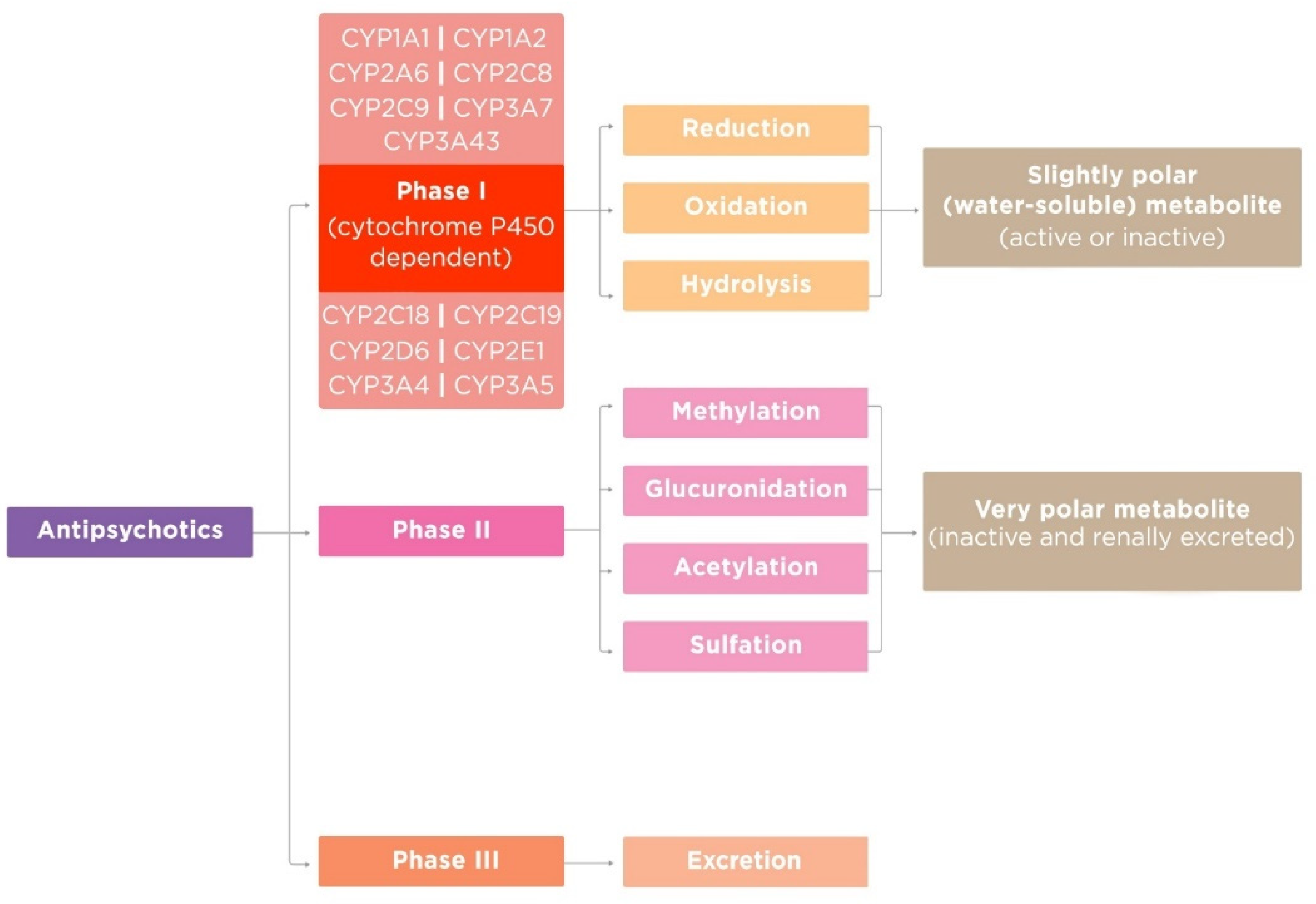
AntipsychoticsАнтипсихотики (APs) are psychotropic drugs that generally have a psycholeptic effect, capable of reducing psychotic symptoms and psychomotor agitation. This class of drugs is widely used in psychiatric practice, especially for the treatment of psychosis in schizophrenia and other psychotic disorders. Most APs pass through a biotransformation process, or metabolism, after they enter the body before being eliminated. There are three phases of AP metabolism. CytochromeАП) являются психотропными препаратами, которые обычно оказывают психолептическое действие, способные уменьшать психотические симптомы и психомоторное возбуждение. Этот класс препаратов широко применяется в психиатрической практике, особенно для лечения психозов при шизофрении и других психотических расстройствах. Большинство AP проходят через процесс биотрансформации или метаболизма после того, как они попадают в организм перед устранением. Существует три фазы метаболизма AP. Монооксигеназа цитохрома P450 (CYP) monooxygenase (mixed-function oxidase) plays a central role in most AP biotransformation. CYP’s functional activity depends on gene–drug and drug–drug interaction and influences on the occurrence of adverse drug reactions (ADRs). So, it is extremely important for a practicing psychiatrist to know the oxidation pathway of APs, since most of them are metabolized in the liver. This is important both to prevent ADRs and to avoid unwanted drug–drug interactions, which will undoubtedly increase the effectiveness and safety of AP therapy(оксидаза со смешанной функцией) играет центральную роль в биотрансформации большинства AP. Функциональная активность CYP зависит от взаимодействия ген-лекарство и лекарство-лекарство и влияет на возникновение побочных реакций на лекарства (ADR). Так, практикующему психиатру крайне важно знать путь окисления АП, так как большинство из них метаболизируются в печени. Это важно как для предотвращения АДР, так и для предотвращения нежелательных лекарственно-медикаментозных взаимодействий, что, несомненно, повысит эффективность и безопасность терапии АП.
- oxidation
- antipsychotics
- cytochrome P450
- enzymes
- -
-
Nutritional status;
- -
-
Hormonal status;
- -
-
Genetic factors;
- -
-
Previous therapy with APs or other classes of drugs;
- -
-
Comorbid somatic, neurological, or mental disorders;
- -
-
Age (for example, very old patients or children often have a greater sensitivity to APs, due in part to the involutional or immature state of the enzyme systems by which APs are metabolized);
- -
-
Functional state of the liver [11].
References
- Finkel, R.; Clark, M.A.; Cubeddu, L.X. Pharmacology, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; p. 151.
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 1–179.
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572.
- Caroff, S.N.; Hurford, I.; Lybrand, J.; Campbell, E.C. Movement Disorders Induced by Antipsychotic Drugs: Implications of the CATIE Schizophrenia Trial. Neurol. Clin. 2011, 29, 127–148.
- Sadock, B.J.; Sadock, V.A.; Ruiz, P. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; pp. 4113–4119.
- Meltzer, H.Y. Update on typical and atypical antipsychotic drugs. An. Rev. Med. 2013, 64, 393–406.
- Sheehan, J.J.; Sliwa, J.K.; Amatniek, J.C.; Grinspan, A.; Canuso, C.M. Atypical antipsychotic metabolism and excretion. Curr. Drug Metab. 2010, 11, 516–525.
- Rourke, J.L.; Sinal, C.J. Biotransformation/Metabolism. Encycl. Toxicol. 2014, 1, 490–502.
- Shanu-Wilson, J.; Evans, L.; Wrigley, S.; Steele, J.; Atherton, J.; Boer, J. Biotransformation: Impact and Application of Metabolism in Drug Discovery. ACS Med. Chem. Lett. 2020, 11, 2087–2107.
- Shen, W.W. The Metabolism of Atypical Antipsychotic Drugs: An Update. Ann. Clin. Psychiatry 1999, 11, 145–158.
- Correia, M.A. Drug biotransformation. In Basic & Clinical Pharmacology, 14th ed.; Katzung, B.G., Ed.; McGraw Hill Education: New York, NY, USA, 2017; Volume 1, pp. 56–74.
- Josephy, D.P.; Guengerich, P.F.; Miners, J.O. “Phase I and Phase II” drug metabolism: Terminology that we should phase out? Drug Metab. Rev. 2005, 37, 575–580.
