Biliary tract cancers (BTC) comprise a group of malignancies originating in the epithelium of the biliary tract. These include cholangiocarcinoma (CCA) and gallbladder carcinoma (GBC). Intrahepatic cholangiocarcinoma or iCCA refers to tumors proximal to the second-order ducts, while extrahepatic cholangiocarcinoma or eCCA refers to tumors arising more distally (perihilar CCA, between second-order ducts and cystic duct and distal CCA, distal to cystic duct). Perihilar CCA represents 50% of the total CCAs, with distal lesions comprising 40% and the final 10% being intrahepatic. BTC are often diagnosed at advanced stages and have a grave outcome due to limited systemic options. Gemcitabine and cisplatin combination (GC) has been the first-line standard for more than a decade. Second-line chemotherapy (CT) options are limited. Targeted therapy or TT (fibroblast growth factor 2 inhibitors or FGFR2, isocitrate dehydrogenase 1 or IDH-1, and neurotrophic tyrosine receptor kinase or NTRK gene fusions inhibitors) have had reasonable success, but <5% of total BTC patients are eligible for them. The use of immune checkpoint inhibitors (ICI) such as pembrolizumab is restricted to microsatellite instability high (MSI-H) patients in the first line. The success of the TOPAZ-1 trial (GC plus durvalumab) is promising, with numerous trials underway that might soon bring targeted therapy (pemigatinib and infrigatinib) and ICI combinations (with CT or TT in microsatellite stable cancers) in the first line.
- cholangiocarcinoma
- gall bladder cancer
- FGFR2
- pemigatinib
- infrigatinib
- HER2
- durvalumab
- gemcitabine
- NTRK
- IDH
1. Introduction
2. Chemotherapy in Biliary Tract Cancers
2.1. Chemotherapy in the First Line
Over 70% BTCs present in advanced stages or aBTC (unresectable or metastatic) and are only eligible to receive palliative therapy. The combination of gemcitabine (Gem) and cisplatin (Cis), or GC, is the current approved first-line therapy [18]. There were no positive first-line trials for over a decade. The standard approach to BTCs is illustrated in Figure 1.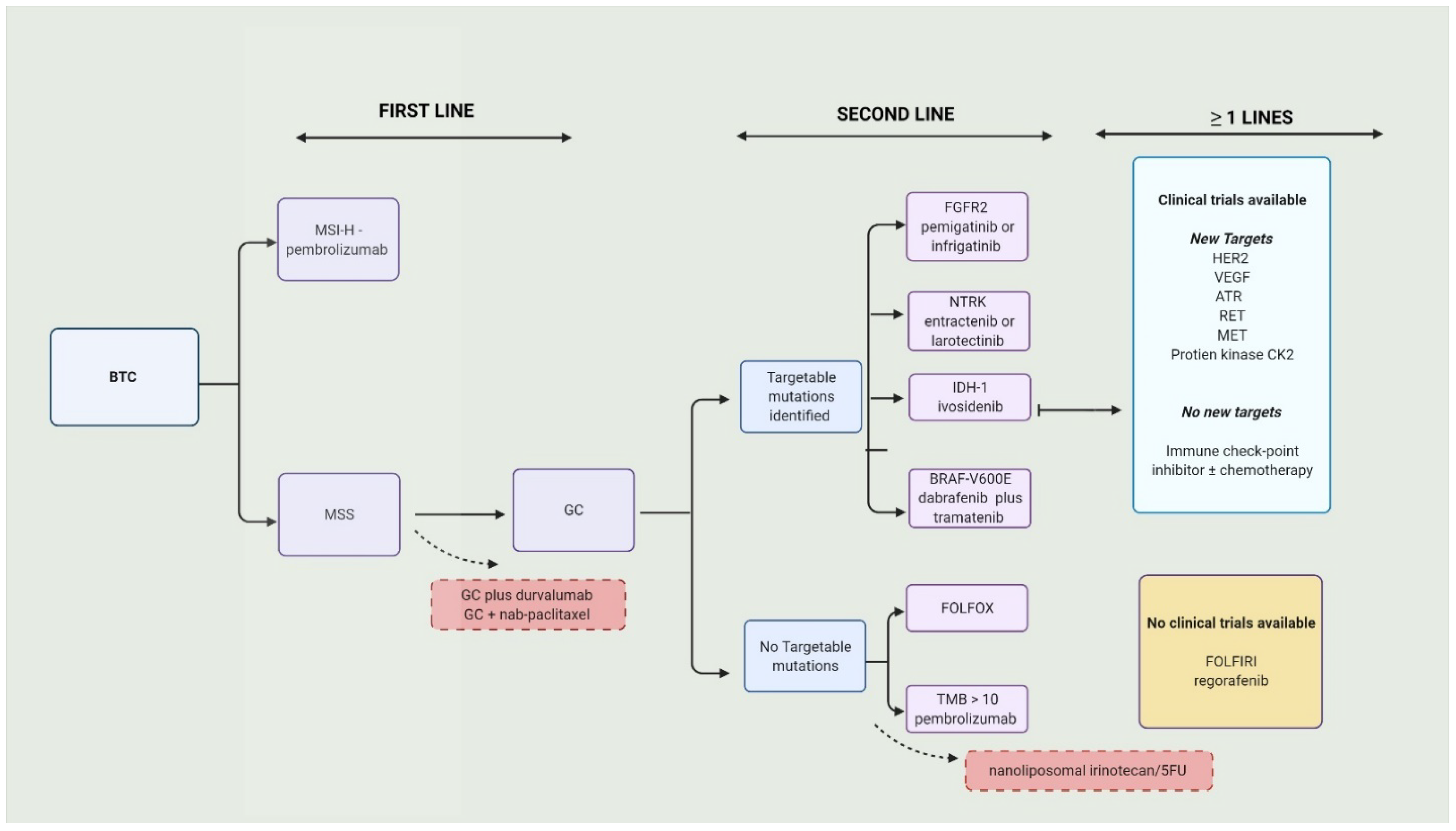
2.2. Chemotherapy in the Second Line
In aBTC (and ampullary cancers), patients who progressed on GC with a preserved performance status (Eastern Cooperative Oncology Group or ECOG scale of 0–1), FOLFOX had a small OS benefit (6.2 months vs. 5.3 months; adjusted hazard ratio = 0.69 [95% CI 0.50–0.97]; p = 0.031) compared to supportive care [28]. The survival rate was higher in the FOLFOX group at 6 months (51% vs. 36%) and 1 year (26% vs. 11%). Subgroup analysis in this trial produced some interesting results. The OS (not PFS) was superior with FOLFOX among the platinum-sensitive (PD after 90 days of completion of first-line chemotherapy) and platinum-resistant/refractory (PD on the first line or in less than 90 days after completion of first-line chemotherapy). Expectedly, high-grade AE were more prevalent in the FOLFOX group (69% vs. 52%). A retrospective study in Italy examined the differences in outcomes after second-line chemotherapy (post-GC) between elderly (≥70 years) and younger (<70 years) patients. There were no significant differences in the outcomes (OS or PFS) between the two groups. The most-used second-line agents in the elderly population were Gem alone or capecitabine alone or a combination of both. Treatment-related toxicity was very high in the elderly population compared to the younger group (48.5% vs. 8.2%; OR 6.31; p < 0.001) [29]. A combination of nanoliposomal irinotecan (Nan-Iri) and 5FU was compared to 5FU alone in the NIFTY trial [30]. It was a multicenter, open-label, randomized, phase IIb trial in which patients progressed on GC. The combination group had a superior PFS (7.1 m vs. 1.4 m; HR = 0.56; 95% CI 0.39–0.81; p = 0.0019) and ORR (19.3% vs. 2.1%) compared to the 5FU group. G3-4 neutropenia (24% vs. 1%) and serious adverse events (42% vs. 24%) occurred more in the combination group than the 5FU-only group. It was concluded that Nan-Iri plus 5-FU could be considered for second-line treatment in patients with BTC who formerly progressed on GC, especially in patients who cannot tolerate platinum agents. On the other hand, mFOLFIRINOX had reasonable efficacy and safety for patients who progressed on GC (≥3 cycles) and is an option for patients with no targetable mutations [31].3. Targeted Therapy in Biliary Tract Cancers
Second-line options in patients who progressed on GC are limited. In the subset of patients with targetable mutations, fibroblast growth factor 2 (FGFR2) inhibitors such as those with pemigatinib and infrigatinib [32], neurotrophic tyrosine receptor kinase (NTRK) gene fusions such as larotrectinib and entrectinib [33][34], and isocitrate dehydrogenase 1 (IDH-1) with ivosidenib [35], are suitable agents which are preferred over chemotherapy in the second line (preferably after GC). Individual targeted therapy options will be discussed in the following text. The reported results of trials and ongoing trials with targeted therapy are summarized in Table 1 and Table 2.Line | Phase | (N) | Clinical Trial Identifier | Treated Cancer Group | Experimental Arm | Target of the Drug (If Applicable) | Comparative Arm | Primary Outcome Studied in the Trial | Top 3 Treatment-Related Adverse Events | Notes | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Line | Phase | Experimental Arm | Clinical Trial Identifier | Treated Cancer Group | Experimental Arm | Comparative Arm | Comparative Arm | Primary Outcome | Primary Outcome | Secondary Outcome (Main) | Secondary Outcome (Main) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
First line | III | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
NCT03875235 | [ | 27] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
First line | III | NCT03773302 | BTC | FGFR rearrangement | Durvalumab (D) + GC | PD-1 | GC + placebo (Pbo) | OS—12.8 m vs. 11.5 m (D vs. Pbo, HR = 0.80; 95% CI, 0.66–0.97; p = 0.021) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
First line | III | NCT04003636 | BTC | CCA | Pembrolizumab + GC | Pemigatinib | GC + placebo | GC | Anemia |
OS | PFS | Low neutrophil count | Low platelet count | PFS-7.2 m vs. 5.7 m (D vs. Pbo, HR, 0.75; 95% CI, 0.64–0.89; p = 0.001); ORR—26.7% vs. 18.7% (D vs. Pbo); Grade 3/4—62.7% vs. 64.9% (D vs. Pbo) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
PFS, ORR, DOR | OS, OR, DOR, DCR | II | NCT03796429 [36] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
BTC | III | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II/III | NCT03773302 | NCT04066491 | FGFR2 fusion/translocation | BTCToripalimab + GC | CCA | PD-1 | Infrigatinib | Single arm | PFS—6.7 m | OS—NR | Leukopenia | GC Anemia | Rash | ORR—21 | DCR—85% | G3/4, non-hematological in 20% and hematological—69% | ||||||||||||||||||||||||||||||||||||||||||||||||||
Bintrafusp alfa | PFS | GC + placebo | OS | DLT | PFS, DOR, ORR | OS. DCR, DOR, BOR | II | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
III | NCT03951597 [37] | NCT04093362 | iCCA with FGFR2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | iCCA | NCT04217954 | Toripalimab + lenvatinib + GemOx + | BTC | iCCA | PD-1 + TKI | HAIC (oxaliplatin + 5-FU) + toripalimab (T) + bevacizumab | Futibatinib | None | Single arm | GC | ORR—80% (1CR and three patients obtained enough control to allow for resection) | PFS, ORR | PFS | OS, AE, CA 19-9, DCE-MRI signal change, DWI MRI signal change | Jaundice | Rash | Proteinuria | ORR. DCR. OS. Safety/Tolerability | DCR—93.3%, | PFS—10 m | OS—NR | DOR—9.8 m | |||||||||||||||||||||||||||||||||||||||||||
II | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | NCT04361331 [38] | iCCA | Lenvatinib + GemOx | NCT03768414 | TKI | None | Single arm | ORR—30% |
ORR | 1/30 was down staged to have resection | Fatigue | Jaundice |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | Not specific | NCT04172402 | BTC |
Vomiting | BTC | GC/NP | None specified | GC | PFS and OS—NR |
OS | PFS, ORR, DCR | DCRc—87% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
TS-1 + gemcitabine + nivolumab | No G5, ≥G3 in 40% | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ib | II | II | NCT02992340 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | NCT03579771 | NCT03898895 | BTC | High risk * | iCCA | Varlitinib + GC | Resectable IHC | Pan-HER 2 | Camrelizumab + radiotherapy | GC/NP | Single arm | None | DLT—1/11 (200 mg); 1/12 (300 mg) | blood and lymphatic system disorders | GCSR | PR = 8/23; SD = 12/23 |
PFS | OS, AE, tumor response | RR, R0; OS; PFS | ORR—35%, DCR—87%, DoR—4 m, PFS—6.8 m | ||||||||||||||||||||||||||||||||||||||||||||||
Ib | II | NCT02128282 [ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
III | 39] | NCT03478488 | CCA | Silmitasertib (CX-4945) + GC | Casein kinase 2 (CK2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Subsequent lines | Single arm | k | PFS 11 m | II | Diarrhea | Neutropenia | NCT04722133 | Nausea | BTC | HER 2 | aBTC | Trastuzumab-pkrb + FOLFOX | None | Compared to GC—Better PFS | Lesser neutropenia | |||||||||||||||||||||||||||||||||||||||||||||||||||
KN035 (PD-L1 antibody) + gemcitabine + oxaliplatin | ORR | PFS, OS, DCR, incidence of TRAE |
GEMOX | OS | PFS, ORR, DCR, DOR, TTP | I | NCT02375880 [40] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | jRCT2031180150 | NCT03796429 | BTC | HER 2 | DKN-01 + GC | BTC | Advanced solid tumors # | Dickkopf-1 (DKK1) | Gemcitabine/S-1 + toripalimab | Trastuzumab and pertuzumab | Single arm | None | Safety—no DLT | ORR | Neutropenia | Thrombocytopenia | Leukopenia | None | PFS, OS | PFS, OS, DoR, safety | ORR—21.3% | PFS—8.7 m | ||||||||||||||||||||||||||||||||||||||||||||
ORR, Safety |
Subsequent lines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | III | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | NCT02091141 | (My Pathway) | NCT04027764 | NCT02989857 (ClarIDHy) [41] | CCA | HER 2 | BTC | Ivosidenib (IVO) | BTC # | IDH-1 | Trastuzumab and pertuzumab | IVO alone vs. | placebo | None | PFS—2.7 m vs. 1.4 m (HR = 0.37; 95% CI 0.25–0.54; |
Toripalimab + S1 and albumin paclitaxel | ORR | p < 0.0001). | Ascites | Fatigue | Anemia | OS in updated analysis 10.3 m IVO vs. 7.5 m (HR = 0.79; 95% CI 0.56–1.12; p = 0.093) | ||||||||||||||||||||||||||||||||||||||||||||
None | ORR | PFS, DCR, OS | DCR, PFS, OS, AE | II | NCT02966821 [42] | BTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | NCT04466891 | Surufatinib | HER 2 | BTC | VEGF | Zanidatamab monotherapy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | NCT04191343 | BTC | Single arm |
Toripalimab + GEMOX | None | PFS rate at 16 wks—46.33% (95%, 24.38–65.73) | None | ORR | Elevated bilirubin |
ORR | Hypertension Proteinuria | PFS—3.7 m |
DoR; DoR > 16 wks; DCR, PFS, OS; incidence of TRAE, PK | OS—6.9 m | ||||||||||||||||||||||||||||||||||||||||||||||||||||
None specified | II | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | ChiCTR1900022003 [ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | 43]. | NCT02999672 | NCT04300959 | BTC | HER 2 | BTC | Anlotinib + | sintlimab | TKI + PD-1 | CCA # | Anlotinib hydrochloride + PD1 + gemcitabine + cisplatin | Trastuzumab emtansine | Gemcitabine Cisplatin | Single arm | None | OS—NR | OS 1 yr | BOR | OS 2 yr, PFS, ORR, AE | Hypertension ** | Diarrhea | Hypothyroidism | PFS, OS, TRAE, SAE, PK | PFS—6.5 m | ORR—40% | DCR—87% | ||||||||||||||||||||||||||||||||||||||||
II | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | NCT02052778 [ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Subsequent lines | 44]. | NCT04482309 | iCCA # | Futibatinib | II | HER2 | FGFR2 |
BTC # | Trastuzumab deruxtecan | Single arm | ORR 37% | NCT03482102 | Hyperphosphatemia | None Diarrhea * | Dry mouth * | DoR—8.3 m and DCR = 82% | ||||||||||||||||||||||||||||||||||||||||||||||||||
HCC, BTC | ORR | DOR, DCR, PFF, OS, AEs, PK and immunogenicity | Tremelimumab + durvalumab + radiation |
II | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
None |
II | NCT03230318 [45] | NCT03839342. | iCCA | Non-V600E BRAF mutations | Derazantinib | Advanced solid tumors | FGFR2—mutations and amplifications | # | Single arm | 3-month PFS rate—76% | Bimimetinib + encorafenib | Not specified | None | DCR = 80% | PFS = 7.3 m | 6-month PFS rate = 50% | |||||||||||||||||||||||||||||||||||||||||||||||||
ORR | Safety, DCR, PFS | II | NCT03797326 [46] | BTC # | Pembrolizumab + lenvatinib | PD-1 + TKI | Single arm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ORR | AE, OS, DCR, PFS, DOR, TTP | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | NCT04238637 | BTC | Durvalumab (D) vs. D + T | None | ORR | II | NCT02428855 | IDH1 mutation | iCCA | Dasatinib | None | ORR—10% | Safety—TRAE in 97% (>G354%) | ORR | Hypertension Dysphonia Diarrhea | PFS, OS, TRAE | DCR—68% | PFS—6.1 m | OS—8.6 m | |||||||||||||||||||||||||||||||||||||||||||||||
II | NCT02265341 [47] | BTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | Ponatinib | FGFR2 | Single arm | ORR—9% | Lymphopenia, Rash | Fatigue (50%) | CR = 0, PR—8%, SD = 36%. PFS—2.4 m and OS—15.7 m | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
NCT02675829 | HER2 amplification | Advanced solid tumors # | Ado-Trastuzumab emtansine | None | ORR | None | II | NCT03834220 [48] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | CCA among Solid tumors | Debio 1347 | FGFR Fusion | Single arm | ORR—2/5 (40%) of CCA | Fatigue | Hyperphosphatemia | Anemia | DoR and PFS were 16.1 weeks and 18.3 weeks (in all patients), respectively. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Safety, DoR, PFS, OS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | NCT02821754 | HCC, BTC | D + T | D +T + TACE | D + T + RFA | D + T + Cryo | PFS | Safety | NCT03207347 | BAP1 and other DDR genes | CCA # | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | NCT02703714 | BTC | Pembrolizumab | and sargramostim (GM-CSF) | None | ORR | AE, PD-L1 positivity, PFS, OS, DOR | Niraparib | None | ORR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
I/II | NCT03937895 | BTC *PFS, OS, TRAE | Allogeneic natural killer cells + pembrolizumab | None | Phase I—DLT | Phase II—ORR |
II | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TTP, toxicity | II | NCT01953926 [49] | NCT03212274 | BTC + AC # | Neratinib | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | IDH1/2 mutation | HER2 or EGFR Exon 18 | Single arm | ORR—12% | CCA | Diarrhea * | Vomiting * | Olaprib | PSS—2.8 m |
None | OS—5.4 m | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
ORR | NCT04306367 | BTC | Pembrolizumab and olaparib | PFS, OS, safety | mFOLFOX-historical control | ORR | DOR, PFS, OS, safety | I/ II | NCT01752920 [50] | iCCA | Derazantinib | FGFR2—fusions | Single arm | Safety—all-grade TRAE in 93% | Fatigue | Eye-toxicity | Hyperphospatemia | ≥3 Grade TRAE in 28% | ORR—27% | DCR—83% | ||||||||||||||||||||||||||||||||||||||||||||||
II | NCT04042831 | DNA repair gene mutation | BTC | Olaparib | None | ORR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | NCT04295317 | iCCA—adjuvant | PD-1 blocking antibody SHR-1210 + capecitabine | NoneOS, PFS, TRAE, DoR | PFS | OS, side effects | I | NCT02699515 [51 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II | ] | NCT03250273 | BTC # | Bintrafusp alfa, | TGF-β and PD-L1 | BTC, PDA | Single arm | Safety—emergent and all adverse events | Rash | Fever | Increased lipase | 63% had TRAE | 37% ≥ G3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
II | NCT03207347 | DNA repair gene mutation | CCA # | Entinostat + nivolumab | Niraparib | NoneNone | ORR | OS, PFS, TRAEs |
ORR | Toxicity, PFS, OS, DOR | I | NCT02892123 [52] | BTC # | ZW25 (Zanidatamab) | bispecific HER2 | Single arm | Safety/tolerability—only G1–G2 reported in 70% | Fatigue ** | Diarrhea | Infusion reaction | ORR—47 | DCR—65% | DoR—6.6 m | |||||||||||||||||||||||||||||||||||||||||||
II | NCT02162914 | VEGF mutation | CCA | Regorafenib | None | PFS | RR, OS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II |
Ib | NCT03996408 [53] | BTC | Anlotinib | TQB2450 | TKI + PDL1 | Single arm | DLT/ MTD | in first 3 weeks (one cycle)—none | RP2D—25 mg | ORR—42% | * Hypertension | Leukopenia | Increased total bilirubin | Neutropenia | PFS—240 days | DCR—75% |
Line | Phase | Clinical Trial Identifier | Target of the Drug | Treated Cancer Group | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
NCT02866383 | ||||||||||||||||||||||||||||||||||||||
BTC, PDA | ||||||||||||||||||||||||||||||||||||||
Nivolumab + ipilimumab + radiotherapy | ||||||||||||||||||||||||||||||||||||||
Nivolumab + radiotherapy | ||||||||||||||||||||||||||||||||||||||
CBR | AE, ORR, PFS, OS, QOL | |||||||||||||||||||||||||||||||||||||
II | NCT03339843 | |||||||||||||||||||||||||||||||||||||
II | CDK 4/6 mutation |
NCT04057365 | BTC | CCA # | DKN-01 + nivolumab | Abemaciclib | None | None | ORR | Anti-tumor activity | PFS, OS, toxicity | |||||||||||||||||||||||||||
PFS, OS | II | NCT04003896 | CDK 4/6 mutation | |||||||||||||||||||||||||||||||||||
II | NCT03639935 | BTC | BTC | Abemaciclib |
Rucaparib + nivolumab | None | None | ORR | PFS, DCR, OS, QoL | |||||||||||||||||||||||||||||
4-month PFS rate | Response rate, PFS, OS | II | NCT02232633 | STAT3 inhibitor | CCA | BBI503 | None | DCR | ||||||||||||||||||||||||||||||
II | NCT04299581 | iCCA | Camrelizumab + cryo | ORR, OS, PFS, PK TRAE | ||||||||||||||||||||||||||||||||||
None | ORR | DOR, PFS, OS, DCR, AE | II | |||||||||||||||||||||||||||||||||||
II | NCT03878095 | NCT03999658 | IDH1/2 mutation | BTC # | CCA # | STI-3031 | anti-PD-L1 antibody | Ceralasertib + olaparib | None | None | ORR | ORRPFS, OS, DoR, Safety | ||||||||||||||||||||||||||
DOR, CR, PFS, 1-year PFS rate, correlative studies | I/II | NCT02273739 | ||||||||||||||||||||||||||||||||||||
II | NCT03801083 | IDH2 mutation | Advanced solid tumors |
BTC | # | Tumor infiltrating lymphocytes (TIL) + aldesleukin | Enasidenib | Enasidenib | None | None | DLT, ECOG | ORR | Plasma concentration metrics | |||||||||||||||||||||||||
CRR, DOR, DCR, PFS, OS, QOL | I | NCT04764084 | HRR mutations | CCA # | Niraparib + anlotinib | |||||||||||||||||||||||||||||||||
I/II | NCT03684811 | BTC # | None | FT-2102 vs. FT-2102 + nivolumab | DLT, MTD | ORR, PFS | ||||||||||||||||||||||||||||||||
None | DLT, Dose, ORR | ORR, AE, PFS, TTP, DOR, OS, TT | I | |||||||||||||||||||||||||||||||||||
I/II | NCT04521686 | NCT03475953 | IDH1 R132-mutant advanced solid tumor types or circulating tumor DNA IDH2 R140 or IDH2 R172 mutation (CCA) | BTC # | CCA # | Regorafenib + avelumab | LY3410738 | LY3410738 + GC | Maximum tolerated dose | None | ORR | Safety and tolerability | Efficacy | PK properties | ||||||||||||||||||||||||
I = dose | II = antitumor activity | MTD, DLT, toxicity, AE, PK and correlative studies | I | NCT02381886 | IDH1 mutation | BTC # | IDH305 | None | DLT | |||||||||||||||||||||||||||||
I/II | NCT03785873 | TRAE, PK, delta 2-hydroxyglutarate, ORR, SAE | ||||||||||||||||||||||||||||||||||||
BTC | Nal-Irinotecan + nivolumab + 5-Fluorouracil + leucovorin | None | I = DLT | II = PFS | AE, ORR, OS | I | NCT03272464 | BRAF-V600E | BTC # | |||||||||||||||||||||||||||||
I | NCT03849469 | JSI-1187 + dabrafenib | None | iCCA #TRAE | XmAb®22841 and pembrolizumab | DOR, OS, PFS, TTP | ||||||||||||||||||||||||||||||||
XmAb | ® | 22841 Monotherapy | Safety and tolerability | None | I | NCT04190628 | ||||||||||||||||||||||||||||||||
I | BRAF-V600E | NCT03257761 | BTC # | ABM-1310 + cobimetinib | None | MTD | TRAE, PK, DOR, OS, PFS, TTP | |||||||||||||||||||||||||||||||
BTC, PDA, HCC | Guadecitabine + durvalumab | None | AE, Tumor response | OS, PFS | I | NCT02451553 | No specific target | BTC # | Afatinib dimaleate + capecitabine | None | AE, DLT, MTD | DOR, OS, PFS, RR, TTP, biomarker profile | ||||||||||||||||||||||||||
I | NCT03507998 | Wnt/β-catenin signaling inhibitors | BTC # | CGX1321 | None | TRAE | PK |
# Basket trial; * T-stage ≥ Ib (Ib-IV); solitary lesion > 5 cm; Multifocal tumors or satellite lesions present; BTC—biliary tract cancers include gall bladder cancers and CCA; iCCA—intrahepatic cholangiocarcinoma; eCCA—extra-hepatic cholangiocarcinoma; CCA—cholangiocarcinoma includes iCCA and eCCA; FGFR2—fibroblast growth factor 2; IDH—isocitrate dehydrogenase-1; VEGF—vascular endothelial growth factor; HER2—human epidermal growth factor receptor 2 inhibitors; STAT—signal transducer and activator of transcription; GC—gemcitabine/cisplatin; DCR—disease control rate; ORR—objective response rate; BOR—best overall response; DOR—duration of response; TTP—time to progression; SR—surgical resect ability; TRAEs—treatment-related adverse events; SAE—serious adverse events; PK—pharmacokinetics; RR—response rate; DLT—dose limiting toxicity MTD—maximum tolerated dose; QoL—quality of life; BOR—best overall response.
4. Immunotherapy in Biliary Tract Cancers
BTC—biliary tract cancers include gall bladder cancers and CCA; iCCA—intrahepatic cholangiocarcinoma; eCCA—extra-hepatic cholangiocarcinoma; CCA—cholangiocarcinoma includes iCCA and eCCA; PDA—pancreatic cancer; HCC—hepatocellular cancer; FGFR2—fibroblast growth factor 2; IDH—isocitrate dehydrogenase-1; VEGF—vascular endothelial growth factor; HER2—human epidermal growth factor receptor 2 inhibitors; HHR—homologous recombination repair; GC—gemcitabine/cisplatin; GM-CSF—granulocyte-macrophage colony-stimulating factor; TACE—transcatheter arterial chemoembolization; RFA—radiofrequency ablation; Cryo—cryotherapy; HAIC—hepatic arterial infusion chemotherapy; CPS—combined positive score; MSI-H—microsatellite instability; DCE—dynamic contrast enhanced; DWI—diffusion weighted imaging; TTP—time to progression; CBR—clinical benefit rate; QOL—quality of life; TTR—time to response; #—basket trials with BTC among them; * at least 1% CPS PD-L1 or MSI-high or dMMR positive.
5. Systemic Therapy in Early-Stage Biliary Tract Cancers
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444.
- Razumilava, N.; Gores, G.J. Classification, diagnosis, and management of cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2013, 11, 13–21.e11.
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762.
- Patel, T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001, 33, 1353–1357.
- Ouyang, G.; Liu, Q.; Wu, Y.; Liu, Z.; Lu, W.; Li, S.; Pan, G.; Chen, X. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: A systematic analysis for the Global Burden of Disease Study 2017. Cancer 2021, 127, 2238–2250.
- Zatonski, W.A.; Lowenfels, A.B.; Boyle, P.; Maisonneuve, P.; Bueno de Mesquita, H.B.; Ghadirian, P.; Jain, M.; Przewozniak, K.; Baghurst, P.; Moerman, C.J.; et al. Epidemiologic aspects of gallbladder cancer: A case-control study of the SEARCH Program of the International Agency for Research on Cancer. J. Natl. Cancer Inst. 1997, 89, 1132–1138.
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017, 24, 1073274817729245.
- Sithithaworn, P.; Yongvanit, P.; Duenngai, K.; Kiatsopit, N.; Pairojkul, C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2014, 21, 301–308.
- Strom, B.L.; Soloway, R.D.; Rios-Dalenz, J.L.; Rodriguez-Martinez, H.A.; West, S.L.; Kinman, J.L.; Polansky, M.; Berlin, J.A. Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer 1995, 76, 1747–1756.
- Florio, A.A.; Ferlay, J.; Znaor, A.; Ruggieri, D.; Alvarez, C.S.; Laversanne, M.; Bray, F.; Mcglynn, K.A.; Petrick, J.L. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer 2020, 126, 2666–2678.
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599.
- Shaib, Y.H.; Davila, J.A.; McGlynn, K.; El-Serag, H.B. Rising incidence of intrahepatic cholangiocarcinoma in the United States: A true increase? J. Hepatol. 2004, 40, 472–477.
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019, 39, 98–107.
- Vogel, A.; Saborowski, A. Current and Future Systemic Therapies in Biliary Tract Cancer. Visc. Med. 2021, 37, 32–38.
- Neumann, U.P.; Schmeding, M. Role of surgery in cholangiocarcinoma: From resection to transplantation. Best Pract Res. Clin. Gastroenterol. 2015, 29, 295–308.
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574.
- Sapisochín, G. Liver transplantation for cholangiocarcinoma: Current status and new insights. World J. Hepatol. 2015, 7, 2396.
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281.
- Valle, J.W.; Wasan, H.; Johnson, P.; Jones, E.; Dixon, L.; Swindell, R.; Baka, S.; Maraveyas, A.; Corrie, P.; Falk, S.; et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: A multicentre randomised phase II study—The UK ABC-01 Study. Br. J. Cancer 2009, 101, 621–627.
- Phelip, J.M.; Desrame, J.; Edeline, J.; Barbier, E.; Terrebonne, E.; Michel, P.; Perrier, H.; Dahan, L.; Bourgeois, V.; Akouz, F.K.; et al. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients with Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J. Clin. Oncol. 2022, 40, 262–271.
- Knox, J.J.; McNamara, M.G.; Goyal, L.; Cosgrove, D.; Springfeld, C.; Sjoquist, K.M.; Park, J.O.; Verdaguer, H.; Braconi, C.; Ross, P.J.; et al. Phase III study of NUC-1031 + cisplatin versus gemcitabine + cisplatin for first-line treatment of patients with advanced biliary tract cancer (NuTide:121). J. Clin. Oncol. 2021, 39, TPS4164.
- Kapacee, Z.A.; Knox, J.J.; Palmer, D.; Blagden, S.P.; Lamarca, A.; Valle, J.W.; Mcnamara, M.G. NUC-1031, use of ProTide technology to circumvent gemcitabine resistance: Current status in clinical trials. Med. Oncol. 2020, 37, 61.
- Assenat, E.; Blanc, J.F.; Bouattour, M.; Gauthier, L.; Touchefeu, Y.; Portales, F.; Borg, C.; Fares, N.; Mineur, L.; Bleuse, J.-P.; et al. 48P (BREGO) Regorafenib combined with modified m-GEMOX in patients with advanced biliary tract cancer (BTC): A phase II randomized trial. Ann. Oncol. 2021, 32, S376–S377.
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers. JAMA Oncol. 2019, 5, 824.
- Cheon, J.; Lee, C.-K.; Sang, Y.B.; Choi, H.J.; Kim, M.H.; Ji, J.H.; Ko, K.H.; Kwon, C.-I.; Kim, D.J.; Choi, S.H.; et al. Real-world efficacy and safety of nab-paclitaxel plus gemcitabine-cisplatin in patients with advanced biliary tract cancers: A multicenter retrospective analysis. Ther. Adv. Med. Oncol. 2021, 13, 175883592110359.
- Sakai, D.; Kanai, M.; Kobayashi, S.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; Ishioka, C.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer (KHBO1401-MITSUBA). Ann. Oncol. 2018, 29, viii205.
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378.
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701.
- Rizzo, A.; Salati, M.; Frega, G.; Merz, V.; Caputo, F.; Ricci, A.D.; Palloni, A.; Messina, C.; Spallanzani, A.; Saccoccio, G.; et al. Second-line chemotherapy (2L) in elderly patients with advanced biliary tract cancer (ABC): A multicenter real-world study. J. Clin. Oncol. 2021, 39, 322.
- Yoo, C.; Kim, K.-P.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): A multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021, 22, 1560–1572.
- Belkouz, A.; de Vos-Geelen, J.; Mathôt, R.A.A.; Eskens, F.A.L.M.; van Gulik, T.M.; van Oijen, M.G.H.; Punt, C.J.A.; Wilmink, J.W.; Klümpen, H.J. Efficacy and safety of FOLFIRINOX as salvage treatment in advanced biliary tract cancer: An open-label, single arm, phase 2 trial. Br. J. Cancer 2020, 122, 634–639.
- Makawita, S.; Abou-Alfa, G.K.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.Y.; Macarulla, T.; et al. Infigratinib in patients with advanced cholangiocarcinoma with. Future Oncol. 2020, 16, 2375–2384.
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282.
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739.
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807.
- Liu, T.; Li, W.; Yu, Y.; Guo, X.; Xu, X.; Wang, Y.; Li, Q.; Wang, Y.; Cui, Y.; Liu, H.; et al. 53P Toripalimab with chemotherapy as first-line treatment for advanced biliary tract tumors: A preliminary analysis of safety and efficacy of an open-label phase II clinical study. Ann. Oncol. 2020, 31, S261.
- Zhou, J.; Fan, J.; Shi, G.; Huang, X.; Wu, D.; Yang, G.; Ge, N.; Hou, Y.; Sun, H.; Huang, X.; et al. 56P Anti-PD1 antibody toripalimab, lenvatinib and gemox chemotherapy as first-line treatment of advanced and unresectable intrahepatic cholangiocarcinoma: A phase II clinical trial. Ann. Oncol. 2020, 31, S262–S263.
- Shi, G.-M.; Jian, Z.; Fan, J.; Huang, X.-Y.; Wu, D.; Liang, F.; Lu, J.-C.; Yang, G.-H.; Chen, Y.; Ge, N.-L.; et al. Phase II study of lenvatinib in combination with GEMOX chemotherapy for advanced intrahepatic cholangiocarcinoma. J. Clin. Oncol. 2021, 39, e16163.
- Borad, M.J.; Bai, L.-Y.; Chen, M.-H.; Hubbard, J.M.; Mody, K.; Rha, S.Y.; Richards, D.A.; Davis, S.L.; Soong, J.; Huang, C.-E.C.-E.; et al. Silmitasertib (CX-4945) in combination with gemcitabine and cisplatin as first-line treatment for patients with locally advanced or metastatic cholangiocarcinoma: A phase Ib/II study. J. Clin. Oncol. 2021, 39, 312.
- Goyal, L.; Sirard, C.; Schrag, M.; Kagey, M.H.; Eads, J.R.; Stein, S.; El-Khoueiry, A.B.; Manji, G.A.; Abrams, T.A.; Khorana, A.A.; et al. Phase I and Biomarker Study of the Wnt Pathway Modulator DKN-01 in Combination with Gemcitabine/Cisplatin in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2020, 26, 6158–6167.
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final results from ClarIDHy, a global, phase III, randomized, double-blind study of ivosidenib (IVO) versus placebo (PBO) in patients (pts) with previously treated cholangiocarcinoma (CCA) and an isocitrate dehydrogenase 1 (IDH1) mutation. J. Clin. Oncol. 2021, 39, 266.
- Bai, Y.; Xu, J.; Sun, H.; Bai, C.; Jia, R.; Li, Y.; Zhang, W.; Liu, L.; Huang, C.; Guan, M.; et al. A single-arm, multicenter, open-label phase 2 trial of surufatinib in patients with unresectable or metastatic biliary tract cancer. J. Clin. Oncol. 2021, 39, e16123.
- Zong, H.; Zhong, Q.; Zhao, R.; Jin, S.; Zhou, C.; Zhang, X.; Shi, J.; Qiao, S.; Han, J.; Jiang, M. Phase II study of anlotinib plus sintlimab as second-line treatment for patients with advanced biliary tract cancers. J. Clin. Oncol. 2021, 39, 307.
- Bridgewater, J.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.; Abrams, T.; Furuse, J.; Kelley, R.K.; Cassier, P.; et al. 54P Efficacy and safety of futibatinib in intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 fusions/other rearrangements: Subgroup analyses of a phase II study (FOENIX-CCA2). Ann. Oncol. 2020, 31, S261–S262.
- Javle, M.M.; Abou-Alfa, G.K.; Macarulla, T.; Personeni, N.; Adeva, J.; Bergamo, F.; Malka, D.; Vogel, A.; Knox, J.J.; Evans, T.R.J.; et al. Efficacy of derazantinib in intrahepatic cholangiocarcinoma patients with FGFR2 mutations or amplifications: Interim results from the phase 2 study FIDES-01. J. Clin. Oncol. 2022, 40, 427.
- Villanueva, L.; Lwin, Z.; Chung, H.C.C.; Gomez-Roca, C.A.; Longo, F.; Yanez, E.; Senellart, H.; Doherty, M.; Garcia-Corbacho, J.; Hendifar, A.E.; et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase 2 LEAP-005 study. J. Clin. Oncol. 2021, 39, 4080.
- Ahn, D.H.; Uson Junior, P.L.S.; Masci, P.; Kosiorek, H.; Halfdanarson, T.R.; Mody, K.; Babiker, H.; DeLeon, T.; Sonbol, M.B.; Gores, G.; et al. A pilot study of Pan-FGFR inhibitor ponatinib in patients with FGFR-altered advanced cholangiocarcinoma. Invest New Drugs. 2022, 40, 134–141.
- Cleary, J.M.; Iyer, G.; Oh, D.-Y.; Mellinghoff, I.K.; Goyal, L.; Ng, M.C.H.; Meric-Bernstam, F.; Matos, I.; Chao, T.-Y.; Sarkouh, R.A.; et al. Final results from the phase I study expansion cohort of the selective FGFR inhibitor Debio 1,347 in patients with solid tumors harboring an FGFR gene fusion. J. Clin. Oncol. 2020, 38, 3603.
- Harding, J.J.; Cleary, J.M.; Quinn, D.I.; Braña, I.; Moreno, V.; Borad, M.J.; Loi, S.; Spanggaard, I.; Park, H.; Ford, J.M.; et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: Results from the phase II SUMMIT ‘basket’ trial. J. Clin. Oncol. 2021, 39, 320.
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171.
- Yoo, C.; Oh, D.-Y.; Choi, H.J.; Kudo, M.; Ueno, M.; Kondo, S.; Chen, L.-T.; Osada, M.; Helwig, C.; Dussault, I.; et al. 73P Long-term follow-up of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. Ann. Oncol. 2020, 31, S268–S269.
- Meric-Bernstam, F.; Hanna, D.L.; El-Khoueiry, A.B.; Kang, Y.-K.; Oh, D.-Y.; Chaves, J.M.; Rha, S.Y.; Hamilton, E.P.; Pant, S.; Javle, M.M.; et al. Zanidatamab (ZW25) in HER2-positive biliary tract cancers (BTCs): Results from a phase I study. J. Clin. Oncol. 2021, 39, 299.
- Zhou, J.; Gong, J.; Cao, Y.; Peng, Z.; Yuan, J.; Wang, X.; LU, M.; Shen, L. Anlotinib plus TQB2450 in patients with advanced refractory biliary tract cancer (BTC): An open-label, dose-escalating, and dose-expansion cohort of phase Ib trial. J. Clin. Oncol. 2021, 39, 292.
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673.
- Ebata, T.; Hirano, S.; Konishi, M.; Uesaka, K.; Tsuchiya, Y.; Ohtsuka, M.; Kaneoka, Y.; Yamamoto, M.; Ambo, Y.; Shimizu, Y.; et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br. J. Surg. 2018, 105, 192–202.
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.-P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.-L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667.
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.G.; Primrose, J.; et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1015–1027.
- Edeline, J.; Hirano, S.; Bertaut, A.; Konishi, M.; Benabdelghani, M.; Uesaka, K.; Watelet, J.; Ohtsuka, M.; Hammel, P.; Kaneoka, Y.; et al. 55P Adjuvant gemcitabine-based chemotherapy for biliary tract cancer: Pooled analysis of the BCAT and PRODIGE-12 studies. Ann. Oncol. 2020, 31, S262.
- Kim, Y.; Amini, N.; Wilson, A.; Margonis, G.A.; Ethun, C.G.; Poultsides, G.; Tran, T.; Idrees, K.; Isom, C.A.; Fields, R.C.; et al. Impact of Chemotherapy and External-Beam Radiation Therapy on Outcomes among Patients with Resected Gallbladder Cancer: A Multi-institutional Analysis. Ann. Surg. Oncol. 2016, 23, 2998–3008.
- Mallick, S.; Benson, R.; Haresh, K.P.; Julka, P.K.; Rath, G.K. Adjuvant radiotherapy in the treatment of gall bladder carcinoma: What is the current evidence. J. Egypt Natl. Canc. Inst. 2016, 28, 1–6.
- Wang, S.J.; Lemieux, A.; Kalpathy-Cramer, J.; Ord, C.B.; Walker, G.V.; Fuller, C.D.; Kim, J.-S.; Thomas, C.R. Nomogram for Predicting the Benefit of Adjuvant Chemoradiotherapy for Resected Gallbladder Cancer. J. Clin. Oncol. 2011, 29, 4627–4632.
- Agrawal, S.; Alam, M.N.; Rastogi, N.; Saxena, R. 59P Evolution of adjuvant therapy in radically resected carcinoma gallbladder (GBC) over a decade: A real world experience from a regional cancer centre. Ann. Oncol. 2020, 31, S264.
- Rizzo, A.; Brandi, G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat. Res. Commun. 2021, 27, 100354.
- Hashimoto, K.; Tono, T.; Nishida, K.; Nonaka, R.; Tsunashima, R.; Fujie, Y.; Fujita, S.; Fujita, J.; Yoshida, T.; Ohnishi, T.; et al. A case of curatively resected advanced intrahepatic cholangiocellular carcinoma through effective response to neoadjuvant chemotherapy. Gan Kagaku Ryoho 2014, 41, 2083–2085.
- Kato, A.; Shimizu, H.; Ohtsuka, M.; Yoshidome, H.; Yoshitomi, H.; Furukawa, K.; Takeuchi, D.; Takayashiki, T.; Kimura, F.; Miyazaki, M. Surgical Resection after Downsizing Chemotherapy for Initially Unresectable Locally Advanced Biliary Tract Cancer: A Retrospective Single-center Study. Ann. Surg. Oncol. 2013, 20, 318–324.
- McMasters, K.M.; Tuttle, T.M.; Leach, S.D.; Rich, T.; Cleary, K.R.; Evans, D.B.; Curley, S.A. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am. J. Surg. 1997, 174, 605–608; discussion 608–609.
- Nelson, J.W.; Ghafoori, A.P.; Willett, C.G.; Tyler, D.S.; Pappas, T.N.; Clary, B.M.; Hurwitz, H.I.; Bendell, J.C.; Morse, M.A.; Clough, R.W.; et al. Concurrent Chemoradiotherapy in Resected Extrahepatic Cholangiocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 148–153.
- Le Roy, B.; Gelli, M.; Pittau, G.; Allard, M.-A.; Pereira, B.; Serji, B.; Vibert, E.; Castaing, D.; Adam, R.; Cherqui, D.; et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br. J. Surg. 2018, 105, 839–847.
- Hong, T.S.; Wo, J.Y.; Yeap, B.Y.; Ben-Josef, E.; Mcdonnell, E.I.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Goyal, L.; et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients with Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Clin. Oncol. 2016, 34, 460–468.
- Tao, R.; Krishnan, S.; Bhosale, P.R.; Javle, M.M.; Aloia, T.A.; Shroff, R.T.; Kaseb, A.O.; Bishop, A.J.; Swanick, C.W.; Koay, E.J.; et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients with Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J. Clin. Oncol. 2016, 34, 219–226.
- Polistina, F.A.; Guglielmi, R.; Baiocchi, C.; Francescon, P.; Scalchi, P.; Febbraro, A.; Costantin, G.; Ambrosino, G. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiother. Oncol. 2011, 99, 120–123.
