Age-related macular degeneration (AMD) is a leading cause of severe visual loss among the elderly. AMD patients are tormented by progressive central blurring/loss of vision and have limited therapeutic options to date. Drusen accumulation causing retinal pigment epithelial (RPE) cell damage is the hallmark of AMD pathogenesis, in which oxidative stress and inflammation are the well-known molecular mechanisms.
- retina
- vision
- ocular stress
- cell damage
- homeostasis
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
Definition: Age-related macular degeneration (AMD) is a leading cause of severe visual loss among the elderly. AMD patients are tormented by progressive central blurring/loss of vision and have limited therapeutic options to date. Drusen accumulation causing retinal pigment epithelial (RPE) cell damage is the hallmark of AMD pathogenesis, in which oxidative stress and inflammation are the well-known molecular mechanisms.
1. Introduction
Age-related macular degeneration (AMD) is a global primary cause of serious blindness [1][2][1,2]. In addition to aging, which is the leading factor of AMD [3], the etiology of AMD may also be ascribed to genetics (e.g. CFH and ARMS2 gene) [4], smoking [5][6][5,6], nutritional disorders [7], chronic light damage [8], and hypertension [9]. The predominant symptom of AMD patients is progressive central vision loss.
AMD is classified into non-neovascular and neovascular types. Non-neovascular AMD is further divided into ‘‘early dry’’ and ‘‘late dry’’ AMD. Neovascular AMD, also called ‘‘wet AMD’’, is a late and serious type of AMD. Among the global AMD population, the proportion of non-neovascular AMD is 80–90%, while the percentage of neovascular AMD is 10–15% [10]. Early dry AMD is defined by the presence of medium-size drusen deposition without pigmentary changes and vision loss. ‘‘Late dry’’ AMD, also called geographic atrophy (GA), is a chronic progressive macular degeneration, with sharply demarcated atrophy in the retina, retinal pigment epithelium (RPE), and choriocapillaries. Neovascular AMD is characterized by choroidal neovascularization which starts from choriocapillaris, extending to the Bruch′s membrane. Overall, vision loss is minimal or nonexistent in early-stage AMD, while late-stage AMD patients has vision loss symptoms.
Drusen accumulation causing RPE cell damage is the hallmark feature of AMD pathology while oxidative stress and inflammation are the well-known molecular mechanisms. However, the underlying mechanisms behind RPE cell stress in response to drusen deposits are still poorly understood. Programmed cell death (PCD) plays an important role in response to stress and the regulation of homeostasis and diseases. Apart from the classical apoptosis, recent studies also revealed the involvement of novel PCD such as pyroptosis, necroptosis, and ferroptosis, which may contribute to the RPE cell death in AMD (Figure 1).
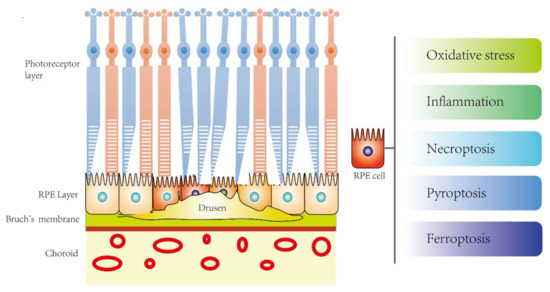
Figure 1. The potential novel disease mechanisms of RPE cell death in age-related macular degeneration (AMD).
2. Prevention and Intervention Strategies of AMD
2.1. Dry AMD
Early dry AMD may not be immediately sight-threatening; it is usually asymptomatic but later will develop symptoms, including visual distortion and reduced central vision. At present, no standard treatment for dry AMD is available in the world. However, several non-drug and drug intervention strategies have been recommended. Initially, maintaining a healthy lifestyle, including a balanced diet, regular exercise, wearing sunglasses, and quitting smoking, reduce the risk of dry AMD progression. In 2001, a randomized, placebo-controlled, age-related eye disease study (AREDS) showed that high-dose supplementation with vitamins C and E, beta carotene, and zinc significantly reduced the rate of visual acuity loss [11][61]. Studies also suggested that antioxidants including vitamins, lutein, and zeaxanthin may reduce the transformation of dry AMD to the reversible wet AMD [12][62]. Moreover, long-term supplementing of vitamins C and E, β-carotene, and zinc could significantly reduce the risk of the development of advanced AMD [13][63]. In 2014, AREDS with a ten-year follow-up reported that both age and the degree of drusen accumulation increased the risk of progression to advanced AMD [14][64]. AREDS2 further reported that the effect of lutein or zeaxanthin supplementation on AMD was better than beta-carotene [15][65]. The antioxidants reduce RPE damage by limiting the generation of free radicals, protecting photoreceptors and acting as retinal tissue nutrients [16][66]. In addition, recent single-center studies showed that photobiomodulation, a recently proposed light therapy, improved symptoms and reversed pathological changes (drusen formation) without causing harmful effects [17][18][67,68], suggesting a novel strategy for the earlier stage of AMD.
2.2. Wet AMD
The current treatments for wet AMD are anti-VEGF drugs, while photodynamic therapy is considered a second-line therapy today. According to the American Academy of Ophthalmology, anti-VEGF treatment improves vision in about one third (1 out of 3) of patients who receive it. For a vast majority (9 out of 10), anti-VEGF can at least stabilize vision [19][69]. However, there are still limitations in the current anti-VEGF treatment; the vision of around two-third of the patients receiving anti-VEGF treatment could not be improved. Therefore, a long-term visual benefit has not been achieved yet, and most treatments are mainly focused on delaying the progression of the diseases.
2.2.1. Anti-VEGF Drugs
Anti-VEGF drugs are typically delivered through intravitreal injection. Recently, a report by the American Academy of Ophthalmology reviewed 28 clinical trials of Anti-VEGF drugs, suggesting considerable safety and efficacy over the two years’ treatment for wet AMD [20][25]. Anti-VEGF drugs, such as ranibizumab and bevacizumab, bind to all VEGF isoforms to inhibit angiogenesis, thereby limiting the development of CNV [21][70]. Nevertheless, the effectiveness can only be sustained by continual periodical intraocular injection. In spite of the efficacy, some cases still showed poor vision outcomes after long-term anti-VEGF therapy. Moreover, several clinical trials also reported that GA developed in neovascular AMD patients after continual treatment with ranibizumab or bevacizumab [21][22][23][24][70,71,72,73]. One study even showed that the cumulative incidence of GA increased from the first (12%) to the fifth year (38%) of treatment. However, the percentages of GA still were significantly lower than the improved rates of visual acuity in these studies [25][26][27][28][74,75,76,77], suggesting anti-VEGF drug is beneficial as a long-term therapy for neovascular AMD. Yet, the development of alternative therapies is warranted in the future.
2.2.2. Photodynamic Therapy (PDT)
PDT combines a light-activated drug with a low-energy laser. The photosensitive drug (verteporfin) is injected through intravenous infusion and travels to the abnormal vessels under the central macula [29][78]. It then attaches to molecules such as low-density lipoprotein, integrin, and monoclonal antibodies that are commonly found in rapidly growing cells, for example new endothelial cells in neovascular AMD [30][79]. Finally, a low-energy laser light is focused directly onto the abnormal vessels, which activates the drug, causing damage specifically to the abnormal blood vessels. PDT can effectively delay the progress of AMD and reduce patients from severe vision loss. Although several treatments are usually required, PDT has largely replaced thermal laser therapy, which may cause permanent damage to the normal retina. However, the recurrence of the neovascularization is high in both PDT and thermal laser therapy. Moreover, PDT cannot restore the damaged macula, and has the risk of causing vascular occlusion, and thereby can further impair the vision [31][80].
2.2.3. Stem Cell Therapy
A stem cell is a type of unlimited self-renewal cell that can differentiate into other types of cells. Induced pluripotent stem cells (iPSCs) and human embryonic stem cells (hESCs) can be differentiated into retinal cells, sharing the same characters with the original ones at both genetic and functional levels. Using stem cells, the damaged retina can be repaired and substituted by the paracrine effect [32][81]. Another advantage of using stem cells is that they are immune friendly to the host. The risk of immunological rejection is significantly lower compared with RPE transplantation.
On-going clinical trials on stem cells for AMD treatment have been conducted in different countries [33][34][35][36][37][82,83,84,85,86]. hESCs and iPSCs derived from AMD patients were first used in the AMD clinical trials in the United States and Japan, respectively [35][38][84,87]. The preliminary and Phase I/II clinical studies showed that hESCs-derived RPE cell therapy has safely and effectively promoted general and peripheral vision, visual acuity and near/distance activities in AMD patients [35][36][84,85]. Moreover, in 2018, a study developed a bioengineered retinal pigment epithelial monolayer to deliver hESCs-derived RPE [34][83] and conducted a Phase I clinical study in advanced stage AMD patients [33][82]. These new technologies contribute to novel therapeutic strategies for AMD and help to improve visual acuity [33][34][82,83]. However, the sample sizes of both studies are relatively small, while the observation period in one study was only 12 months [33][82], which is not sufficient for safety and tumorigenicity evaluation. In fact, although stem cell therapy is a regenerative treatment option, it is costly in time and money.
Taken together, to date, some but not all pathogenic processes of AMD have been revealed and therapies for preventing these processes are being used. In spite of the implementation of current therapies, the reoccurrence rate is high and no existing therapeutic strategy can cure the disease, which may lead to excessive health care expenditures and substantial socio-economic burden worldwide. Consequently, improved understanding of the underlying mechanisms in RPE response after drusen exposure that allows exploration of novel strategies to prevent and tackle AMD is urgently needed.
