Oxidative stress has long been considered one of the pathophysiological mechanisms involved in numerous diseases, which has led to the investigation of the antioxidant systems as a promising therapy more than two decades ago. A useful antioxidant must meet specific characteristics; it must be capable of interacting with biologically relevant oxidants and free radicals; its reaction by-products should be harmless; and finally, it must reach a sufficiently high concentration in the tissue and cell compartments to ensure its activity is quantitatively relevant.
- Oxidative Stress
- Antioxidants
- Non-Enzymatic
1. Introduction[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47]
An imbalance of reactive species (RS) production and reactive intermediates detoxification generates oxidative stress. Oxidative stress affects cellular function by targeting nucleic acids, lipids, and proteins, all of which are constituents of cell organelles [1]. RS are generated by biological processes within the cell in normal and stressful conditions [2]. RS have a double and opposed function on cell fate. Under physiological conditions, a moderate RS increase plays an essential role in promoting cell proliferation and survival. However, when RS exceed baseline levels, surpassing the cells’ antioxidant capacity, cell metabolic processes are affected. Usually, antioxidant defenses counteract oxidative stress; antioxidants along with the turnover of oxidated macromolecules and organelles, leading to cell survival. If oxidative stress persists, it can trigger cell death, including neuronal cell death or permanent cell damage producing cellular transformation [3]. To protect itself from this damage, the cell has evolved and developed a complex antioxidant defense system, that includes various antioxidant enzymes such as copper/zinc and manganese-dependent superoxide dismutase (SOD), iron-dependent catalase (CAT), selenium-dependent glutathione peroxidase (GPx), and glutathione reductase (GR) [4]. In addition, the antioxidant scavenging system includes several non-enzymatic antioxidants (Figure 1Fig. 1), which are easy to obtain and modify the administration dose, including mitochondria-targeted antioxidant (MitoQ) [5], coenzyme Q10 [6], and carnosine [7], which are targeted to the mitochondria, the main source of free radicals; some endogenous antioxidants precursors like inosine, which is metabolized into uric acid (UA) [8], and N-acetylcysteine (NAC) [9], a glutathione (GSH) precursor; GSH itself [10], known as “the master antioxidant”; and vitamins C and E [11].
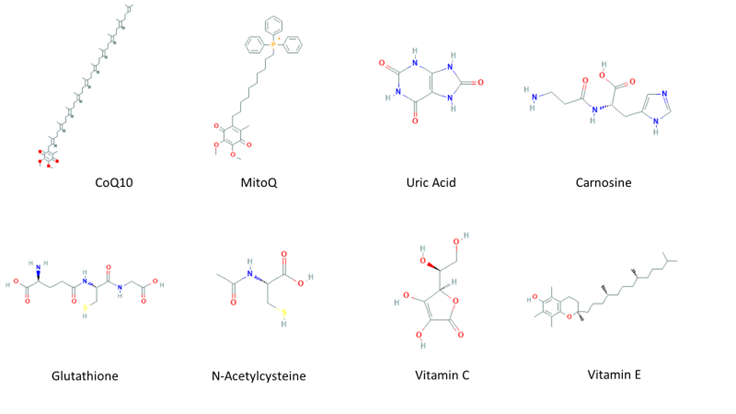
Figure 1. Molecular structures of non-enzymatic antioxidants.
2. Mitochondria-targeted antioxidants
2.1 Coenzyme Q10
Coenzyme Q10 (CoQ10) is a parabenzoquinone (also known as ubiquinone) ubiquitous in virtually all cells; it is a crucial component of the oxidative phosphorylation process in the mitochondria but is not just an agent for energy transduction. It is also well located close to the membranes unsaturated lipids, acting as a primary scavenger of free radicals to avoid lipid peroxidation [6], protecting biological membranes and DNA from oxidative damage [12].
The chemical structure of CoQ10 is very similar to vitamins; however, it is not considered one of them because it is the only lipid-soluble antioxidant that can be synthesized de novo by animal cells [13].
Ubiquinone endogenous levels depend on the production and consumption rates within the organism. The dietary intake of CoQ10 is minimal with daily contributions of around 3-5 mg [14], which explains why supplementation with CoQ10 has been recommended in cases of deficiency. Despite this, oral administration of CoQ10 has a poor absorption efficiency due to its hydrophobic nature. Therefore, various formulations have been created to improve its bioavailability [15].
2.2 Mitochondria-Targeted antioxidant (MitoQ)
An orally available derivative of mitochondrial-targeted coenzyme Q10 is a therapeutic compound termed Mitoquinone (MitoQ), which is an ubiquinone synthesized from the union of its oxidized (mitoquinone) and reduced (mitoquinol) form and a covalent bond to a lipophilic thriphenylphosphonium cation through an aliphatic carbon chain [5].
The MitoQ oral formulations have shown suitable pharmacokinetics behavior; doses of 1 mg/kg reach a maximum plasma concentration of 33.15 ng/ml after one hour of administration [16]. MitoQ is rapidly cleared from plasma and accumulates in the heart, skeletal muscle, liver, and brain; its accumulation came to a steady-state after 7 to 10 days of administration [17].
MitoQ quickly crosses the blood-brain barrier and cell membranes and concentrates on mitochondria because of its high membrane potential across the inner mitochondrial membrane [18]. MitoQ is rapidly absorbed by the mitochondria, driven by the membrane potential. Once inside, almost all the accumulated MitoQ is adsorbed to the inner membrane’s matrix surface, where it is reduced to the active antioxidant ubiquinol by complex II in the respiratory chain. MitoQ scavenges peroxyl radicals (ROO•), ONOO- and O2•- and protects mitochondria against lipid peroxidation. It is also a poor substrate for complex I and has no reactivity with complex III, so MitoQ remains in its reduced form ubiquinol, being more efficient than CoQ10 [19].
2.3 Carnosine
Carnosine (β-alanine-L-histidine) is anendogenous dipeptide of excitable tissues (skeletal muscle, heart, and brain); it is highly hydrophilic, penetrates the blood-brain barrier easily, and has significant antioxidant properties. Carnosine is electrochemically active as a reducing agent; it shows peroxyl radical-trapping activity; inhibits deoxyguanosine oxidative hydroxylation induced by copper ions; and acts as a metal ion chelator, quenching singlet oxygen and binding hydroperoxides [7].
Carnosine concentration in human brain is currently lacking in the literature. Homocarnosine (γ-aminobutyryl-L-histidine) is a novel alternative imidazole peptide with structural similarity to carnosine. Homocarnosine concentration in human brain is quite high (0.4-1.0 µM). Both, carnosine and homocarnosine are synthesized by carnosine synthase and degraded by carnosinase [20].[20]
3. Inosine
Inosine, also known as hypoxanthosine or panholic-L, belongs to the organic compounds known as purine nucleosides. It is an intermediate in the degradation of purines and purine nucleosides to UA and purine salvage pathways.
Inosine is a urate precursor, which has an antioxidant effect in vitro [8] and in vivo [21]. UA represents about 60% of total plasma antioxidant capacity [22]; it scavenges singlet oxygen (1O2), OH•, H2O2, and ONOO- [23]. However, its effect is not limited to eliminating free radicals. UA-mediated neuroprotection has been improved by high K+-induced depolarization through a mechanism involving Ca2+ increasing and extracellular signal-regulated kinase 1/2 (ERK1/2) activation, which is involved in neuronal survival [24]. UA also interacts and stabilizes other antioxidant systems, including SOD [25]. UA has metal-complexing properties, too; it chelates iron by forming stable complexes with Fe3+ and blocking iron-dependent oxidation reactions [26].
4. Cysteine-based antioxidant
4.1 Glutathione
GSH is a tripeptide (cysteine, glycine, and glutamic acid) found in high concentrations in most cells’ mitochondrial and cytoplasmatic compartments. It is synthesized in the cytoplasm by the sequential addition of cysteine to glutamic acid, followed by glycine addition. GSH functions as an antioxidant, a free radical scavenger, and a detoxifying agent; it is a GSH peroxidase cofactor, acts as a substrate for GSH S-transferase, and maintains exogenous antioxidants, such as vitamins C and E, in their reduced (active) forms [27][28][29][27-29]. The cysteine’s sulfhydryl group is essential for GSH function as it participates in the reduction, oxidation, and conjugation reactions [30]. This antioxidant exists in two states: reduced (GSH) and oxidized (GSSG); in the reduced state, the cysteine’s thiol group can donate an electron to unstable molecules such as ROS; by donating this electron, the GSH oxidizes and react with another GSH to form GSH disulfide (GSSG). Therefore, the ratio of GSH/GSSG determines the cells’ redox status [30][31].
4.2 N-Acetylcysteine (NAC)
Acetylcysteine is a synthetic N-acetyl derivative of the endogenous amino acid L-cysteine, a GSH precursor (PubChem). NAC has been used for more than 50 years in the clinic to replenish hepatic GSH after acetaminophen overdose [31][32], as a mucolytic in lung diseases [32][33], and as a disease modifier in infectious diseases [33][34]. NAC’s antioxidant activity is attributed to its rapid reaction with OH•, NO•2, CO3, and thiyl radicals, as well as to the detoxification of semiquinones, hypochlorous acid (HOCl), nitroxyl (HNO), and heavy metals [9].
5. Vitamins
5.1 Vitamin C
Vitamin C (Vit C), also known as ascorbic acid, ascorbate, or L-ascorbate, is a natural water-soluble vitamin that plays significant roles as a free radical scavenger and as a cofactor of several enzymes reactions, including catecholamine synthesis [34][35]. Its effect as an antioxidant comes from its actions as a non-enzymatic reducer of O2 •-, hydroxyl (HO•), alkoxyl (RO• ), peroxyl (ROO•), and other radicals [35][36]. Vit C also reacts with the radical tocopheroxyl, which results from Vitamin E oxidation when it scavenges free radicals in lipid membranes, regenerating Vitamin E (Vit E) [36][37].
Unlike most molecular low-weight compounds, the absorption, distribution, and metabolism of Vit C are complex. Its uptake in tissues and distribution mainly occurs through the sodium-dependent vitamin C transporter family of proteins [37][38]. These transporters’ differential expression between tissues leads to nonlinear pharmacokinetics of Vit C under physiological conditions, and its distribution is highly compartmentalized [38][39]. Vit C distribution pattern has a wide range of concentrations in the different tissues ranging from 0.2 mM in muscle and heart to 10 mM in the adrenal glands and brain [39][40].
5.2 Vitamin E
Vit E is the generic term for eight substances or trocochromanols, four tocopherols, four tocotrienols [40][123], and the major lipid-soluble antioxidant [41]. As this vitamin cannot be synthesized in the human body, it must be supplied by the diet. The antioxidant effects exerted by each Vit E isoform are complicated, and their mechanisms are still not well understood. However, researchers theorize that Vit E may protect key cell components by reducing free radicals and breaking lipid peroxidation chain reaction. Thus, cell membranes are protected by lipid repair and replacement [42].
As mentioned above, the antioxidant effects of Vit E and its mechanism of action are not well understood. Vit E absorption efficiency varies from 10 to 33% [43] and is affected by several factors including the food matrix, genetic factors, and metabolic fate, altering its bioavailability [44].[44]
6. Conclusions
Oxidative stress has long been considered one of the pathophysiological mechanisms involved in numerous diseases, which has led to the investigation of the antioxidant systems as a promising therapy more than two decades ago. A useful antioxidant must meet specific characteristics; it must be capable of interacting with biologically relevant oxidants and free radicals; its reaction by-products should be harmless; and finally, it must reach a sufficiently high concentration in the tissue and cell compartments to ensure its activity is quantitatively relevant (Figure 2Fig. 2). Antioxidant bioavailability is related to the antioxidant structure, interaction with other molecules, half-life, and delivery efficiency into the brain. Increasing the antioxidant dosage not necessary would increase its tissue-specific concentration; it may produce adverse effects. To improve the antioxidant half-life, tissue-specific delivery, and bioavailability, antioxidants can be incorporated in a biocompatible substrate to surpass the administration limitations and increase its therapeutic effect.
Recently, antioxidants delivery and transportation systems were developed for specific subcellular targeting [45] by encapsulation into liposomes or linkage to nanoparticles including nanovesicles, solid lipid nanoparticles, nanostructured lipid carriers, nanoemulsions, and polymeric nanoparticles to make them more stable [46][47][46, 47]. These are up-and-coming systems to be evaluated in preclinical and clinical studies to obtain the most significant antioxidants benefits, with the least toxicity and side effects.
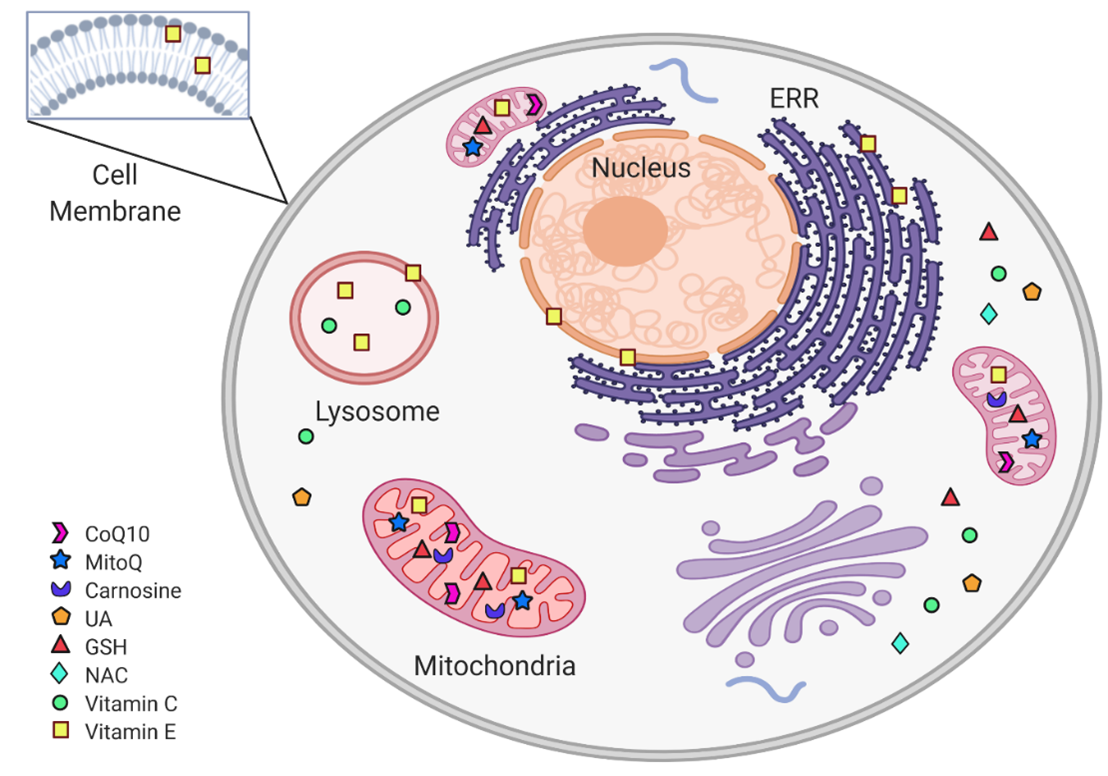
Figure 2. Subcellular non-enzymatic antioxidants targets. Within the cell, non-enzymatic antioxidants are distributed according to their subcellular target. The mitochondria are protected from oxidative stress by CoQ10, MitoQ, carnosine, and Vit C. In the cytoplasm, the antioxidant system includes GSH, NAC, and UA. Vit E is found in cytoplasmic, ERR, and mitochondrial membranes, as well as in lysosomes. (Figure created in BioRender.com)
- AJ, S., Radiation Chemistry of Organic Compounds, in Radiation Chemistry of Organic Compounds, S. THOMSON, Editor. 1960.
- Tanaka, M. and L. Vécsei, Monitoring the Redox Status in Multiple Sclerosis. Biomedicines, 2020. 8(10).
- Cairns, R.A., I.S. Harris, and T.W. Mak, Regulation of cancer cell metabolism. Nat Rev Cancer, 2011. 11(2): p. 85-95.
- Krishnamurthy, P., & Wadhwani, A., Antioxidant Enzymes and Human Health. Antioxidant Enzyme. 2012: IntechOpen.
- Kelso, G.F., et al., Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem, 2001. 276(7): p. 4588-96.
- Crane, F.L., Biochemical functions of coenzyme Q10. J Am Coll Nutr, 2001. 20(6): p. 591-8.
- Kohen, R., et al., Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci U S A, 1988. 85(9): p. 3175-9.
- Zhang, N., et al., Nrf2 signaling contributes to the neuroprotective effects of urate against 6-OHDA toxicity. PLoS One, 2014. 9(6): p. e100286.
- Samuni, Y., et al., The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta, 2013. 1830(8): p. 4117-29.
- Beutler, E., Nutritional and metabolic aspects of glutathione. Annu Rev Nutr, 1989. 9: p. 287-302.
- Sies, H. and W. Stahl, Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr, 1995. 62(6 Suppl): p. 1315S-1321S.
- Ernster, L. and G. Dallner, Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta, 1995. 1271(1): p. 195-204.
- Bhagavan, H.N. and R.K. Chopra, Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res, 2006. 40(5): p. 445-53.
- Kaikkonen, J., et al., Determinants of plasma coenzyme Q10 in humans. FEBS Lett, 1999. 443(2): p. 163-6.
- Chopra, R.K., et al., Relative bioavailability of coenzyme Q10 formulations in human subjects. Int J Vitam Nutr Res, 1998. 68(2): p. 109-13.
- Li, Y., et al., Quantitation and metabolism of mitoquinone, a mitochondria-targeted antioxidant, in rat by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom, 2007. 21(13): p. 1958-64.
- Smith, R.A., et al., Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A, 2003. 100(9): p. 5407-12.
- Murphy, M.P. and R.A. Smith, Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol, 2007. 47: p. 629-56.
- James, A.M., et al., Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem, 2005. 280(22): p. 21295-312.
- Boldyrev, A.A., G. Aldini, and W. Derave, Physiology and pathophysiology of carnosine. Physiol Rev, 2013. 93(4): p. 1803-45.
- Gong, L., et al., Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson's disease: linking to Akt/GSK3β signaling pathway. J Neurochem, 2012. 123(5): p. 876-85.
- Yeum, K.J., et al., Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys, 2004. 430(1): p. 97-103.
- Becker, B.F., Towards the physiological function of uric acid. Free Radic Biol Med, 1993. 14(6): p. 615-31.
- Guerreiro, S., et al., Protection of midbrain dopaminergic neurons by the end-product of purine metabolism uric acid: potentiation by low-level depolarization. J Neurochem, 2009. 109(4): p. 1118-28.
- Hink, H.U., et al., Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol, 2002. 22(9): p. 1402-8.
- Davies, K.J., et al., Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J, 1986. 235(3): p. 747-54.
- Fernández-Checa, J.C., et al., Mitochondrial glutathione: importance and transport. Semin Liver Dis, 1998. 18(4): p. 389-401.
- Ursini, F. and A. Bindoli, The role of selenium peroxidases in the protection against oxidative damage of membranes. Chem Phys Lipids, 1987. 44(2-4): p. 255-76.
- Pompella, A., et al., The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol, 2003. 66(8): p. 1499-503.
- Meister, A., Biosynthesis and functions of glutathione, an essential biofactor. J Nutr Sci Vitaminol (Tokyo), 1992. Spec No: p. 1-6.
- Pizzorno, J., Glutathione! Integr Med (Encinitas), 2014. 13(1): p. 8-12.
- Smilkstein, M.J., et al., Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med, 1988. 319(24): p. 1557-62.
- Moldéus, P., I.A. Cotgreave, and M. Berggren, Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration, 1986. 50 Suppl 1: p. 31-42.
- Staal, F.J., et al., Glutathione deficiency and human immunodeficiency virus infection. Lancet, 1992. 339(8798): p. 909-12.
- Linster, C.L. and E. Van Schaftingen, Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J, 2007. 274(1): p. 1-22.
- Njus, D., et al., Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic Biol Med, 2020. 159: p. 37-43.
- Packer, J.E., T.F. Slater, and R.L. Willson, Direct observation of a free radical interaction between vitamin E and vitamin C. Nature, 1979. 278(5706): p. 737-8.
- Tsukaguchi, H., et al., A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature, 1999. 399(6731): p. 70-5.
- Frei, B., I. Birlouez-Aragon, and J. Lykkesfeldt, Authors' perspective: What is the optimum intake of vitamin C in humans? Crit Rev Food Sci Nutr, 2012. 52(9): p. 815-29.
- Hasselholt, S., P. Tveden-Nyborg, and J. Lykkesfeldt, Distribution of vitamin C is tissue specific with early saturation of the brain and adrenal glands following differential oral dose regimens in guinea pigs. Br J Nutr, 2015. 113(10): p. 1539-49.
- Burton, G.W., A. Joyce, and K.U. Ingold, First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet, 1982. 2(8293): p. 327.
- Colombo, M.L., An update on vitamin E, tocopherol and tocotrienol-perspectives. Molecules, 2010. 15(4): p. 2103-13.
- Bruno, R.S., et al., Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled alpha-tocopheryl acetate. Am J Clin Nutr, 2006. 83(2): p. 299-304.
- Borel, P., D. Preveraud, and C. Desmarchelier, Bioavailability of vitamin E in humans: an update. Nutr Rev, 2013. 71(6): p. 319-31.
- Jin, H., et al., Mitochondria-targeted antioxidants for treatment of Parkinson's disease: preclinical and clinical outcomes. Biochim Biophys Acta, 2014. 1842(8): p. 1282-94.
- Khalil, I., et al., Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants (Basel), 2019. 9(1).
- Mohd Zaffarin, A.S., et al., Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. Int J Nanomedicine, 2020. 15: p. 9961-9974.
References
- AJ, S., Radiation Chemistry of Organic Compounds, in Radiation Chemistry of Organic Compounds, S. THOMSON, Editor. 1960.
- Tanaka, M. and L. Vécsei, Monitoring the Redox Status in Multiple Sclerosis. Biomedicines, 2020. 8(10).
- Cairns, R.A., I.S. Harris, and T.W. Mak, Regulation of cancer cell metabolism. Nat Rev Cancer, 2011. 11(2): p. 85-95.
- Krishnamurthy, P., & Wadhwani, A., Antioxidant Enzymes and Human Health. Antioxidant Enzyme. 2012: IntechOpen.
- Kelso, G.F., et al., Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem, 2001. 276(7): p. 4588-96.
- Crane, F.L., Biochemical functions of coenzyme Q10. J Am Coll Nutr, 2001. 20(6): p. 591-8.
- Kohen, R., et al., Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci U S A, 1988. 85(9): p. 3175-9.
- Zhang, N., et al., Nrf2 signaling contributes to the neuroprotective effects of urate against 6-OHDA toxicity. PLoS One, 2014. 9(6): p. e100286.
- Samuni, Y., et al., The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta, 2013. 1830(8): p. 4117-29.
- Beutler, E., Nutritional and metabolic aspects of glutathione. Annu Rev Nutr, 1989. 9: p. 287-302.
- Sies, H. and W. Stahl, Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr, 1995. 62(6 Suppl): p. 1315S-1321S.
- Ernster, L. and G. Dallner, Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta, 1995. 1271(1): p. 195-204.
- Bhagavan, H.N. and R.K. Chopra, Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res, 2006. 40(5): p. 445-53.
- Kaikkonen, J., et al., Determinants of plasma coenzyme Q10 in humans. FEBS Lett, 1999. 443(2): p. 163-6.
- Chopra, R.K., et al., Relative bioavailability of coenzyme Q10 formulations in human subjects. Int J Vitam Nutr Res, 1998. 68(2): p. 109-13.
- Li, Y., et al., Quantitation and metabolism of mitoquinone, a mitochondria-targeted antioxidant, in rat by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom, 2007. 21(13): p. 1958-64.
- Smith, R.A., et al., Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A, 2003. 100(9): p. 5407-12.
- Murphy, M.P. and R.A. Smith, Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol, 2007. 47: p. 629-56.
- James, A.M., et al., Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem, 2005. 280(22): p. 21295-312.
- Boldyrev, A.A., G. Aldini, and W. Derave, Physiology and pathophysiology of carnosine. Physiol Rev, 2013. 93(4): p. 1803-45.
- Gong, L., et al., Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson's disease: linking to Akt/GSK3β signaling pathway. J Neurochem, 2012. 123(5): p. 876-85.
- Yeum, K.J., et al., Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys, 2004. 430(1): p. 97-103.
- Becker, B.F., Towards the physiological function of uric acid. Free Radic Biol Med, 1993. 14(6): p. 615-31.
- Guerreiro, S., et al., Protection of midbrain dopaminergic neurons by the end-product of purine metabolism uric acid: potentiation by low-level depolarization. J Neurochem, 2009. 109(4): p. 1118-28.
- Hink, H.U., et al., Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol, 2002. 22(9): p. 1402-8.
- Davies, K.J., et al., Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J, 1986. 235(3): p. 747-54.
- Fernández-Checa, J.C., et al., Mitochondrial glutathione: importance and transport. Semin Liver Dis, 1998. 18(4): p. 389-401.
- Ursini, F. and A. Bindoli, The role of selenium peroxidases in the protection against oxidative damage of membranes. Chem Phys Lipids, 1987. 44(2-4): p. 255-76.
- Pompella, A., et al., The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol, 2003. 66(8): p. 1499-503.
- Pizzorno, J., Glutathione! Integr Med (Encinitas), 2014. 13(1): p. 8-12.Meister, A., Biosynthesis and functions of glutathione, an essential biofactor. J Nutr Sci Vitaminol (Tokyo), 1992. Spec No: p. 1-6.
- Smilkstein, M.J., et al., Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med, 1988. 319(24): p. 1557-62.Pizzorno, J., Glutathione! Integr Med (Encinitas), 2014. 13(1): p. 8-12.
- Moldéus, P., I.A. Cotgreave, and M. Berggren, Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration, 1986. 50 Suppl 1: p. 31-42.Smilkstein, M.J., et al., Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med, 1988. 319(24): p. 1557-62.
- Staal, F.J., et al., Glutathione deficiency and human immunodeficiency virus infection. Lancet, 1992. 339(8798): p. 909-12.Moldéus, P., I.A. Cotgreave, and M. Berggren, Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration, 1986. 50 Suppl 1: p. 31-42.
- Linster, C.L. and E. Van Schaftingen, Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J, 2007. 274(1): p. 1-22.Staal, F.J., et al., Glutathione deficiency and human immunodeficiency virus infection. Lancet, 1992. 339(8798): p. 909-12.
- Njus, D., et al., Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic Biol Med, 2020. 159: p. 37-43.Linster, C.L. and E. Van Schaftingen, Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J, 2007. 274(1): p. 1-22.
- Packer, J.E., T.F. Slater, and R.L. Willson, Direct observation of a free radical interaction between vitamin E and vitamin C. Nature, 1979. 278(5706): p. 737-8.Njus, D., et al., Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic Biol Med, 2020. 159: p. 37-43.
- Tsukaguchi, H., et al., A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature, 1999. 399(6731): p. 70-5.Packer, J.E., T.F. Slater, and R.L. Willson, Direct observation of a free radical interaction between vitamin E and vitamin C. Nature, 1979. 278(5706): p. 737-8.
- Frei, B., I. Birlouez-Aragon, and J. Lykkesfeldt, Authors' perspective: What is the optimum intake of vitamin C in humans? Crit Rev Food Sci Nutr, 2012. 52(9): p. 815-29.Tsukaguchi, H., et al., A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature, 1999. 399(6731): p. 70-5.
- Hasselholt, S., P. Tveden-Nyborg, and J. Lykkesfeldt, Distribution of vitamin C is tissue specific with early saturation of the brain and adrenal glands following differential oral dose regimens in guinea pigs. Br J Nutr, 2015. 113(10): p. 1539-49.Frei, B., I. Birlouez-Aragon, and J. Lykkesfeldt, Authors' perspective: What is the optimum intake of vitamin C in humans? Crit Rev Food Sci Nutr, 2012. 52(9): p. 815-29.
- Martínez Banaclocha, M. N-acetylcysteine elicited increase in complex I activity in synaptic mitochondria from aged mice: Implications for treatment of Parkinson’s disease. Brain Res. 2000, 859, 173–175.Hasselholt, S., P. Tveden-Nyborg, and J. Lykkesfeldt, Distribution of vitamin C is tissue specific with early saturation of the brain and adrenal glands following differential oral dose regimens in guinea pigs. Br J Nutr, 2015. 113(10): p. 1539-49.
- Burton, G.W., A. Joyce, and K.U. Ingold, First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet, 1982. 2(8293): p. 327.
- Colombo, M.L., An update on vitamin E, tocopherol and tocotrienol-perspectives. Molecules, 2010. 15(4): p. 2103-13.
- Bruno, R.S., et al., Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled alpha-tocopheryl acetate. Am J Clin Nutr, 2006. 83(2): p. 299-304.
- Borel, P., D. Preveraud, and C. Desmarchelier, Bioavailability of vitamin E in humans: an update. Nutr Rev, 2013. 71(6): p. 319-31.
- Jin, H., et al., Mitochondria-targeted antioxidants for treatment of Parkinson's disease: preclinical and clinical outcomes. Biochim Biophys Acta, 2014. 1842(8): p. 1282-94.
- Khalil, I., et al., Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants (Basel), 2019. 9(1).
- Mohd Zaffarin, A.S., et al., Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. Int J Nanomedicine, 2020. 15: p. 9961-9974.
