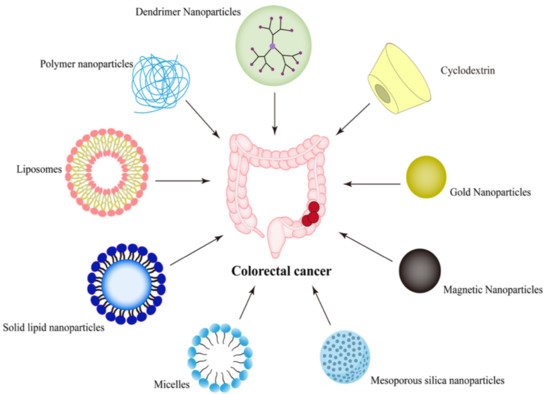
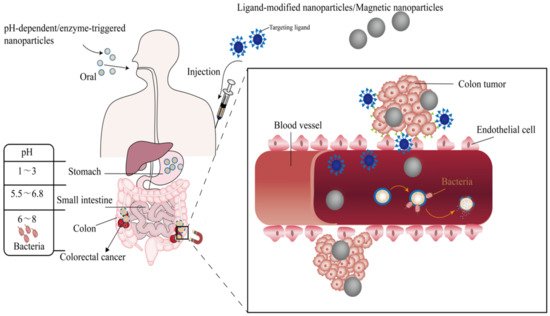
2. Nano-Drug Delivery Systems Targeting CRC
2.1. Passive Targeting Nanoparticles
It is generally believed that the anatomy and pathophysiology of solid tumors differ from that of normal tissue. For example, solid tumors have a high blood vessel density to meet the nutrition and oxygen required by tumor cells growth. Furthermore, the tumor lacks functional lymphatic vessels and the gap between tumor endothelial cells is relatively large, which can extravasate or retain macromolecular drugs. The phenomenon that makes nanoparticles accumulate in tumor cells is called the enhanced permeability and retention (EPR) effect
[14][15][16][14,15,16].
The discovery of this phenomenon has promoted the emergence of passive targeting nanoparticles, which can target tumors through the EPR effect. In research, oxaliplatin was encapsulated into N, O-carboxymethyl chitosan nanoparticles by ionic crosslinking, and resveratrol was encapsulated into them by emulsification crosslinking. The particle size of the former was about 190.0 nm and that of the latter was about 164.2 nm. The nanoparticles enhanced the solubility, stability, and EPR effect of oxaliplatin and resveratrol, showing stronger anti-CRC activity in subcutaneous tumor-bearing mice compared to the free drug
[17].
However, according to research findings, nanoparticles smaller than 10 nm are filtered out by the kidneys, and particles larger than 100 nm are captured by the liver, so the ideal nanoparticles should be between 10 nm and 100 nm in size, which can circulate for a long time to increase the possibility of uptake by tumors
[18]. Moreover, Anitha et al.
[19] mentioned that the degree of vascularization in CRC is low. It can be seen that there are controversies about the EPR effect in CRC. In recent years, scientists have gradually realized that the tumor-targeting mediated by EPR is highly heterogeneous. Hence, it is necessary to enhance the targeting ability of nanoparticles based on EPR by combining them with other targeting mechanisms
[20][21][20,21].
2.2. Active Targeting Nanoparticles
Ligand-modified nanoparticles can actively accumulate in the tumor through a ligand–receptor binding mechanism, thus delivering the drug to the target. These nanoparticles are called active targeting nanoparticles
[22]. Cancer cells overexpress some types of receptors and secrete biomolecules that can promote cell proliferation of cancer and surrounding tissues through paracrine or autocrine pathways
[23]. In studies, there are two main methods to combine ligands with nanoparticles: one is to modify them chemically during the synthesis of nanoparticles, and the other is to bind ligands with polymers before the synthesis of nanoparticles
[24][25][24,25]. Ligand-modified nanoparticles can accumulate in the tumor through passive targeting, and then enter tumor cells through active targeting, resulting in a more selective and enhanced therapeutic effect.
In the recent three years, the active targeting design idea of nano-drug targeted delivery systems for CRC mainly adopt the receptor–ligand binding strategy, which involved many highly expressed receptors in CRC, such as folate receptor
[26], EGFR
[27], CD44, epithelial cell adhesion molecule (EpCAM)
[28], CD133, αvβ3 integrin receptor, carcinoembryonic antigen
[29], nucleolin
[30], mannose receptor
[31], hyaluronic acid receptor
[32],
N-acetyl-
d-glucosamine
[33], transferrin receptor
[34], checkpoint kinase 2
[35], CXCR4+
[36], lipoprotein receptor-related protein-1
[37], MUC1
[38], NRP-1
[39],
P-selectin
[40], sigma-2 receptors
[41], SSTRs
[42], and glucocorticoid receptor
[43][43]. The characteristics of these nanoparticles are shown in Table 1. Among them, the nanoparticles targeting EpCAM, folate receptor, epidermal growth factor, and CD44 were studied more.
Table 1. Actively targeting nano delivery system for the treatment of colorectal cancer.
2.2.1. Nanoparticles Targeting EpCAM
Epithelial cell adhesion molecule (EpCAM) is a 314 amino acid and 39 kDa transmembrane glycoprotein that almost only exists on epithelial cells playing a role in adhering cells in normal cells
[44][45][44,45]. As early as 1979, it has been reported as the main surface antigen of CRC
[46], Later, scholars have found that overexpression of EpCAM also existed in human adenocarcinoma cells, and it could also carry out intercellular signal transduction
[47].
In many kinds of research, to make nanoparticles target CRC after systemic administration, a DNA EpCAM aptamer (SYL3C) having a strong affinity with EpCAM protein and cancer cells expressing EpCAM was modified on nanoparticles. To reduce drug toxicity and improve therapeutic effect, Ge et al.
[28] synthesized biological conjugates loaded with celastrol, which could be captured by CRC overexpressed with EpCAM. EpCAM aptamer, PEG, and dendrimers constituted the conjugates. The results showed that SW620 would undergo extensive apoptosis when exposed to biological conjugates. Moreover, in the xenograft mice and zebrafish models, the biological conjugate showed low toxicity. The dendrimer used in the study is composed of biocompatible components and has an excellent multivalent effect, which can improve the safety of chemotherapeutic drugs.
In another study, the author prepared a cationic liposome to deliver miR-139-5p. The main lipids contained in it are HSPC, DOTAP, Chol, and DSPE-PEG2000-COOH. Moreover, to make the nanoparticles target CRC, the surface of it was modified with EpCAM aptamer. It is found that nanoparticles had inhibitory effects on HCT cells, consistent with the experimental results of animal pharmacodynamics experiments. They also restrained the tumor growth of CRC mice injected subcutaneously with HCT8
[48].
In addition to the specific targets of CRC, some targets of other tumors are also expressed in CRC, which can be used as the targets of nano-drug delivery systems in the targeted therapy of CRC.
2.2.2. Nanoparticles Targeting Folate Receptor
The folate receptor is a cell membrane-anchored protein, which is slightly expressed or absent in normal cells, but overexpressed in various malignant tumor cells, especially in CRC
[22]. Therefore, it can be used as a target. Natural polymers chitosan and chondroitin sulfate have the advantages of biocompatibility and biodegradability. Soe et al.
[26] prepared self-assembled nanoparticles using these two materials to incorporate the hydrophobic drug, bortezomib, and modified the nanoparticles to actively target folate receptors.
Folate was attached to DSPE-PEG and then coupled with nanoparticles. It was found that DSPE-PEG can not only promote blood circulation, but also improve core drug loading and stability. In the pharmacodynamic experiment of animal models, researchers found that nanoparticles had a strong inhibitory effect on the transplanted tumor and were almost non-toxic to other parts outside the tumor.
2.2.3. Nanoparticles Targeting EGFR
The overexpression of epidermal growth factor receptor (EGFR) occurs in about one-third of epithelial malignancies. It can stimulate tumor growth, proliferation, promote angiogenesis, and promote cell invasion and metastasis. Therefore, the poor prognosis of CRC may have a great relationship with the overexpression of EGFR in CRC
[49]. In order to solve this problem, targeted therapy using monoclonal antibodies to block biological pathways has been used to treat CRC.
The commonly used monoclonal antibody is cetuximab, which is used for CRC under FDA approval
[50]. It can selectively target the EGFR to block signal transduction in vivo
[49]. This characteristic also makes cetuximab widely used to modify nano-drug delivery systems, actively target CRC, and increase the accumulation in CRC
[51].
Sankha Bhattacharya
[27] formulated PLGA-PEG-coated nanoparticles to deliver anti-epidermal growth factor receptor-5-fluorouracil (5-FU), which could improve the pharmacodynamics and distribution of the drug in vivo. The Anti-EGFR mAb was bound to the polymeric nanoparticles by using m-maleimidobenzoyl-N hydroxysuccinimide ester to increase target specificity. On the other hand, polymeric nanoparticles consisting of PLGA and PEG can block opsonic action through the reticular epithelial system. These nanoparticles have great clinical value because their preparation methods are solvent emulsification and evaporation, which are simple and rapid
[52].
Another attractive carrier is gold nanoparticles, which are commercially available and easily functionalized. Hallal et al.
[53] found that AuNPs loaded with cetuximab had better EGFR endocytosis and could inhibit downriver signaling pathways compared with cetuximab and gold nanoparticles alone, which could inhibit cell proliferation and accelerate cell apoptosis. This finding is of great help in solving the problem of drug resistance in the treatment of EGFR monoclonal antibodies.
此外,许多研究发现,将M
oreover, many studies found that the therapeutic
effect of monoclonal antibodies could be improved by combining McAb with chemotherapeutic drugs [54][55]. Ab与化疗药物联合使用可以改善单克隆抗体的治疗效果[54,55]。Chen
et al. [56] constructed a hybrid nano delivery system to deliver 等人[56]构建了一种混合纳米递送系统来递送5-FU
, including mesoporous silica nanoparticles (,包括介孔二氧化硅纳米颗粒(MSN
), a supported lipid bilayer (SLB), and cetuximab. Furthermore, the ),负载脂质双层(SLB)和西妥昔单抗。此外,SLB-MSN
was functionalized with hydrophilic PEG, which made the nanoparticles more stable, circulate longer in the blood, and more likely to accumulate in the tumor through the EPR effect. Then, to enable the nanoparticles to actively target CRC, cetuximab was coupled to the PEG terminal in 用亲水性PEG功能化,使纳米颗粒更稳定,在血液中循环时间更长,并且更容易通过EPR效应在肿瘤中积累。然后,为了使纳米颗粒能够主动靶向CRC,西妥昔单抗被偶联到SLB-MSN
. The 中的PEG末端。SLB-MSN
has the advantages of both MSN and liposome, showing significant biocompatibility. It can also encapsulate much drug, achieve controlled release, and its stability can be greatly improved.
具有MSN和脂质体的优点,显示出显着的生物相容性。它还可以封装很多药物,实现控释,其稳定性可以大大提高。
2.2.4. Nanoparticles Targeting 以CD44
为目标的奈米颗粒
CD44
is a transmembraneous glycoprotein that exists as an adhesion molecule on the cell surface and takes part in intercellular and cytomatrix interactions, as well as cell adhesion and migration [57]. The overexpression of 是一种跨膜糖蛋白,以粘附分子的形式存在于细胞表面,并参与细胞间和细胞基团相互作用,以及细胞粘附和迁移[57]。CD44
in 在CRC
cells is closely related to tumor metastasis [58][59]. Therefore, it is also a biomarker for 细胞中的过表达与肿瘤转移密切相关[58,59]。因此,它也是CRC
as well as a target. 的生物标志物以及靶标。透明质酸(H
yaluronic acid (HA) is the main ligand of CD44, consisting of units of D-glucuronic acid and N-acetyl-D-glucosamine alternately. Especially, it is very popular with researchers, because of its non-toxic, excellent biocompatibility, and biodegradability [60].
A)是CD44的主要配体,由D-葡萄糖醛酸和N-乙酰基-D-氨基葡萄糖交替组成。特别是,它因其无毒,优异的生物相容性和可生物降解性而非常受研究人员的欢迎[60]。Wang
et al. [58] synthesized 等人[58]合成了HA
-d修饰的聚多巴胺纳米颗粒,将氯e
corated polydopamine nanoparticles to deliver chlorin e6 to the CRC. By coupling the HA with nanoparticles, the nanoparticles were easily internalized via endocytosis under the guidance of CD44. Moreover, chlorin e6 [61], the second-generation PS approved by 6递送到CRC。通过将HA与纳米颗粒偶联,在CD44的指导下,纳米颗粒通过内吞作用容易内化。此外,FDA
, and polydopamin批准的第二代PS氯e
, the photothermal agent with excellent light-thermal conversion efficiency were conjugated to promote the synergistic effect of photodynamic therapy and photothermal therapy by avoiding the toxicity induced by mental-ion. The result showed that the tumor growth was inhibited strongly in 6[61]与光热转换效率优异的光热剂聚多巴胺共轭,通过避免精神离子诱导的毒性,促进光动力疗法和光热疗法的协同效应。结果表明,HCT-116
肿瘤小鼠肿瘤生长受到强烈抑制,这也证实了两种治疗方法可以提高抗肿瘤效率。仿生奈米输送系统
除了配体-受体结合策略外,纳米颗粒还可以通过仿生技术主动靶向肿瘤,该技术主要利用生物膜来包覆纳米颗粒。这种策略不仅可以防止纳米颗粒被免疫系统鉴定,还可以利用膜蛋白,糖蛋白和同源粘附使纳米颗粒特异性地积聚在肿瘤中。近来,报道的仿生细胞膜纳米颗粒主要有红细胞膜[62]、癌细胞膜[63]、白细胞膜[64]等。此外,为了使纳米颗粒具有更多的性能,最好用杂化细胞膜伪装纳米颗粒。
[63] tumor mice, which also confirmed that the antitumor efficiency could be enhanced by combining the two treatments.
2.2.5. Biomimetic Nano Delivery Systems
In addition to the ligand–receptor binding strategy, the nanoparticles can also actively target the tumor by bionic technology, which mainly uses biofilms to coat nanoparticles. This strategy can not only prevent nanoparticles from being identified by the immune system, but also make use of membrane proteins, glycoproteins, and homologous adhesion to make nanoparticles specifically accumulate in the tumor. Recently, the bionic cell membranes used in the reported nanoparticles are mainly erythrocyte membrane [62], cancer cell membrane [63], leukocyte membrane [64], and so on. Furthermore, to make the nanoparticles endowed with more properties, it is better to camouflage the nanoparticles with hybrid cell membranes.
Wang et al. [63] prepared Zn制备锌1.25Ga加语1.5Ge通用 电气0.25O
4:: Cr铬3+,, Yb
3+, Er的3+ persistent luminescence nanoparticles coated with mesoporous silica to deliver 用介孔二氧化硅包覆的持久发光纳米颗粒以传递IR825
as well as irinotecan and encapsulated the nanoparticles with a cancer cell-macrophage hybrid membrane. The membrane made the nanoparticles actively target the tumor, then the nanoparticles continued to glow at the tumor, which could provide an accurate position for subsequent photothermal therapy. Moreover, after the CT26 tumor-bearing mice were treated with three months of nanoparticles and laser irradiation, the relative tumor volume of mice decreased significantly.
In a study, hollow long persistence luminescence nanomaterials loaded with cisplatin were synthesized and coated with a hybrid cell membrane, which was mainly composed of a customized erythrocyte membrane that could prevent nanoparticles from being recognized by the immune system and biofilm expressing 以及伊立替康,并用癌细胞 - 巨噬细胞杂交膜封装纳米颗粒。该膜使纳米颗粒主动靶向肿瘤,然后纳米颗粒继续在肿瘤上发光,这可以为随后的光热疗法提供准确的位置。此外,CT26荷瘤小鼠接受三个月的纳米颗粒和激光照射治疗后,小鼠的相对肿瘤体积显着降低。
在一项研究中,合成了含有顺铂的空心长持久性发光纳米材料,并涂覆了杂交细胞膜,该杂化细胞膜主要由可阻止纳米颗粒被免疫系统识别的定制红细胞膜和表达PD-1
that gave nanoparticles targeting ability. Then, this team evaluated their efficacy through in vitro anti-cancer experiments and concluded that these nanoplatforms, which played the role of immunotherapy and chemotherapy, were efficient in 的生物膜组成,该生物膜具有纳米颗粒的靶向能力。然后,研究小组通过体外抗癌实验评估了它们的疗效,并得出结论,这些起免疫治疗和化疗作用的纳米平台在CT26
tumor-bearing mice [65]. These studies indicated that bionic technology may be an effective method for tumor-targeted therapy.
荷瘤小鼠中是有效的[65]。这些研究表明,仿生技术可能是肿瘤靶向治疗的有效方法。