Vector-borne infectious diseases (e.g., malaria, dengue fever, and yellow fever) result from a parasite transmitted to humans and other animals by blood-feeding arthropods. They are major contributors to the global disease burden, as they account for nearly a fifth of all infectious diseases worldwide. The interaction between vectors and their hosts plays a key role driving vector-borne disease transmission.
- haemosporidian
- mosquitoes
- parasite manipulation hypothesis
- preen oil
- vector attractants
1. Avian Haemosporidians and Their Vectors
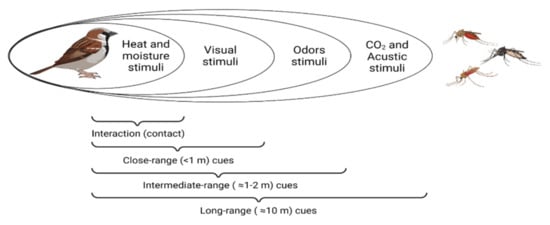
2. Cues Followed by Haemosporidian Vectors to Locate Their Hosts
|
Stimulus |
Host |
Vector |
Effect |
Explanation |
Reference |
|
|---|---|---|---|---|---|---|
|
Visual |
Colour |
49 North American bird species |
Culex pipiens |
+ |
Mosquitoes fed preferably on birds with lighter-coloured plumage. |
[36] |
|
Motion |
Cyanistes caeruleus |
Biting midges |
+ |
Abundance of biting midges was positively associated with parental provisioning effort (increased motion activity). |
[37] |
|
|
Size |
49 North American bird species |
Culex pipiens |
+ |
Mosquitoes fed preferably on birds with longer tarsi. |
[36] |
|
|
Heat and moisture |
Temperature |
Ficedula hypoleuca |
Biting midges |
+ |
Abundance of biting midges increased with temperature inside the bird nests. |
[38] |
|
Temperature |
Parus major |
Culex pipiens |
− |
Birds with a lower body temperature were preferentially chosen by mosquitoes. |
[39] |
|
|
Metabolic rate |
Passer domesticus |
Culex pipiens |
− |
House sparrows with lower metabolic rate suffered more mosquito bites. |
[40] |
|
|
Moisture and temperature |
Cyanistes caerules |
Biting midges and black flies |
0 |
No higher abundance of biting midges and black flies in nests with higher temperature and lower humidity. |
[41] |
|
|
Acoustic |
Bird calls |
Passer, Fringila, Emberiza |
Culex territans |
+ |
60% of female mosquitoes oriented toward the bird songs in phonotaxis experiments. |
[42] |
|
Auditory stimulus |
Upupa epops |
Mosquitoes, blackflies and biting midges |
0 |
Auditory cues of nestling hoopoes did not affect the abundance of vectors. |
[43] |
|
|
Olfactory |
Carbon dioxide (CO2) |
Cyanistes caeruleus |
Biting midges |
+ |
Higher biting midge abundance in nests boxes with CO2 levels higher than in the forest air. |
[44] |
|
Uropygial gland secretions |
Uropygial secretion |
Gavia immer |
Simulium euryadminiculum |
+ |
Black flies were attracted to the odour of the common loon’s uropygial gland. |
[45] |
|
Uropygial secretion |
Gavia immer |
Simulium euryadminiculum |
+ |
Higher attraction of black flies to a combination of ether extract of the uropygial glands and CO2 than to CO2 alone. |
[46] |
|
|
Ether extract |
Gavia immer |
Simulium euryadminiculum |
+ |
Black flies were attracted to ether components of the uropygial gland. |
[47] |
|
|
Cotton swabs coated with uropygial secretions |
Corvus brachyrhynchus |
Culex pipiens, Culex restuans |
+ |
CDC traps baited with uropygial secretions captured more mosquitos than control traps. |
[48] |
|
|
Diol volatile compounds from Natasauropygial gland secretion |
Culex quinquefasciatus Culex tarsalis, Culex nigripalpus, Aedes aegypti |
0 |
Meso-2,3-butanediol, 2,3-butanediol, and 2,3- docosanediol were not attractive to mosquitoes. |
[49] |
||
|
Uropygial secretions |
Columba livia Cyanistes caeruleus |
Biting midges and black flies |
0 |
No differences in the number of vectors captured in CDC traps or nests with this stimulus. |
[50] |
|
|
Uropygial secretions |
Passer domesticus |
Culex pipiens, Aedes caspius |
0 |
Mosquitoes were attracted equally to the ports containing uropygial secretion and to the control in olfactometer assays. |
[51] |
|
|
Uropygial secretions |
Upupa epops |
Biting midges |
− |
Traps baited with uropygial secretion in pine forest significantly captured less biting midges than control traps. |
[43] |
|
|
Haemosporidian infection |
Bird infected with malaria |
Serinus canaria |
Culex pipiens |
+ |
Chronically infected birds attracted more vectors than either uninfected or acutely infected birds. |
[52] |
|
Bird infected with malaria |
Passer domesticus |
Culex pipiens |
+ |
Higher feeding preference of mosquitoes on infected sparrows. |
[53] |
|
|
Bird infected with malaria |
Passer domesticus |
Culex pipiens |
+ |
Mosquitoes were more attracted to the odour of malaria-infected sparrows. |
[54] |
|
|
Bird infected with malaria |
Cyanistes caeruleus |
Biting midges |
− |
Higher abundance of biting midges in the nest attended by medicated birds with reduced parasitaemia. |
[37] |
|
|
Bird infected with malaria |
Parus major |
Culex pipiens |
− |
Plasmodium-infected birds attracted significantly fewer mosquitoes than the uninfected ones. |
[55] |
|
|
Bird infected with malaria |
Corvus monedula Passer domesticus |
Culex pipiens, Aedes caspius |
0 |
Similar biting rates of mosquitoes on malaria infected and uninfected birds. |
[56] |
|
