The assessment of psoriatic nail changes in everyday practice is based exclusively on clinical symptoms that do not reflect the entire disease process in the nail apparatus. The use of imaging methods, especially widely available and inexpensive ultrasonography, creates the possibility of additional revealing and assessing grayscale of morphological changes of the ventral nail plate, nail bed, and matrix, as well as the attachment of the finger extensor tendon to the distal phalanx. What is more, it enables the assessment of inflammation severity in the power Doppler technique. A qualitative classification of nail plate morphological changes corresponding to the severity of psoriatic nail changes has been developed so far and attempts are being made to develop a quantitative method to assess not only the presence of changes but also the severity of inflammation.
- nail psoriasis
- ultrasonography
- psoriasis assessment
1. Introduction
2. The Structure of the Nail Apparatus
The nail apparatus consists of the nail plate, the reproductive part formed by the nail matrix and nail bed, and the soft tissues surrounding the nail plate (Figure 1).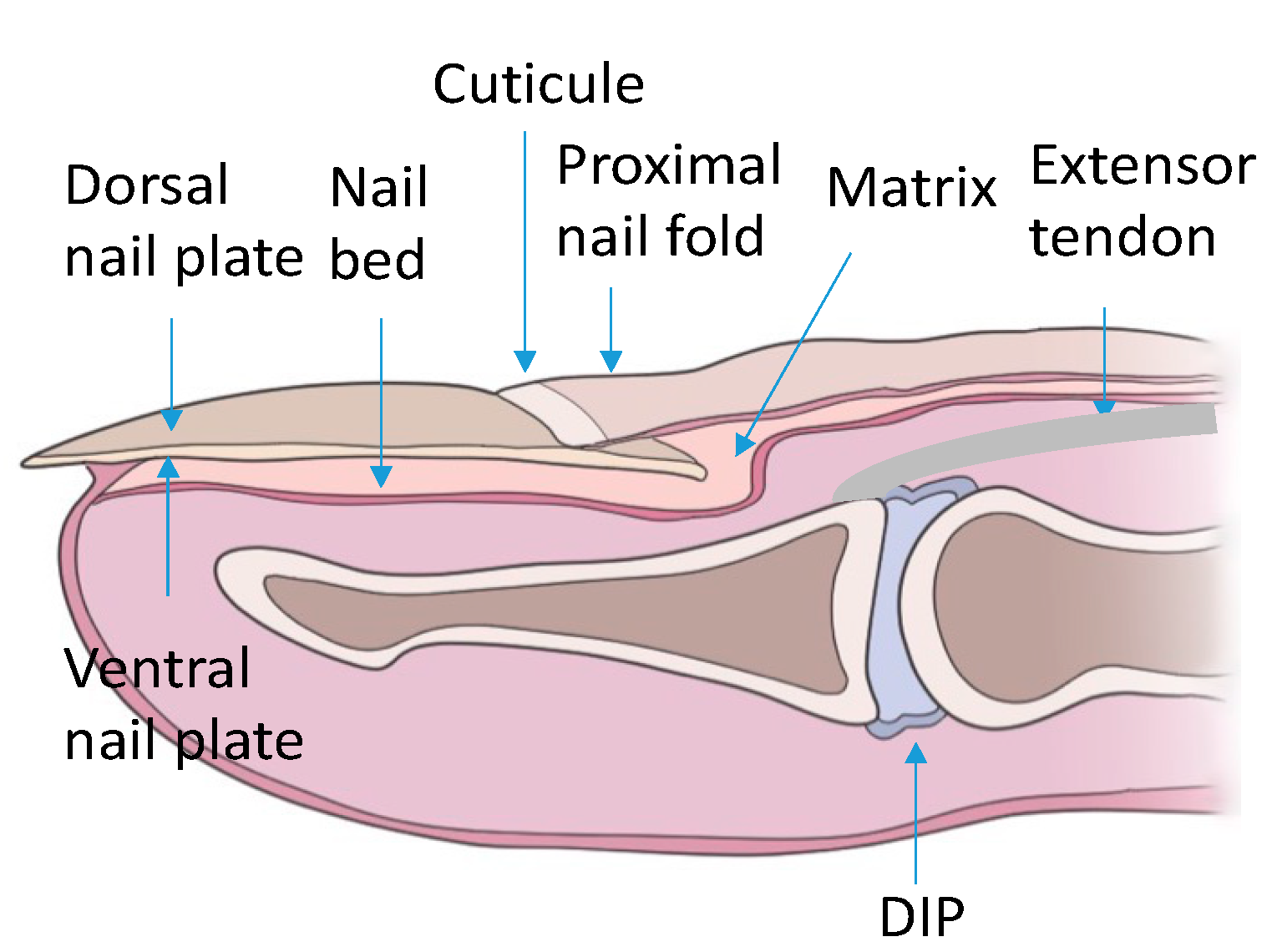
3. Psoriatic Nail Changes
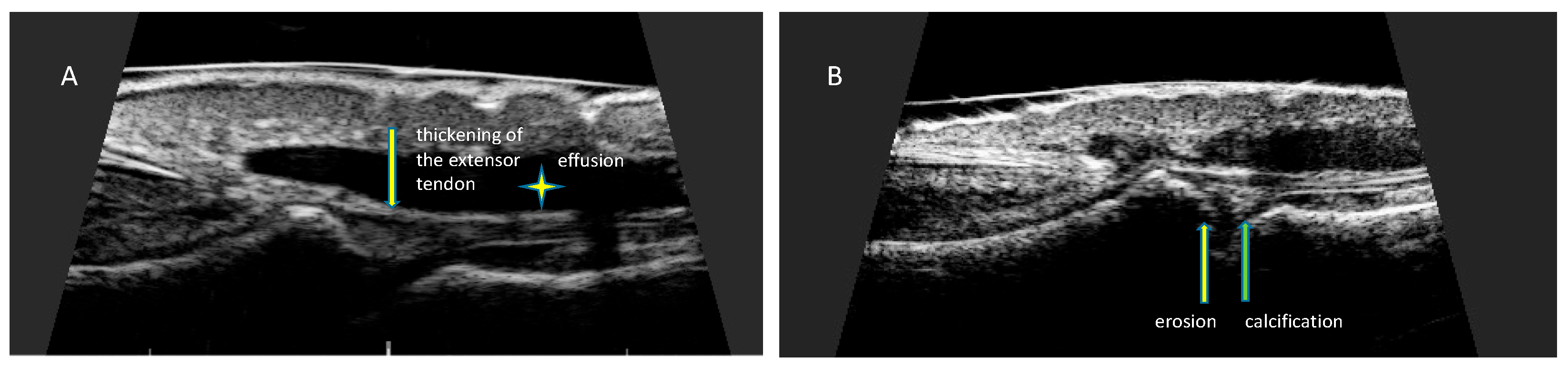
The evaluated psoriatic nail changes depend on the structure of the nail apparatus which is involved in the disease process. The following are associated with matrix involvement: thinning—small, superficial depressions in the nail plate, leukonychia—small, white spots in the nail plate, red spots in the rim, nail plate dystrophy characterized by increased brittleness and crumbling of the nail and Beau lines—transverse depressions in the plate. In the course of the psoriatic nail bed involvement, they are present: onycholysis—separation of the nail plate from the nail bed, oil spots (salmon spots)—brown-red discoloration under the nail plate, linear subungual hemorrhages, and subungual hyperkeratosis caused by an accumulation of non-exfoliated cells under the nail plate (Figure 2) [16]. The inflammatory process may extend beyond the nail plate to the proximal and lateral nail shafts.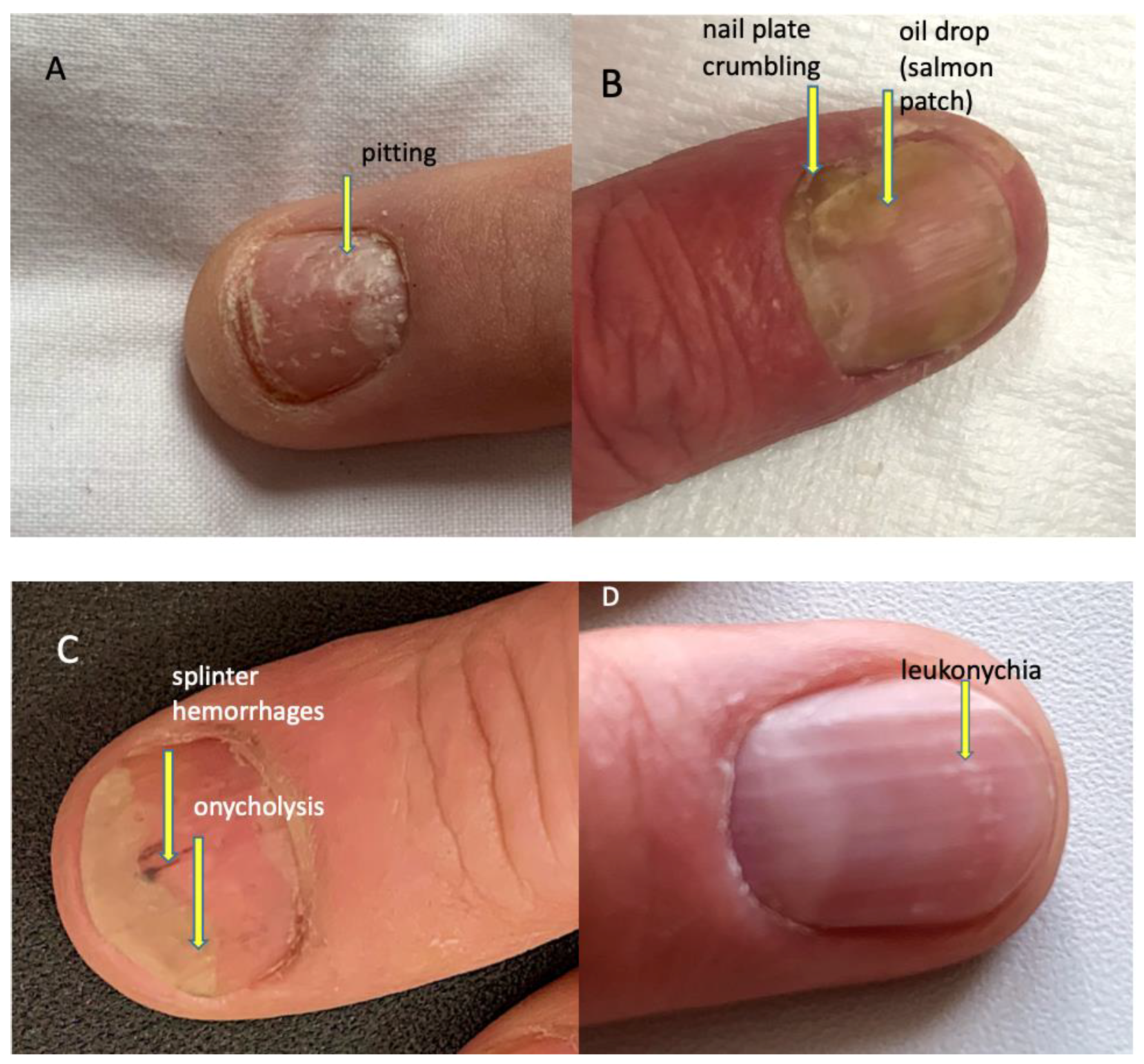
4. Ultrasonographic Image of the Nail Apparatus
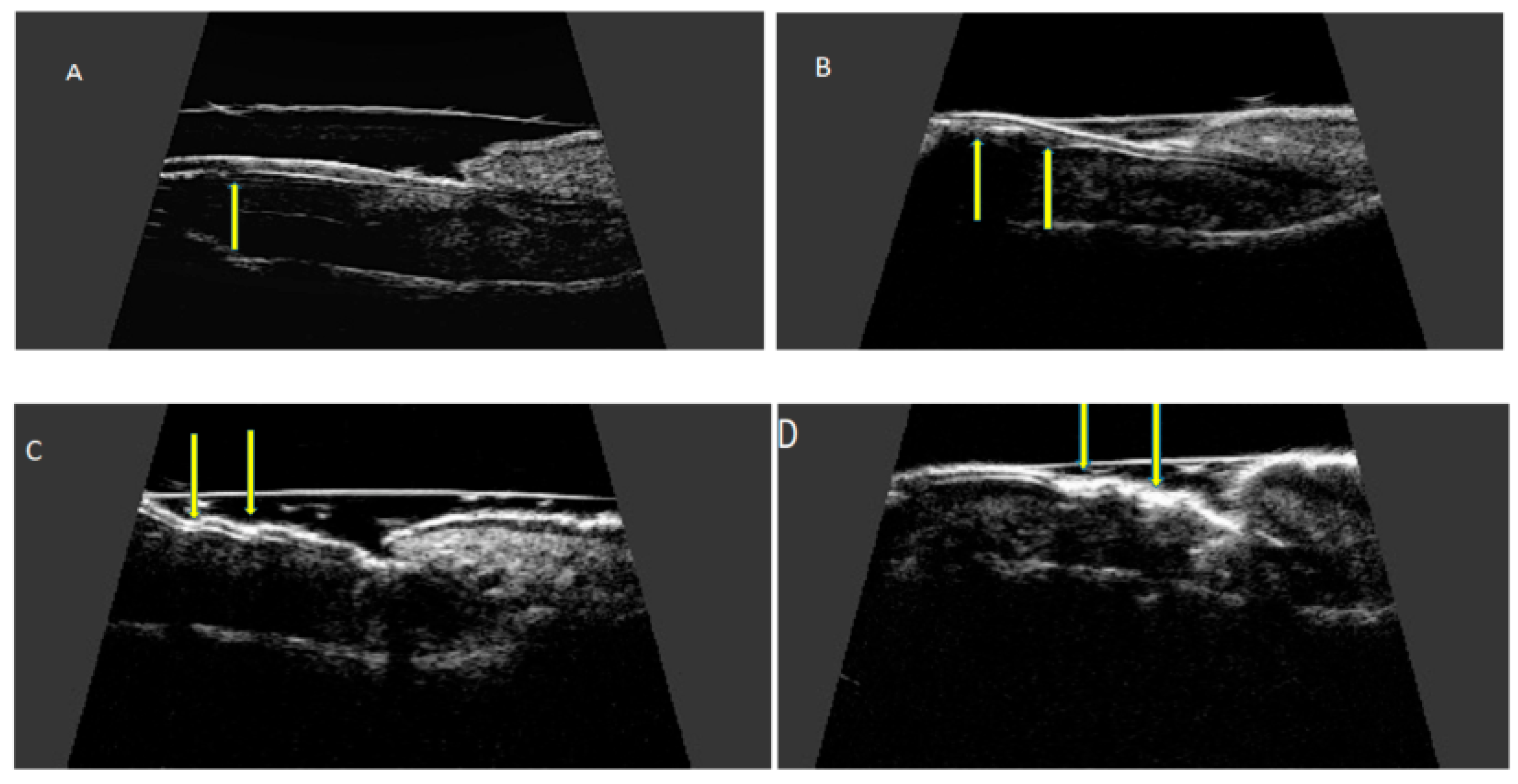
The nail is a structure consisting of two parallel hyperechoic (white) plates: dorsal and ventral. The plates are separated from each other by a hypoechoic interlaminar space. The thickness of a normal plate varies between 0.3 and 0.65 mm [17]. The nail matrix is an isoechogenic (light grey) structure in the proximal part of the nail, 1–5.3 mm long [12]. The thickness of the hypoechoic (dark gray) nail bed of a healthy nail, measured between the ventral nail plate and the periosteum of the distal phalanx, is 0.7–6.5 mm [17]. Under the nail bed, a hyperechoic band is clearly visible on ultrasound, which is the dorsal surface of the distal phalanx. In the proximal part of the dorsal surface of the distal phalanx, a site of attachment of the finger extensor tendon is located (Figure 3).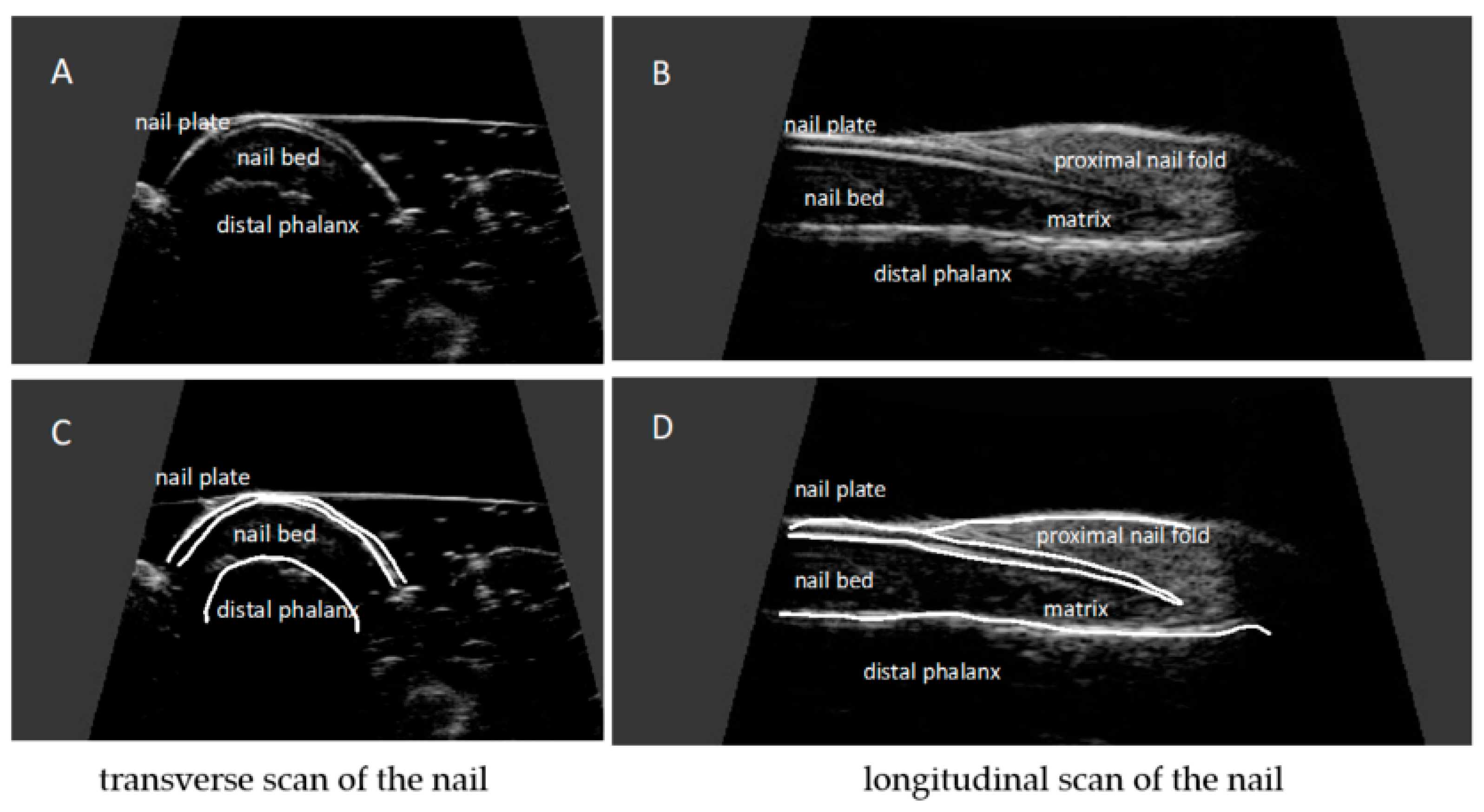
5. Examination Technique
For ultrasonographic examination of the nail apparatus, a high-frequency linear head, above 15 MHz, is used [11,12,13][11][12][13]. Fingernail examination is performed in a seated position with hands placed on a table. The hand nail apparatus is assessed in the sagittal plane, from the dorsal side of the nails, usually in the longitudinal section. It is not recommended to use gel pads to avoid pressure on superficial tissues—the proper distance of the head from the skin, allowing imaging of superficial structures, is maintained only with an appropriate amount of gel. The following parameters are measured: nail plate thickness, nail bed thickness, nail matrix thickness, and finger extensor tendon thickness. Nail thickness is measured as the maximum distance between the dorsal and ventral hyperechoic plates of the nail. The thickness of the hypoechogenic nail bed is measured as the maximum distance between the ventral plate of the nail and the edge of the phalangeal bone. The thickness of the isoechogenic area of the nail matrix is measured at the proximal end of the nail bed. Increased vascularization, a sign of inflammation, is assessed with the power Doppler technique. Tendon attachments are assessed in accordance with OMERACT (outcome measures in rheumatology) recommendations on the scale of grayness. The thickness of the extensor tendon is measured at the place where an extensor tendon is attached to the distal phalanx [13,18,19][13][18][19].6. Ultrasonographic Changes of the Nail Apparatus in Psoriasis and Psoriatic Arthritis
High-resolution ultrasonography can accurately image morphological psoriatic changes in the nail apparatus and objectively assess clinical progression, the severity of active inflammation, and response to treatment. Ultrasonographic images of nails with psoriatic changes depend on the nature of clinical changes (Figure 4).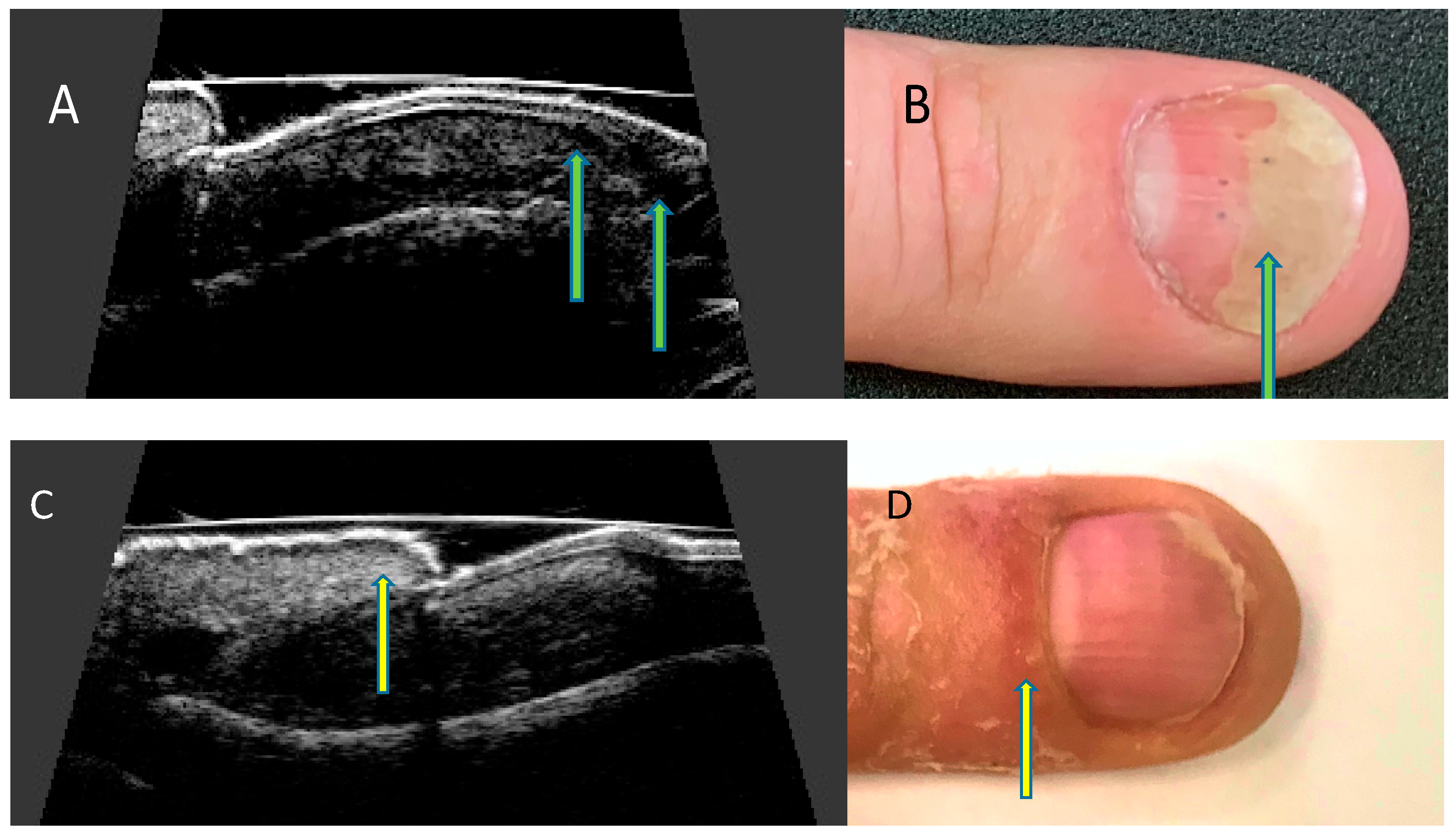

References
- Wilson, F.C.; Icen, M.; Crowson, C.S.; McEvoy, M.T.; Gabriel, S.E.; Kremers, H.M. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: A population-based study. Arthritis Rheum. 2009, 61, 233–239.
- Williamson, L.; Dalbeth, N.; Dockerty, J.L.; Gee, B.C.; Weatherall, R.; Wordsworth, B.P. Extended report: Nail disease in psoriatic arthritis--clinically important, potentially treatable and often overlooked. Rheumatology 2004, 43, 790–794.
- McGonagle, D. Enthesitis: An autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J. Eur. Acad. Dermatol. Venereol. 2009, 23 (Suppl. 1), 9–13.
- McGonagle, D.; Tan, A.L.; Benjamin, M. The nail as a musculoskeletal appendage--implications for an improved understanding of the link between psoriasis and arthritis. Dermatology 2009, 218, 97–102.
- Aydin, S.Z.; Ash, Z.R.; Tinazzi, I.; Castillo-Gallego, C.; Kwok, C.; Wilson, C.; Goodfield, M.; Gisondi, P.; Tan, A.L.; Marzo-Ortega, H.; et al. The link between enthesitis and arthritis in psoriatic arthritis: A switch to a vascular phenotype at insertions may play a role in arthritis development. Ann. Rheum. Dis. 2013, 72, 992–995.
- Benjamin, M.; McGonagle, D. The enthesis organ concept and its relevance to the spondyloarthropathies. Adv. Exp. Med. Biol. 2009, 649, 57–70.
- Rich, P.; Scher, R.K. Nail Psoriasis Severity Index: A useful tool for evaluation of nail psoriasis. J. Am. Acad. Dermatol. 2003, 49, 206–212.
- Cassell, S.E.; Bieber, J.D.; Rich, P.; Tutuncu, Z.N.; Lee, S.J.; Kalunian, K.C.; Wu, C.W.; Kavanaugh, A. The modified Nail Psoriasis Severity Index: Validation of an instrument to assess psoriatic nail involvement in patients with psoriatic arthritis. J. Rheumatol. 2007, 34, 123–129.
- Bandinelli, F.; Prignano, F.; Bonciani, D.; Bartoli, F.; Collaku, L.; Candelieri, A.; Lotti, T.; Matucci-Cerinic, M. Ultrasound detects occult entheseal involvement in early psoriatic arthritis independently of clinical features and psoriasis severity. Clin. Exp. Rheumatol. 2013, 31, 219–224.
- Soscia, E.; Scarpa, R.; Cimmino, M.A.; Atteno, M.; Peluso, R.; Sirignano, C.; Costa, L.; Iervolino, S.; Caso, F.; Del Puente, A.; et al. Magnetic resonance imaging of nail unit in psoriatic arthritis. J. Rheumatol. Suppl. 2009, 83, 42–45.
- Berritto, D.; Iacobellis, F.; Rossi, C.; Reginelli, A.; Cappabianca, S.; Grassi, R. Ultra high-frequency ultrasound: New capabilities for nail anatomy exploration. J. Dermatol. 2017, 44, 43–46.
- Szymoniak-Lipska, M.; Polańska, A.; Jenerowicz, D.; Lipski, A.; Żaba, R.; Adamski, Z.; Dańczak-Pazdrowska, A. High-Frequency Ultrasonography and Evaporimetry in Non-invasive Evaluation of the Nail Unit. Front. Med. 2021, 8, 686470.
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W.; Wojtkiewicz, M.; Wiktorowicz, A.; Wojtkiewicz, J. Ultrasound Assessment of Changes in Nails in Psoriasis and Psoriatic Arthritis. BioMed Res. Int. 2018, 2018, 8251097.
- Perrin, C. Nail Anatomy, Nail Psoriasis, and Nail Extensor Enthesitis Theory: What Is the Link? Am. J. Dermatopathol. 2019, 41, 399–409.
- de Berker, D. Nail anatomy. Clin. Dermatol. 2013, 31, 509–515.
- Ji, C.; Wang, H.; Bao, C.; Zhang, L.; Ruan, S.; Zhang, J.; Gong, T.; Cheng, B. Challenge of Nail Psoriasis: An Update Review. Clin. Rev. Allergy Immunol. 2021, 61, 377–402.
- Cecchini, A.; Montella, A.; Ena, P.; Meloni, G.B.; Mazzarello, V. Ultrasound anatomy of normal nails unit with 18 mHz linear transducer. Ital. J. Anat. Embryol. 2009, 114, 137–144.
- Wortsman, X.; Gutierrez, M.; Saavedra, T.; Honeyman, J. The role of ultrasound in rheumatic skin and nail lesions: A multi-specialist approach. Clin. Rheumatol. 2011, 30, 739–748.
- Wakefield, R.J.; Balint, P.V.; Szkudlarek, M.; Filippucci, E.; Backhaus, M.; D’Agostino, M.A.; Sanchez, E.N.; Iagnocco, A.; Schmidt, W.A.; Bruyn, G.A.; et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J. Rheumatol. 2005, 32, 2485–2487.
- Cunha, J.S.; Qureshi, A.A.; Reginato, A.M. Nail Enthesis Ultrasound in Psoriasis and Psoriatic Arthritis: A Report from the 2016 GRAPPA Annual Meeting. J. Rheumatol. 2017, 44, 688–690.
- Gutierrez, M.; Filippucci, E.; De Angelis, R.; Filosa, G.; Kane, D.; Grassi, W. A sonographic spectrum of psoriatic arthritis: “The five targets”. Clin. Rheumatol. 2010, 29, 133–142.
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W.; Wojtkiewicz, M.; Wiktorowicz, A.; Wojtkiewicz, J. Distal interphalangeal joint extensor tendon enthesopathy in patients with nail psoriasis. Sci. Rep. 2019, 9, 3628.
- Gisondi, P.; Idolazzi, L.; Girolomoni, G. Ultrasonography reveals nail thickening in patients with chronic plaque psoriasis. Arch. Dermatol. Res. 2012, 304, 727–732.
- Aydin, S.Z.; Castillo-Gallego, C.; Ash, Z.R.; Marzo-Ortega, H.; Emery, P.; Wakefield, R.J.; Wittmann, M.; McGonagle, D. Ultrasonographic assessment of nail in psoriatic disease shows a link between onychopathy and distal interphalangeal joint extensor tendon enthesopathy. Dermatology 2012, 225, 231–235.
- Idolazzi, L.; Zabotti, A.; Fassio, A.; Errichetti, E.; Benini, C.; Vantaggiato, E.; Rossini, M.; De Vita, S.; Viapiana, O. The ultrasonographic study of the nail reveals differences in patients affected by inflammatory and degenerative conditions. Clin. Rheumatol. 2019, 38, 913–920.
- Scarpa, R.; Soscia, E.; Peluso, R.; Atteno, M.; Manguso, F.; Del Puente, A.; Spanò, A.; Sirignano, C.; Oriente, A.; Di Minno, M.N.; et al. Nail and distal interphalangeal joint in psoriatic arthritis. J. Rheumatol. 2006, 33, 1315–1319.
- Acosta-Felquer, M.L.; Ruta, S.; Rosa, J.; Marin, J.; Ferreyra-Garrot, L.; Galimberti, M.L.; Galimberti, R.; Garcia-Monaco, R.; Soriano, E.R. Ultrasound entheseal abnormalities at the distal interphalangeal joints and clinical nail involvement in patients with psoriasis and psoriatic arthritis, supporting the nail-enthesitis theory. Semin. Arthritis Rheum. 2017, 47, 338–342.
- Moya Alvarado, P.; Roé Crespo, E.; Muñoz-Garza, F.Z.; López-Ferrer, A.; Laiz Alonso, A.; Vilarrassa Rull, E.; Casademont I Pou, J.; Puig Sanz, L. Subclinical enthesopathy of extensor digitorum tendon is highly prevalent and associated with clinical and ultrasound alterations of the adjacent fingernails in patients with psoriatic disease. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1728–1736.
