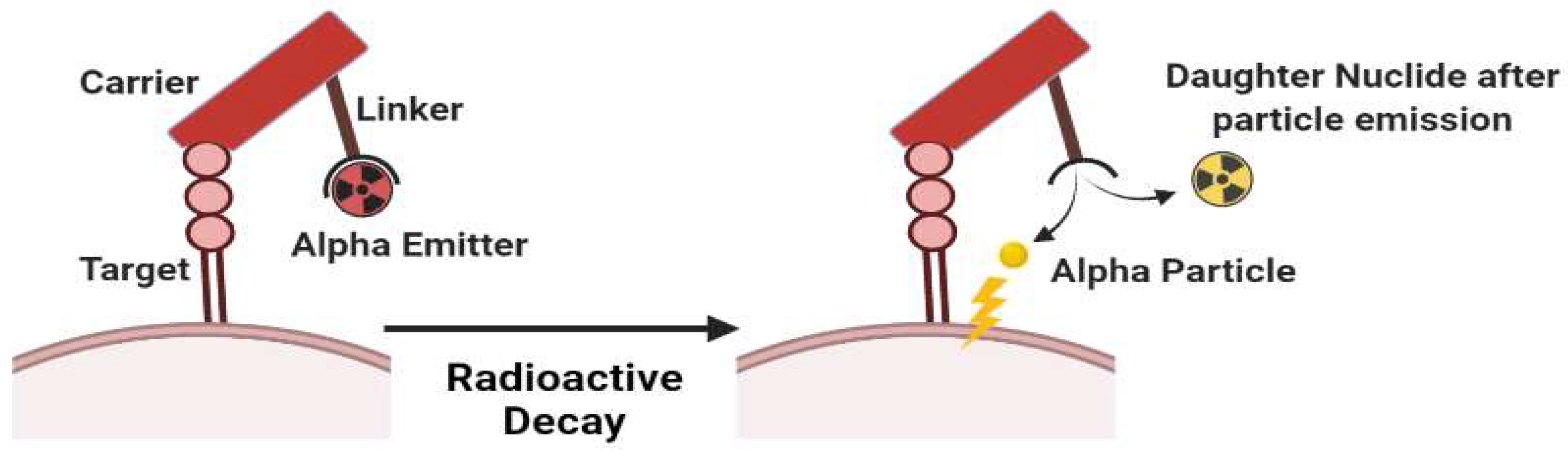
Theranostic Radiopharmaceuticals (Radiotheranostics) is a term in the medical field to define the combination of therapeutic and diagnostic techniques by a suitable radiopharmaceutical agent. Radionuclides are isotopes that emit radiation or have excess nuclear energy, making them chemically unstable and tend to change into another atom. Various types of radiation can be emitted by radionuclides e.g. alpha particles, beta particles, and gamma energy. In radiotheranostics, a pharmaceutical agent (drug) is needed to be a carrier molecule that introduces the radionuclide to its target. Radionuclides are then used as a source of radiation in radiotheranostics that are responsible for diagnosing or treating various diseases.
- radiopharmaceuticals
- theranostics
- radionuclide
- chelator
1. Radionuclides for Diagnosis Purposes
| Radionuclide | Half-Life | Mode of Decay | Energy (KeV) (%Abundance) | Indication (in Radiopharmaceutical Form) | References |
|---|---|---|---|---|---|
| 99mTc | 6.02 h | γ | 140.5 (89%) | (l,l-[99mTc]Tc-ECD) Functional imaging of the brain *, [99m Tc-MDP] bone scintigraphy * | [6][7][8] |
| 111In | 67.3 h | EC | 171 (90%) 295 (94%) |
(111In-pentetreotide) imaging of neuroendocrine tumors *, (Capromab Pendetide) for metastatic prostate cancer *, and leukocyte marking for invitro purposes * | [9][10][11][12][13] |
| 18F | 109.7 min | β+ EC |
635 (97%) 1655 (EC) 3% |
FDGPET radionuclide for cancer * and Piflufolastat PET radionuclide for protate cancer imaging * | [14][15] |
| 11C | 20.4 min | β+ | 960 (100%) | Imaging of tyrosine kinase receptor *****, [11C]Flumazenil for GABA **** imaging, [11C]mZIENT for imaging serotonin receptor *****, and 11C-coenzyme Q10 myocardial imaging ***** | [16][17] |
| 133Xe | 5.27 days | γ | 81 (38%) | Cerebral blood flow, Xe Technegas for lung perfusion imaging ** | [18][19] |
| 201Tl | 73 h | γ | 135 and 167 | imaging of soft tissue and bone tumors, detection of recurrence in gliomas | [20] |
| 51Cr | 27.7 days | γ | 320 (9.8%) | Red blood cell labeling, 51-EDTA for GFR measurement *** | [21][22] |
| 67Ga | 78.3 h | EC γ |
EC (100%) γ (93 (39%), 300 (17%), and 185 (21%)) |
Imaging skeletal infection, 67Ga–Citrate for CSF flow imaging **** |
[23][24][25] |
| 68Ga | 68 min | β+ | 890 (90%) | Diagnosis or imaging of myocardial perfusion use Ga-68 Galmydar ****, pulmonary perfusion ****, and PSMA for prostate cancer *. | [26][27] |
| 123I | 13 h | EC | 159 | Ioflupane I-123 Injection * Injection Dopamine transporter for parkinson’s diagnosis |
[28][29] |
| 125I | 59.4–60.2 d | EC | 28.5 | Evaluation of glomerular filtration rate and imaging of thyroid, and 125 Iodine Seeds for brachytherapy in solid tumor *. | [30][31][32][33][34] |
| 82Rb | 75 s | β+ | 776 | 82Rb(Rb)+**** for myocardial ischemia and brain tumors imaging. | [35][36] |
| 13N | 9.97 min | β+ | 492 (100%) | 13N-ammonia * for myocardial perfusion and blood flow imaging in tissue. | [37] |
| 166Ho | 26.8 h | β− γ |
1.774 (50%) 80.57 (6.6%) |
166Ho-chitosan ***** for diagnosis of liver cancer | [38][39] |
| 89Zr | 78.4 h | β+ | 395 (23%) | Diagnosis of various types of tumor and cancer (pancreatic, lymphoma, liver, colorectal, and prostate) (89Zr-trastuzumab, 89Zr-J951, 89Zr-lumretuzumab) ***** | [40] |
| 61Cu | 3.3 h | β+ EC γ |
1220, 1150 (62%); 940, 560 (38%); 380 γ (3%) |
61Cu-ATSM ***** imaging of tumor hypoxia. | [41] |
| 64Cu | 12.7 h | β+ β− γ |
657 (19%), 141 (38%) 511 (43%), |
64Cu-SAR-bisPSMA *** Imaging for prostate, 64Cu-DOTA-Trastuzumab *** breast cancer, 64Cu-ATSM *** diagnosis of cervical cancer, 64Cu-DOTA-Daratumumab **** multiple myeloma, and 64Cu-Cl2 urological malignancy. | [42][43] |
- a.
-
99m-Technetium
- b.
-
111Indium
- c.
-
67Galium, and 68Gallium
- d.
-
61Copper and 64Copper
- e.
-
89Zirconium
- f.
-
18Fluorine
2. Radionuclides for Therapy Purposes
| Radionuclide | Half-Life | Mode of Decay |
Energy (KeV) | Indication (in Radiopharmaceutical Form) | References |
|---|---|---|---|---|---|
| 90Y | 64.10 h | β− β+ γ |
2270 (100%) 739 (0.003%) 511 (0.006%) |
90Y-microsphere (TheraSphere® and SIR-Spheres®) * radiotherapy for hepatic metastasis, 90Y-ibritumomab tiuxetan ** for lymphoma, and 90Y-hydroxypatite and 90Y-citrate colloid ** for leukemia PVNS (synovitis). | [71][72] |
| 117mSn | 13.6 d | IT | 130 150 |
117mSn-DTPA *** for bone tumor treatment and palliative therapy. | [71] |
| 131I | 8.02 d | β−; γ | 606 (89.3%); 364 (81.2%) | 131I (radioactive iodine therapy) * use for therapy in thyroid cancer, for hyperthyroidism, RIT for NHL, and therapy for malignant pheochromocytoma neuroblastoma | [71][73] |
| 153Sm | 46.5 h | β− | 808 (20%); 710 (50%) | 153Sm-EDTMP * for painful bone metastasis and synovitis tratment. | [71][72][74][75] |
| 177Lu | 6.73 d | β− | 498 (78%) | 177Lu-HA **** for synovitis treatment, 177Lu-PSMA-617 (Pluvicto) * for prostate cancer, 177Lu-DOTATATE (Luthatera ®) * for neuroendocrine tumor. | [71][72] |
| 225Ac | 10 d | α | 5793 (18.1%) 5830 (50.7%) |
225Ac-PSMA-617 **** for prostate cancer, 225Ac-lintuzumab *** for leukemia, and 225Ac-NOTA-trastuzumab ***** for breast cancer treatment | [76] |
| 186Re | 3.72 d | EC, β− | 1965 β− (25.6%) | 186Re-HEDP *** for painful skeletal metastasis and painful arthritis | [71][72] |
| 188Re | 17.00 h | β−, γ | 2120 (71.1%) | 188Re-HEDP *** for painful bone metastasis, rheumatoid arthritis, and treatments for RIT with various cancers | [71][72] |
| 223Ra | 11.44 d | α | 5979 (100%) | 223Ra-dichloride (Xofigo®) * for bone metastasis | [77] |
| 166Ho | 26.8 h | β− γ |
1774 (49.9%) 80.57 (6.6%) |
166Ho-chitosan ***** for liver cancer | [39] |
- a.
-
186Rhenium and 188Rhenium
- b.
-
225Actinium
- c.
-
90Yttrium
- d.
-
177Lutetium
- e.
-
153Samarium
References
- MacPherson, D.S.; Fung, K.; Cook, B.E.; Francesconi, L.C.; Zeglis, B.M. A Brief Overview of Metal Complexes as Nuclear Imaging Agents. Dalton Trans. 2019, 48, 14547–14565.
- Rahmim, A.; Qi, J.; Sossi, V. Resolution Modeling in PET Imaging: Theory, Practice, Benefits, and Pitfalls: Resolution Modeling in PET Imaging. Med. Phys. 2013, 40, 064301.
- Calais, J.; Kishan, A.U.; Cao, M.; Fendler, W.P.; Eiber, M.; Herrmann, K.; Ceci, F.; Reiter, R.E.; Rettig, M.B.; Hegde, J.V.; et al. Potential Impact of 68Ga-PSMA-11 PET/CT on the Planning of Definitive Radiation Therapy for Prostate Cancer. J. Nucl. Med. 2018, 59, 1714–1721.
- Ljungberg, M.; Pretorius, P.H. SPECT/CT: An Update on Technological Developments and Clinical Applications. Br. J. Radiol. 2018, 91, 20160402.
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902.
- Boschi, A.; Uccelli, L.; Martini, P. A Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging. Appl. Sci. 2019, 9, 2526.
- Rahmanian, N.; Hosseinimehr, S.J.; Khalaj, A.; Noaparast, Z.; Abedi, S.M.; Sabzevari, O. 99mTc-Radiolabeled GE11-Modified Peptide for Ovarian Tumor Targeting. DARU 2017, 25, 13.
- Chokkappan, K.; Kannivelu, A.; Srinivasan, S.; Babu, S. Review of Diagnostic Uses of Shunt Fraction Quantification with Technetium-99m Macroaggregated Albumin 20 Perfusion Scan as Illustrated by a Case of Osler-Weber-Rendu Syndrome. Ann. Thorac. Med. 2016, 11, 155.
- Hope, T.A.; Calais, J.; Zhang, L.; Dieckmann, W.; Millo, C. 111In-Pentetreotide Scintigraphy versus 68Ga-Dotatate Pet: Impact on Krenning Scores and Effect of Tumor Burden. J. Nucl. Med. 2019, 60, 1266–1269.
- Rosenkranz, A.A.; Slastnikova, T.A.; Karmakova, T.A.; Vorontsova, M.S.; Morozova, N.B.; Petriev, V.M.; Abrosimov, A.S.; Khramtsov, Y.V.; Lupanova, T.N.; Ulasov, A.V.; et al. Antitumor Activity of Auger Electron Emitter 111In Delivered by Modular Nanotransporter for Treatment of Bladder Cancer with EGFR Overexpression. Front. Pharmacol. 2018, 9, 1331.
- Lütje, S.; Van Rij, C.M.; Franssen, G.M.; Fracasso, G.; Helfrich, W.; Eek, A.; Oyen, W.J.; Colombatti, M.; Boerman, O.C. Targeting Human Prostate Cancer with 111In-Labeled D2B IgG, F (Ab′) 2 and Fab Fragments in Nude Mice with PSMA-Expressing Xenografts. Contrast Media Mol. Imaging 2015, 10, 28–36.
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-Human Molecular Imaging of HER2 Expression in Breast Cancer Metastases Using the 111In-ABY-025 Affibody Molecule. J. Nucl. Med. 2014, 55, 730–735.
- Tahara, N.; Zandbergen, H.R.; de Haas, H.J.; Petrov, A.; Pandurangi, R.; Yamaki, T.; Zhou, J.; Imaizumi, T.; Slart, R.H.J.A.; Dyszlewski, M.; et al. Noninvasive Molecular Imaging of Cell Death in Myocardial Infarction Using 111In-GSAO. Sci. Rep. 2014, 4, 6826.
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjugate Chem. 2015, 26, 1–18.
- Koç, Z.P.; Kara, P.Ö.; Dağtekin, A. Detection of unknown primary tumor in patients presented with brain metastasis by F-18 fluorodeoxyglucose positron emission tomography/computed tomography. CNS Oncol. 2018, 7, CNS12.
- Antoni, G. The Radiopharmaceutical Chemistry of Carbon-11: Basic Principles. In Radiopharmaceutical Chemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 207–220.
- Goud, N.S.; Bhattacharya, A.; Joshi, R.K.; Nagaraj, C.; Bharath, R.D.; Kumar, P. Carbon-11: Radiochemistry and Target-Based PET Molecular Imaging Applications in Oncology, Cardiology, and Neurology. J. Med. Chem. 2021, 64, 1223–1259.
- Zheng, Y.; Zhou, Z. SPECT and PET in Vascular Dementia. In PET and SPECT in Neurology; Springer: Cham, Switzerland, 2021; pp. 563–575.
- Apple, M.; Waksman, R.; Chan, R.C.; Vodovotz, Y.; Fournadjiev, J.; Bass, B.G. Radioactive 133-Xenon Gas-Filled Balloon to Prevent Restenosis: Dosimetry, Efficacy, and Safety Considerations: Dosimetry, Efficacy, and Safety Considerations. Circulation 2002, 106, 725–729.
- Mettler, F.A.; Guiberteau, M.J. Essentials of Nuclear Medicine Imaging: Expert Consult—Online and Print; W B Saunders: London, UK, 2012.
- Korell, J.; Coulter, C.V.; Duffull, S.B. Evaluation of Red Blood Cell Labelling Methods Based on a Statistical Model for Red Blood Cell Survival. J. Theor. Biol. 2011, 291, 88–98.
- Frei, R.; Gaucher, C.; Poulton, S.W.; Canfield, D.E. Fluctuations in Precambrian Atmospheric Oxygenation Recorded by Chromium Isotopes. Nature 2009, 461, 250–253.
- Ferreira, C.L.; Lamsa, E.; Woods, M.; Duan, Y.; Fernando, P.; Bensimon, C.; Kordos, M.; Guenther, K.; Jurek, P.; Kiefer, G.E. Evaluation of Bifunctional Chelates for the Development of Gallium-Based Radiopharmaceuticals. Bioconjugate Chem. 2010, 21, 531–536.
- Al-Suqri, B.; Al-Bulushi, N. Gallium-67 Scintigraphy in the Era of Positron Emission Tomography and Computed Tomography: Tertiary Centre Experience. Sultan Qaboos Univ. Med. J. 2015, 15, e338–e343.
- Othman, M.F.B.; Verger, E.; Costa, I.; Tanapirakgul, M.; Cooper, M.S.; Imberti, C.; Lewington, V.J.; Blower, P.J.; Terry, S.Y.A. In Vitro Cytotoxicity of Auger Electron-Emitting Ga-Trastuzumab. Nucl. Med. Biol. 2020, 80–81, 57–64.
- Velikyan, I. 68Ga-Based Radiopharmaceuticals: Production and Application Relationship. Molecules 2015, 20, 12913–12943.
- Meisenheimer, M.; Saenko, Y.; Eppard, E. Gallium-68: Radiolabeling of Radiopharmaceuticals for PET Imaging—A Lot to Consider. In Medical Isotopes; IntechOpen: London, UK, 2021.
- Frigerio, B.; Franssen, G.; Luison, E.; Satta, A.; Seregni, E.; Colombatti, M.; Fracasso, G.; Valdagni, R.; Mezzanzanica, D.; Boerman, O.; et al. Full Preclinical Validation of the 123I-Labeled Anti-PSMA Antibody Fragment ScFvD2B for Prostate Cancer Imaging. Oncotarget 2017, 8, 10919–10930.
- Bajaj, N.; Hauser, R.A.; Seibyl, J.; Kupsch, A.; Plotkin, M.; Chen, C.; Grachev, I.D. Association between Hoehn and Yahr, Mini-Mental State Examination, Age, and Clinical Syndrome Predominance and Diagnostic Effectiveness of Ioflupane I 123 Injection (DaTSCANTM) in Subjects with Clinically Uncertain Parkinsonian Syndromes. Alzheimer’s Res. Ther. 2014, 6, 67.
- Stevens, L.A.; Claybon, M.A.; Schmid, C.H.; Chen, J.; Horio, M.; Imai, E.; Nelson, R.G.; Van Deventer, M.; Wang, H.-Y.; Zuo, L.; et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration Equation for Estimating the Glomerular Filtration Rate in Multiple Ethnicities. Kidney Int. 2011, 79, 555–562.
- Schwarz, S.B.; Thon, N.; Nikolajek, K.; Niyazi, M.; Tonn, J.C.; Belka, C.; Kreth, F.W. Iodine-125 Brachytherapy for Brain Tumours-a Review. Radiat. Oncol. 2012, 7, 30.
- Binder, C.; Mruthyunjaya, P.; Schefler, A.C.; Seider, M.I.; Crilly, R.; Hung, A.; Meltsner, S.; Mowery, Y.; Kirsch, D.G.; Teh, B.S.; et al. Practice Patterns for the Treatment of Uveal Melanoma with Iodine-125 Plaque Brachytherapy: Ocular Oncology Study Consortium Report 5. Ocul. Oncol. Pathol. 2020, 6, 210–218.
- Suchorska, B.; Hamisch, C.; Treuer, H.; Mahnkopf, K.; Lehrke, R.E.; Kocher, M.; Ruge, M.I.; Voges, J. Stereotactic Brachytherapy Using Iodine 125 Seeds for the Treatment of Primary and Recurrent Anaplastic Glioma WHO III. J. Neurooncol. 2016, 130, 123–131.
- Drozdovitch, V.; Brill, A.B.; Callahan, R.J.; Clanton, J.A.; DePietro, A.; Goldsmith, S.J.; Greenspan, B.S.; Gross, M.D.; Hays, M.T.; Moore, S.C.; et al. Use of Radiopharmaceuticals in Diagnostic Nuclear Medicine in the United States: 1960–2010. Health Phys. 2015, 108, 520–537.
- Dorbala, S.; Hachamovitch, R.; Curillova, Z.; Thomas, D.; Vangala, D.; Kwong, R.Y.; Di Carli, M.F. Incremental Prognostic Value of Gated Rb-82 Positron Emission Tomography Myocardial Perfusion Imaging over Clinical Variables and Rest LVEF. JACC Cardiovasc. Imaging 2009, 2, 846–854.
- Kostenikov, N.A.; Zhuikov, B.L.; Chudakov, V.M.; Iliuschenko, Y.R.; Shatik, S.V.; Zaitsev, V.V.; Sysoev, D.S.; Stanzhevskiy, A.A. Application of 82 Sr/82 Rb Generator in Neurooncology. Brain Behav. 2019, 9, e01212.
- Fathala, A.; Aboulkheir, M.; Shoukri, M.M.; Alsergani, H. Diagnostic Accuracy of 13N-Ammonia Myocardial Perfusion Imaging with PET-CT in the Detection of Coronary Artery Disease. Cardiovasc. Diagn. Ther. 2019, 9, 35–42.
- Vente, M.A.; Wit, T.C.; Van Den Bosch, M.A.; Bult, W.; Seevinck, P.R.; Zonnenberg, B.A. Holmium-166 Poly (L-Lactic Acid) Microsphere Radioembolization of the Liver: Technical Aspects Studied in a Large Animal Model. Eur. Radiol. 2010, 20, 862–869.
- Mishiro, K.; Hanaoka, H.; Yamaguchi, A.; Ogawa, K. Radiotheranostics with Radiolanthanides: Design, Development Strategies, and Medical Applications. Coord. Chem. Rev. 2019, 383, 104–131.
- Watering, F.C.J.; Rijpkema, M.; Perk, L.; Brinkmann, U.; Oyen, W.J.G.; Boerman, O.C. Zirconium-89 Labeled Antibodies: A New Tool for Molecular Imaging in Cancer Patients. BioMed Res. Int. 2014, 2014, 203601.
- Yip, C.; Blower, P.J.; Goh, V.; Landau, D.B.; Cook, G.J.R. Molecular Imaging of Hypoxia in Non-Small-Cell Lung Cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 956–976.
- Fodero-Tavoletti, M.T.; Villemagne, V.L.; Paterson, B.M.; White, A.R.; Li, Q.-X.; Camakaris, J.; O’Keefe, G.; Cappai, R.; Barnham, K.J.; Donnelly, P.S. Bis(Thiosemicarbazonato) Cu-64 Complexes for Positron Emission Tomography Imaging of Alzheimer’s Disease. J. Alzheimers Dis. 2010, 20, 49–55.
- Niccoli Asabella, A.; Cascini, G.L.; Altini, C.; Paparella, D.; Notaristefano, A.; Rubini, G. The Copper Radioisotopes: A Systematic Review with Special Interest to 64Cu. Biomed Res. Int. 2014, 2014, 786463.
- Cohen, I.; Robles, A.; Mendoza, P.; Airas, R.; Montoya, E. Experimental Evidences of 95mTc Production in a Nuclear Reactor. Appl. Radiat. Isot. 2018, 135, 207–211.
- Costa, I.M.; Siksek, N.; Volpe, A.; Man, F.; Osytek, K.M.; Verger, E.; Schettino, G.; Fruhwirth, G.O.; Terry, S.Y.A. Relationship of in Vitro Toxicity of Technetium-99m to Subcellular Localisation and Absorbed Dose. Int. J. Mol. Sci. 2021, 22, 13466.
- Qin, H.; Shao, W. The Recent Research Progress on 99 Mo/99 Tc m Generator. Labeled Immunoass. Clin. Med. 2016, 23, 949–953.
- Rathmann, S.M.; Ahmad, Z.; Slikboer, S.; Bilton, H.A.; Snider, D.P.; Valliant, J.F. The Radiopharmaceutical Chemistry of Technetium-99m. In Radiopharmaceutical Chemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 311–333.
- Alberto, R. From Oxo to Carbonyl and Arene Complexes; A Journey through Technetium Chemistry. J. Organomet. Chem. 2018, 869, 264–269.
- Qaiser, S.; Khan, A.; Khan, M. Synthesis, Biodistribution and Evaluation of 99m Tc-Sitafloxacin Kit: A Novel Infection Imaging Agent. J. Radioanal. Nucl. Chem. 2010, 284, 189–193.
- Herrero Álvarez, N.; Bauer, D.; Hernández-Gil, J.; Lewis, J.S. Recent Advances in Radiometals for Combined Imaging and Therapy in Cancer. ChemMedChem 2021, 16, 2909–2941.
- Terova, O.S. Characterization and Inertness Studies of Gallium (III) and Indium (III) Complexes of Dicarboxymethyl Pendantarmed Cross-Bridged Cyclam; University of New Hampshire: Durham, NH, USA, 2008.
- Spang, P.; Herrmann, C.; Roesch, F. Bifunctional Gallium-68 Chelators: Past, Present, and Future. Semin. Nucl. Med. 2016, 46, 373–394.
- Fani, M.; André, J.P.; Maecke, H.R. 68Ga-PET: A Powerful Generator-Based Alternative to Cyclotron-Based PET Radiopharmaceuticals. Contrast Media Mol. Imaging 2008, 3, 67–77.
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, Limitations and Challenges. Nucl. Med. Commun. 2008, 29, 193–207.
- Shetty, D.; Lee, Y.-S.; Jeong, J.M. (68)Ga-Labeled Radiopharmaceuticals for Positron Emission Tomography. Nucl. Med. Mol. Imaging 2010, 44, 233–240.
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2018, 119, 902–956.
- Bin Othman, M.F.; Mitry, N.R.; Lewington, V.J.; Blower, P.J.; Terry, S.Y. Re-Assessing Gallium-67 as a Therapeutic Radionuclide. Nucl. Med. Biol. 2017, 46, 12–18.
- de Andrade Martins, P.; Osso, J.A., Jr. Thermal Diffusion of 67Ga from Irradiated Zn Targets. Appl. Radiat. Isot. 2013, 82, 279–282.
- Meulen, N.P.; Dolley, S.G.; Steyn, G.F.; Walt, T.N.; Raubenheimer, H.G. The Use of Selective Volatization in the Separation of 68Ge from Irradiated Ga Targets. Appl. Radiat. Isot. 2011, 69, 727–731.
- Velikyan, I. Prospective of 68Ga-Radiopharmaceutical Development. Theranostics 2013, 4, 47–80.
- Prata, I.M. Gallium-68: A New Trend in PET Radiopharmacy. Curr. Radiopharm. 2012, 5, 142–149.
- Blower, J.E.; Cooper, M.S.; Imberti, C.; Ma, M.T.; Marshall, C.; Young, J.D.; Blower, P.J. The Radiopharmaceutical Chemistry of the Radionuclides of Gallium and Indium. In Radiopharmaceutical Chemistry; Springer: Cham, Switzerland, 2019; pp. 255–271.
- Khosravifarsani, M.; Ait-Mohand, S.; Paquette, B.; Sanche, L.; Guérin, B. Design, Synthesis, and Cytotoxicity Assessment of Cu-NOTA-Terpyridine Platinum Conjugate: A Novel Chemoradiotherapeutic Agent with Flexible Linker. Nanomaterials 2021, 11, 2154.
- Wu, N.; Kang, C.S.; Sin, I.; Ren, S.; Liu, D.; Ruthengael, V.C.; Lewis, M.R.; Chong, H.-S. Promising Bifunctional Chelators for Copper 64-PET Imaging: Practical (64)Cu Radiolabeling and High in Vitro and in Vivo Complex Stability. J. Biol. Inorg. Chem. 2016, 21, 177–184.
- Pichler, V.; Berroterán-Infante, N.; Philippe, C.; Vraka, C.; Klebermass, E.-M.; Balber, T.; Pfaff, S.; Nics, L.; Mitterhauser, M.; Wadsak, W. An Overview of PET Radiochemistry, Part 1: The Covalent Labels 18F, 11C, and 13N. J. Nucl. Med. 2018, 59, 1350–1354.
- Archibald, S.J.; Allott, L. The Aluminium-Fluoride Revolution: Simple Radiochemistry with a Big Impact for Radiolabelled Biomolecules. EJNMMI Radiopharm. Chem. 2021, 6, 30.
- Cieslak, J.A.; Sibenaller, Z.A.; Walsh, S.A.; Ponto, L.L.B.; Du, J.; Sunderland, J.J.; Cullen, J.J. Fluorine-18-Labeled Thymidine Positron Emission Tomography (FLT-PET) as an Index of Cell Proliferation after Pharmacological Ascorbate-Based Therapy. Radiat. Res. 2016, 185, 31–38.
- McBride, W.J.; D’Souza, C.A.; Sharkey, R.M.; Karacay, H.; Rossi, E.A.; Chang, C.-H.; Goldenberg, D.M. Improved 18F labeling of peptides with a fluoride-aluminum-chelate complex. Bioconjugate Chem. 2010, 21, 1331–1340.
- Deng, X.; Rong, J.; Wang, L.; Vasdev, N.; Zhang, L.; Josephson, L.; Liang, S.H. Chemistry for Positron Emission Tomography: Recent Advances in 11 C-, 18 F-, 13 N-, and 15 O-Labeling Reactions. Angew. Chem. Int. Ed. 2019, 58, 2580–2605.
- Ramogida, C.F.; Orvig, C. Tumour Targeting with Radiometals for Diagnosis and Therapy. Chem. Commun. 2013, 49, 4720–4739.
- Tickner, B.J.; Stasiuk, G.J.; Duckett, S.B.; Angelovski, G. The Use of Yttrium in Medical Imaging and Therapy: Historical Background and Future Perspectives. Chem. Soc. Rev. 2020, 49, 6169–6185.
- Das, T.; Pillai, M.R.A. Options to Meet the Future Global Demand of Radionuclides for Radionuclide Therapy. Nucl. Med. Biol. 2013, 40, 23–32.
- Tong, A.K.T.; Kao, Y.H.; Too, C.W.; Chin, K.F.W.; Ng, D.C.E.; Chow, P.K.H. Yttrium-90 Hepatic Radioembolization: Clinical Review and Current Techniques in Interventional Radiology and Personalized Dosimetry. Br. J. Radiol. 2016, 89, 20150943.
- Sciacca, F. Samarium-153. 2020. Available online: https://radiopaedia.org/articles/samarium-153 (accessed on 24 April 2021).
- Kasbollah, A.; Amiroudine, M.Z.A.M.; Karim, J.A.; Hamid, S.S.A.; Ghazi, S.A.F.W.S.M.; Awang, W.A.W.; Ali, M.R. Samarium-153 Production Using (n,γ) Reaction at Triga Puspati Research Reactor. In Application of Mathematics in Technical and Natural Sciences: Proceedings of the 12th International On-line Conference for Promoting the Application of Mathematics in Technical and Natural Sciences—AMiTaNS’20; AIP Publishing: Melville, NY, USA, 2020.
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in Targeted Alpha-Particle Therapeutic Applications. Curr. Radiopharm. 2011, 4, 306–320.
- Gupta, N.; Devgan, A.; Bansal, I.; Olsavsky, T.D.; Li, S.; Abdelbaki, A.; Kumar, Y. Usefulness of Radium-223 in Patients with Bone Metastases. Proc. Bayl. Univ. Med. Cent. 2017, 30, 424–426.
- Argyrou, M.; Valassi, A.; Andreou, M.; Lyra, M. Rhenium-188 Production in Hospitals, by W-188/Re-188 Generator, for Easy Use in Radionuclide Therapy. Int. J. Mol. Imaging 2013, 2013, 290750.
- Lepareur, N.; Lacœuille, F.; Bouvry, C.; Hindré, F.; Garcion, E.; Chérel, M.; Noiret, N.; Garin, E.; Knapp, F.F.R., Jr. Rhenium-188 Labeled Radiopharmaceuticals: Current Clinical Applications in Oncology and Promising Perspectives. Front. Med. 2019, 6, 132.
- Wick, R.R.; Nekolla, E.A.; Gaubitz, M.; Schulte, T.L. Increased Risk of Myeloid Leukaemia in Patients with Ankylosing Spondylitis Following Treatment with Radium-224. Rheumatology 2008, 47, 855–859.
- Piotrowska, A.; Leszczuk, E.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized NaA Nanozeolites Labeled with (224,225)Ra for Targeted Alpha Therapy. J. Nanoparticle Res. 2013, 15, 2082.
- Cordier, D.; Forrer, F.; Bruchertseifer, F.; Morgenstern, A.; Apostolidis, C.; Good, S.; Müller-Brand, J.; Mäcke, H.; Reubi, J.C.; Merlo, A. Targeted Alpha-Radionuclide Therapy of Functionally Critically Located Gliomas with 213Bi-DOTA--Substance P: A Pilot Trial. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1335–1344.
- Wright, C.L.; Zhang, J.; Tweedle, M.F.; Knopp, M.V.; Hall, N.C. Theranostic Imaging of Yttrium-90. BioMed Res. Int. 2015, 2015, 481279.
- Goffredo, V.; Paradiso, A.; Ranieri, G.; Gadaleta, C.D. Yttrium-90 (90Y) in the Principal Radionuclide Therapies: An Efficacy Correlation between Peptide Receptor Radionuclide Therapy, Radioimmunotherapy and Transarterial Radioembolization Therapy. Ten Years of Experience (1999–2009). Crit. Rev. Oncol. Hematol. 2011, 80, 393–410.
- Rösch, F.; Herzog, H.; Qaim, S.M. The Beginning and Development of the Theranostic Approach in Nuclear Medicine, as Exemplified by the Radionuclide Pair 86Y and 90Y. Pharmaceuticals 2017, 10, 56.
- Kam, B.L.R.; Teunissen, J.J.M.; Krenning, E.P.; de Herder, W.W.; Khan, S.; van Vliet, E.I.; Kwekkeboom, D.J. Lutetium-Labelled Peptides for Therapy of Neuroendocrine Tumours. Eur. J. Nucl. Med. Mol. Imaging 2012, 39 (Suppl. S1), S103–S112.
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schaefers, M.; Essler, M.; Krause, B.J. German Multicenter Study Investigating Lu-177-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90.
- Bober, B.; Saracyn, M.; Zaręba, K.; Lubas, A.; Mazurkiewicz, P.; Wilińska, E.; Kamiński, G. Early Complications of Radioisotope Therapy with Lutetium-177 and Yttrium-90 in Patients with Neuroendocrine Neoplasms-A Preliminary Study. J. Clin. Med. 2022, 11, 919.
- Dash, A.; Pillai, M.R.A.; Knapp, F.F., Jr. Production of (177)Lu for Targeted Radionuclide Therapy: Available Options. Nucl. Med. Mol. Imaging 2015, 49, 85–107.
- Banerjee, S.; Pillai, M.R.A.; Knapp, F.F.R. Lutetium-177 Therapeutic Radiopharmaceuticals: Linking Chemistry, Radiochemistry, and Practical Applications. Chem. Rev. 2015, 115, 2934–2974.
