl, A.; Chamulitrat, W.; Komposch, G.; Tomakidi, P. Discrimination of epithelium-like and fibroblast-like phenotypes derived from ethanol-treated immortalised human gingival keratinocytes in epithelial equivalents. Cell Tissue Res. 2008, 332, 57–71.
- Sun, S.; Yang, H.; Wang, F.; Zhao, S. Oct4 downregulation-induced inflammation increases the migration and invasion rate of oral squamous cell carcinoma. Acta Biochim. Biophys. Sin. 2021, 53, 1440–1449.
- Teh, M.-T.; Ma, H.; Liang, Y.-Y.; Solomon, M.C.; Chaurasia, A.; Patil, R.; Tekade, S.A.; Mishra, D.; Qadir, F.; Yeung, J.-Y.S. Molecular Signatures of Tumour and Its Microenvironment for Precise Quantitative Diagnosis of Oral Squamous Cell Carcinoma: An International Multi-Cohort Diagnostic Validation Study. Cancers 2022, 14, 1389.
- Yen, W.-C.; Chang, I.Y.-F.; Chang, K.-P.; Ouyang, C.N.; Liu, C.-R.; Tsai, T.-L.; Zhang, Y.-C.; Wang, C.-I.; Wang, Y.-H.; Yu, A.L. Genomic and molecular signatures of successful patient-derived xenografts for oral cavity squamous cell carcinoma. Front. Oncol. 2022, 12, 792297.
- Han, J.; Menicanin, D.; Gronthos, S.; Bartold, P. Stem cells, tissue engineering and periodontal regeneration. Aust. Dent. J. 2014, 59 (Suppl. S1), 117–130.
- Lam, V.K.L.; Wong, J.Y.H.; Chew, S.Y.; Chan, B.P. Rac1-GTPase regulates compression-induced actin protrusions (CAPs) of mesenchymal stem cells in 3D collagen micro-tissues. Biomaterials 2021, 274, 120829.
- Bjørge, I.M.; de Sousa, B.M.; Patrício, S.G.; Silva, A.S.; Nogueira, L.P.; Santos, L.F.; Vieira, S.I.; Haugen, H.J.; Correia, C.R.; Mano, J.F. Bioengineered Hierarchical Bonelike Compartmentalized Microconstructs Using Nanogrooved Microdiscs. ACS Appl. Mater. Interfaces 2022, 14, 19116–19128.
- Wang, L.; Wang, C.; Wu, S.; Fan, Y.; Li, X. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges. Biomater. Sci. 2020, 8, 2714–2733.
- Vasconcelos e Cruz, J.; Delgado, A.H.; Félix, S.; Brito, J.; Gonçalves, L.; Polido, M. Improving Properties of an Experimental Universal Adhesive by Adding a Multifunctional Dendrimer (G-IEMA): Bond Strength and Nanoleakage Evaluation. Polymers 2022, 14, 1462.
- Na, S.; Collin, O.; Chowdhury, F.; Tay, B.; Ouyang, M.; Wang, Y.; Wang, N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. USA 2008, 105, 6626–6631.
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82.
- Kanemoto, Y.; Miyaji, H.; Nishida, E.; Miyata, S.; Mayumi, K.; Yoshino, Y.; Kato, A.; Sugaya, T.; Akasaka, T.; Nathanael, A.J. Periodontal tissue engineering using an apatite/collagen scaffold obtained by a plasma-and precursor-assisted biomimetic process. J. Periodontal Res. 2022, 57, 205–218.
- Kwack, K.H.; Ji, J.Y.; Park, B.; Heo, J.S. Fucoidan (Undaria pinnatifida)/Polydopamine Composite-Modified Surface Promotes Osteogenic Potential of Periodontal Ligament Stem Cells. Mar. Drugs 2022, 20, 181.
- Tryba, A.M.; Krok-Borkowicz, M.; Kula, M.; Piergies, N.; Marzec, M.; Wegener, E.; Frączyk, J.; Jordan, R.; Kolesińska, B.; Scharnweber, D. Surface Functionalization of Poly (L-lactide-co-glycolide) Membranes with RGD-Grafted Poly (2-oxazoline) for Periodontal Tissue Engineering. J. Funct. Biomater. 2022, 13, 4.
- Wang, J.; Chen, Y.; Li, J.; Chen, Z.; Fan, M.; Lin, F.; Xie, Y. Electrospun Polysaccharides for Periodontal Tissue Engineering: A Review of Recent Advances and Future Perspectives. Ann. Biomed. Eng. 2022, 1–25.
- Amano, A. Molecular Interaction of Porphyromonas gingivalis with Host Cells: Implication for the Microbial Pathogenesis of Periodontal Disease. J. Periodontol. 2003, 74, 90–96.
- Sela, M.N. Role of Treponema denticola in periodontal diseases. Crit. Rev. Oral Biol. Med. 2001, 12, 399–413.
- Sharma, A. Virulence mechanisms of Tannerella forsythia. Periodontol. 2000 2010, 54, 106.
- Kesic, L.; Petrovic, M.; Obradovic, R.; Pejcic, A. The importance of aggregatibacter actinomycetemcomitans in etiology of periodontal disease—Mini review. Acta Med. Median. 2009, 48, 35–37.
- Lagosz-Cwik, K.B.; Wielento, A.; Lipska, W.; Kantorowicz, M.; Darczuk, D.; Kaczmarzyk, T.; Gibbs, S.; Potempa, J.; Grabiec, A.M. hTERT-immortalized gingival fibroblasts respond to cytokines but fail to mimic primary cell responses to Porphyromonas gingivalis. Sci. Rep. 2021, 11, 10770.
- Rocha, F.G.; Ottenberg, G.; Eure, Z.G.; Davey, M.E.; Gibson III, F.C. Sphingolipid-containing outer membrane vesicles serve as a delivery vehicle to limit macrophage immune response to Porphyromonas gingivalis. Infect. Immun. 2021, 89, e00614-20.
- Hammami, C.; Nasri, W. Antibiotics in the Treatment of Periodontitis: A Systematic Review of the Literature. Int. J. Dent. 2021, 2021.
- Lu, H.; He, L.; Jin, D.; Zhu, Y.; Meng, H. Effect of adjunctive systemic antibiotics on microbial populations compared with scaling and root planing alone for the treatment of periodontitis: A pilot randomized clinical trial. J. Periodontol. 2021, 93, 570–583.
- Povšič, K.; Čuk, K.; Milavec, S.; Erčulj, V.; Seme, K.; Gašperšič, R. Systemic azithromycin as an adjunct to scaling and root planing in patients with stage III/IV periodontitis: 12-month results of a randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5997–6006.
- Rubio, F.; Wienecke, F.; Arnabat-Domínguez, J.; Betancourt, P. Photobiomodulation therapy and endodontic treatment of teeth with apical periodontitis using 940-nm diode laser. Report of two cases. J. Clin. Exp. Dent. 2022, 14, e298.
- Sterczała, B.; Grzech-Leśniak, K.; Michel, O.; Trzeciakowski, W.; Dominiak, M.; Jurczyszyn, K. Assessment of human gingival fibroblast proliferation after laser stimulation in vitro using different laser types and wavelengths (1064, 980, 635, 450, and 405 nm)—Preliminary report. J. Pers. Med. 2021, 11, 98.
- Almoudi, M.M.; Hussein, A.S.; Abu-Hassan, M.I.; Saripudin, B.; Mohamad, M.S.F. The Association of Early Childhood Caries with Salivary Antimicrobial Peptide LL37 and Mutans Streptococci. J. Clin. Pediatric Dent. 2021, 45, 330–336.
- Khabbaz, H.; Karimi-Jafari, M.H.; Saboury, A.A.; BabaAli, B. Prediction of antimicrobial peptides toxicity based on their physico-chemical properties using machine learning techniques. BMC Bioinform. 2021, 22, 549.
- Zou, P.; Laird, D.; Riga, E.K.; Deng, Z.; Dorner, F.; Perez-Hernandez, H.-R.; Guevara-Solarte, D.L.; Steinberg, T.; Al-Ahmad, A.; Lienkamp, K. Antimicrobial and cell-compatible surface-attached polymer networks—How the correlation of chemical structure to physical and biological data leads to a modified mechanism of action. J. Mater. Chem. B 2015, 3, 6224–6238.
- Moreno Sancho, F.; Leira, Y.; Orlandi, M.; Buti, J.; Giannobile, W.V.; D’Aiuto, F. Cell-based therapies for alveolar bone and periodontal regeneration: Concise review. Stem Cells Transl. Med. 2019, 8, 1286–1295.
- Assis, R.I.; Racca, F.; Ferreira, R.S.; Ruiz, K.G.; da Silva, R.A.; Clokie, S.J.; Wiench, M.; Andia, D.C. Osteogenic commitment of human periodontal ligament cells is predetermined by methylation, chromatin accessibility and expression of key transcription factors. Cells 2022, 11, 1126.
- Antarianto, R.D.; Pragiwaksana, A.; Septiana, W.L.; Mazfufah, N.F.; Mahmood, A. Hepatocyte Differentiation from iPSCs or MSCs in Decellularized Liver Scaffold: Cell–ECM Adhesion, Spatial Distribution, and Hepatocyte Maturation Profile. Organogenesis 2022, 18, 2061263.
- Li, N.; Xie, T.; Sun, Y. Towards organogenesis and morphogenesis in vitro: Harnessing engineered microenvironment and autonomous behaviors of pluripotent stem cells. Integr. Biol. 2018, 10, 574–586.
- Huang, D.; Li, R.; Ren, J.; Luo, H.; Wang, W.; Zhou, C. Temporal induction of Lhx8 by optogenetic control system for efficient bone regeneration. Stem Cell Res. Ther. 2021, 12, 339.
- Spagnuolo, G.; Genovese, F.; Fortunato, L.; Simeone, M.; Rengo, C.; Tatullo, M. The impact of optogenetics on regenerative medicine. Appl. Sci. 2019, 10, 173.
- Lai, Y.S.; Chang, Y.H.; Chen, Y.Y.; Xu, J.; Yu, C.S.; Chang, S.J.; Chen, P.S.; Tsai, S.J.; Chiu, W.T. Ca2+-regulated cell migration revealed by optogenetically engineered Ca2+ oscillations. J. Cell. Physiol. 2021, 236, 4681–4693.
- Wong, C.-W.; Ko, L.-N.; Huang, H.-J.; Yang, C.-S.; Hsu, S.-H. Engineered Bacteriorhodopsin May Induce Lung Cancer Cell Cycle Arrest and Suppress Their Proliferation and Migration. Molecules 2021, 26, 7344.
- Paradowska-Stolarz, A.; Wieckiewicz, M.; Owczarek, A.; Wezgowiec, J. Natural Polymers for the Maintenance of Oral Health: Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 10337.
- Samiei, M.; Alipour, M.; Khezri, K.; Saadat, Y.R.; Forouhandeh, H.; Abdolahinia, E.D.; Vahed, S.Z.; Sharifi, S.; Dizaj, S.M. Application of collagen and mesenchymal stem cells in regenerative dentistry. Curr. Stem Cell Res. Ther. 2021.
- Yamada, S.; Shanbhag, S.; Mustafa, K. Scaffolds in Periodontal Regenerative Treatment. Dent. Clin. 2022, 66, 111–130.
- Dehghan-Baniani, D.; Mehrjou, B.; Chu, P.K.; Wu, H. A Biomimetic Nano-Engineered Platform for Functional Tissue Engineering of Cartilage Superficial Zone. Adv. Healthc. Mater. 2021, 10, 2001018.
- Steinberg, T.; Dannewitz, B.; Tomakidi, P.; Hoheisel, J.; Müssig, E.; Kohl, A.; Nees, M. Analysis of interleukin-1β-modulated mRNA gene transcription in human gingival keratinocytes by epithelia-specific cDNA microarrays. J. Periodontal Res. 2006, 41, 426–446.
- Ali, M.; Yang, F.; Jansen, J.A.; Walboomers, X.F. Lipoxin suppresses inflammation via the TLR4/MyD88/NF-κB pathway in periodontal ligament cells. Oral Dis. 2020, 26, 429–438.
- Yang, J.W.; Shin, Y.Y.; Seo, Y.; Kim, H.-S. Therapeutic functions of stem cells from oral cavity: An update. Int. J. Mol. Sci. 2020, 21, 4389.
- Lima, R.L.; Holanda-Afonso, R.C.; Moura-Neto, V.; Bolognese, A.M.; DosSantos, M.F.; Souza, M.M. Human dental follicle cells express embryonic, mesenchymal and neural stem cells markers. Arch. Oral Biol. 2017, 73, 121–128.
- Angelopoulos, I.; Brizuela, C.; Khoury, M. Gingival mesenchymal stem cells outperform haploidentical dental pulp-derived mesenchymal stem cells in proliferation rate, migration ability, and angiogenic potential. Cell Transplant. 2018, 27, 967–978.
- Andrukhov, C.B.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J. Stem Cells 2019, 11, 604.
- Misawa, M.Y.O.; Silverio Ruiz, K.G.; Nociti, F.H., Jr.; Albiero, M.L.; Saito, M.T.; Nóbrega Stipp, R.; Condino-Neto, A.; Holzhausen, M.; Palombo, H.; Villar, C.C. Periodontal ligament-derived mesenchymal stem cells modulate neutrophil responses via paracrine mechanisms. J. Periodontol. 2019, 90, 747–755.
- Hossein-Khannazer, N.; Hashemi, S.M.; Namaki, S.; Ghanbarian, H.; Sattari, M.; Khojasteh, A. Study of the immunomodulatory effects of osteogenic differentiated human dental pulp stem cells. Life Sci. 2019, 216, 111–118.
- Liu, N.; Zhou, M.; Zhang, Q.; Yong, L.; Zhang, T.; Tian, T.; Ma, Q.; Lin, S.; Zhu, B.; Cai, X. Effect of substrate stiffness on proliferation and differentiation of periodontal ligament stem cells. Cell Prolif. 2018, 51, e12478.
- Proksch, S.; Steinberg, T.; Schulz, S.; Sauerbier, S.; Hellwig, E.; Tomakidi, P. Environmental biomechanics substantiated by defined pillar micropatterns govern behavior of human mesenchymal stem cells. Cell Transplant. 2012, 21, 2455–2469.
- Proksch, S.; Steinberg, T.; Stampf, S.; Schwarz, U.; Hellwig, E.; Tomakidi, P. Crosstalk on cell behavior in interactive cocultures of hMSCs with various oral cell types. Tissue Eng. A 2012, 18, 2601–2610.
- Proksch, S.; Steinberg, T.; Vach, K.; Hellwig, E.; Tomakidi, P. Shaping oral cell plasticity to osteogenic differentiation by human mesenchymal stem cell coculture. Cell Tissue Res. 2014, 356, 159–170.
- Proksch, S.; Bittermann, G.; Vach, K.; Nitschke, R.; Tomakidi, P.; Hellwig, E. hMSC-Derived VEGF Release Triggers the Chemoattraction of Alveolar Osteoblasts. Stem Cells 2015, 33, 3114–3124.
- Sumi, K.; Abe, T.; Kunimatsu, R.; Oki, N.; Tsuka, Y.; Awada, T.; Nakajima, K.; Ando, K.; Tanimoto, K. The effect of mesenchymal stem cells on chemotaxis of osteoclast precursor cells. J. Oral Sci. 2018, 60, 221–225.
- Lin, H.; Chen, H.; Zhao, X.; Chen, Z.; Zhang, P.; Tian, Y.; Wang, Y.; Ding, T.; Wang, L.; Shen, Y. Advances in mesenchymal stem cell conditioned medium-mediated periodontal tissue regeneration. J. Transl. Med. 2021, 19, 456.
- Qiu, J.; Wang, X.; Zhou, H.; Zhang, C.; Wang, Y.; Huang, J.; Liu, M.; Yang, P.; Song, A. Enhancement of periodontal tissue regeneration by conditioned media from gingiva-derived or periodontal ligament-derived mesenchymal stem cells: A comparative study in rats. Stem Cell Res. Ther. 2020, 11, 42.
- Nagata, M.; Iwasaki, K.; Akazawa, K.; Komaki, M.; Yokoyama, N.; Izumi, Y.; Morita, I. Conditioned medium from periodontal ligament stem cells enhances periodontal regeneration. Tissue Eng. A 2017, 23, 367–377.
- Xu, J.; Wang, W.; Kapila, Y.; Lotz, J.; Kapila, S. Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev. 2009, 18, 487–496.
- Proksch, S.; Kirsch, K.; Vach, K.; Hellwig, E.; Tomakidi, P. Comparative differentiation analysis of distinct oral tissue-derived cells in response to osteogenic stimulation. Clin. Oral Investig. 2019, 23, 1077–1089.
- Gottwald, E.; Giselbrecht, S.; Augspurger, C.; Lahni, B.; Dambrowsky, N.; Truckenmüller, R.; Piotter, V.; Gietzelt, T.; Wendt, O.; Pfleging, W. A chip-based platform for the in vitro generation of tissues in three-dimensional organization. Lab Chip 2007, 7, 777–785.
- Altmann, B.; Steinberg, T.; Giselbrecht, S.; Gottwald, E.; Tomakidi, P.; Bachle-Haas, M.; Kohal, R.J. Promotion of osteoblast differentiation in 3D biomaterial micro-chip arrays comprising fibronectin-coated poly(methyl methacrylate) polycarbonate. Biomaterials 2011, 32, 8947–8956.
- Lee, S.; Kim, J.-E.; Seo, H.-J.; Jang, J.-H. Design of fibronectin type III domains fused to an elastin-like polypeptide for the osteogenic differentiation of human mesenchymal stem cells. Acta Biochim. Biophys. Sin. 2019, 51, 856–863.
- Escoda-Francolí, J.; Sánchez-Garcés, M.Á.; Gimeno-Sandig, Á.; Muñoz-Guzón, F.; Barbany-Cairó, J.R.; Badiella-Busquets, L.; Gay-Escoda, C. Guided bone regeneration using beta-tricalcium phosphate with and without fibronectin—An experimental study in rats. Clin. Oral Implants Res. 2018, 29, 1038–1049.
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16.
- Wang, H.; Du, T.; Li, R.; Main, R.P.; Yang, H. Interactive effects of various loading parameters on the fluid dynamics within the lacunar-canalicular system for a single osteocyte. Bone 2022, 158, 116367.
- Altmann, B.; Löchner, A.; Swain, M.; Kohal, R.-J.; Giselbrecht, S.; Gottwald, E.; Steinberg, T.; Tomakidi, P. Differences in morphogenesis of 3D cultured primary human osteoblasts under static and microfluidic growth conditions. Biomaterials 2014, 35, 3208–3219.
- Radović, K.; Brković, B.; Roganović, J.; Ilić, J.; Milić Lemić, A.; Jovanović, B. Salivary VEGF and post-extraction wound healing in type 2 diabetic immediate denture wearers. Acta Odontol. Scand. 2022, 80, 9–14.
- Müller, K.; Engesser, R.; Metzger, S.; Schulz, S.; Kämpf, M.M.; Busacker, M.; Steinberg, T.; Tomakidi, P.; Ehrbar, M.; Nagy, F. A red/far-red light-responsive bi-stable toggle switch to control gene expression in mammalian cells. Nucleic Acids Res. 2013, 41, e77.
- Müller, K.; Engesser, R.; Schulz, S.; Steinberg, T.; Tomakidi, P.; Weber, C.C.; Ulm, R.; Timmer, J.; Zurbriggen, M.D.; Weber, W. Multi-chromatic control of mammalian gene expression and signaling. Nucleic Acids Res. 2013, 41, e124.
- Zhou, C.; Yang, G.; Chen, M.; Wang, C.; He, L.; Xiang, L.; Chen, D.; Ling, J.; Mao, J.J. Lhx8 mediated Wnt and TGFβ pathways in tooth development and regeneration. Biomaterials 2015, 63, 35–46.
- Kim, J.-Y.; Choung, P.-H. USP1 inhibitor ML323 enhances osteogenic potential of human dental pulp stem cells. Biochem. Biophys. Res. Commun. 2020, 530, 418–424.
More
