You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Klaudia Niemczyk.
Gold nanoparticles (AuNPs) have the ability to absorb and scatter light, and can convert optical energy into heat using nonradiative electron relaxation dynamics and surface chemistry. Moreover, gold nanoparticles can be used as drug carriers, making them very attractive and versatile nanoparticles. The features of AuNPs that make them particularly attractive in biomedicine are their excellent stability and biocompatibility, ease to functionalize their surfaces, their low toxicity, and their drug transferability. Other features, such as shape and size adaptation, have certainly drawn attention for the use of gold nanoparticles in many fields.
- diagnosing
- gold nanoparticles
- imaging
1. Imaging and Diagnosing
Increasingly, refined metal (gold) nanoparticles are being applied in imaging to visualize key subcellular compartments and/or for diagnostics (Figure 31). The fundamental aim of imaging is to recognize and localize specific targets, very often by accumulation of a specific imaging compound at the specific part of the cell/body or disease site, in a harmless and non-invasive way [28][1]. For in vivo imaging, either high avidity of the NPs in target binding or their payload delivery and capacity for multiplexing with therapeutics and other agents are highly desired. Moreover, the ability of NPs to induce changes in electromagnetic or sound waves to transmit biological signals to monitoring devices is also desired [29][2]. Among the various metal NPs, owing to their high atomic number, high X-ray absorption coefficient, and unique optical properties, gold nanoparticles have received considerable attention. These make this nanomaterial ideal for electron microscopy, computed tomography, or colorimetric-biosensing approaches [30,31][3][4].
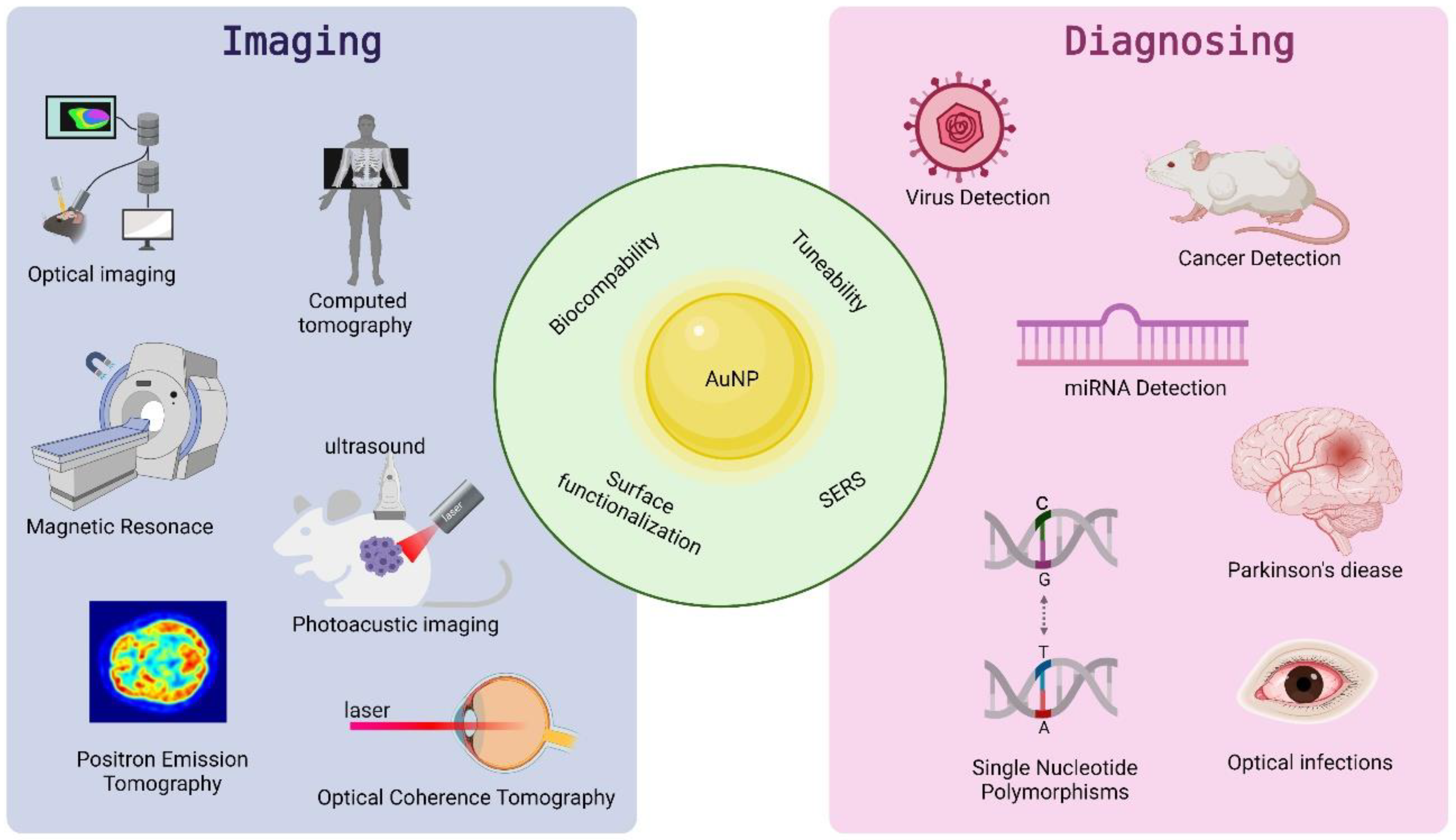
Figure 31.
Properties and application of AuNPs in imaging and diagnosing.
Computed tomography (CT), a non-invasive system, is founded on the exploitation of X-ray scanning, its attenuation in tissues, and computed image reconstruction to obtain morphologic and vascular information within the body [32,33][5][6]. This is obtained due to the fact that various tissues have different affinities to absorb X-rays [34,35][7][8]. The absorption and dispersion of X-ray radiation are, collectively, referred to as attenuation. The attenuation of any substance in CT imaging is defined on the Hounsfield scale and is expressed in terms of Hounsfield units according to the following equation: Attenuation (HU) = 1000 × (μx − μwater)/(μwater − μair) [36][9]. Currently, in CT imaging, contrast agents predominantly based on iodine containing molecules, are used to enhance absorbing X-rays. However, they are not specifically targeted since they cannot be bioconjugated with other components. Moreover, they exhibit very short imaging times due to rapid clearance by the kidney and poor contrast in large patients [37,38][10][11]. Thus, the combination of CT with gold nanoparticles as a contrast agent, due to the strong X-ray attenuation and bioconjugation, has recently seen significant development [39][12]. One of the key factors determining the effectiveness of CT contrast agents is a high atomic number—the higher it is, the better the resulting CT contrast. This makes AuNPs ideal candidates for CT contrast agents, as the high atomic number of gold (Z = 79) can induce strong X-ray suppression [39][12]. Galper et al. reported that gold nanoparticles provide almost 1.9 times greater contrast than iodine when scanning at 120 kV in water with a Brilliance iCT scanner with a gold NP attenuation of 5.14 HU/mM [40][13]. These results are consistent with other research where, for gold at a 120 kV attenuation rate, the value was 5.4 HU/mM, and for iodine it was 2.2 HU/mM [41][14]. For instance, the AuNP colloid has been introduced for the imaging of cardiovascular diseases. This is due to its extended blood circulation time, which, in turn, allows longer imaging times and the delineation of blood vessels [38][11]. The great advantage of gold nanoparticles is the significantly longer blood half-life. For commercially available iodine preparations, it is less than 10 min, while for AuNP-PEG it can reach 14.57 ± 3.27 h [42][15]. Nanosized gold can passively accumulate in tumor tissues more readily than in normal tissues due to the enhanced permeability and retention effect (EPR) of tumors. Therefore, an improvement in the contrast between normal and cancer cells can be observed [43,44][16][17]. Moreover, AuNPs can be actively targeted to a specific type of cancer by loading antibodies, peptides, or ligands on them, which enables tumor detection with CT imaging [39][12]. Usually, antibodies bound to conventional CT contrast agents (i.e., iodine) have not provided sufficient targeting CT contrast and load around three iodine atoms per antibody. Thus, using AuNPs seems to be much more promising since these nanoparticles can load antibodies with greater numbers of heavier atoms [45][18]. Gold nanoparticles are also consider as an ideal blood pool CT agents due to their ability to overcome biological barriers and remain in the blood stream for a prolonged period of time, compared to molecules containing iodine [45][18]. However, the incapability to uptake into the brain, due to the BBB, is the main problem in the treatment and diagnosing of neurodegenerative diseases. Shilo et al. demonstrated that, after injection of the insulin-targeted gold NPs into rat, they were found in a specific brain region using a micro-CT scanner. Therefore, gold NPs have been suggested for imaging and therapy of neurodegenerative disorders as a nanodevice that can overcome the restrictive mechanism of the BBB [46][19].
Optical imaging, as a much more rapid and inexpensive method, is one of the most preferable techniques since optical microscopy approaches can achieve astounding spatial resolutions [47][20]. This can be achieved through the generation of colorimetric contrast between specific cells/organelles/biocompounds by these imaging agents, mostly through the use of organic fluorophores [31][4]. However, the limits of the latter method are prone to photobleaching and a broad emission window [48][21]. Moreover, compared with typical fluorophores, gold nanoparticles show a light-scattering power equivalent to the signal arising from nearly 106 fluorescein molecules, and, more importantly, does not photobleach [49][22]. Thus, gold NPs, with their unique optical properties originating from surface plasmon resonance (SPR), are considered an excellent biomedical diagnostic tool due to their large absorption and scattering cross-sections when excited [50][23]. With the development of microscopic methods, gold nanoparticles have been extensively used for the direct or indirect visualization of biological systems. Starting from imaging the intracellular location of the AuNPs inside cells, through the real-time tracking of biointeractions, ending with measuring biomolecular dynamics and mechanics [50][23]. Among various AuNP-assisted imaging techniques, the special optical properties of AuNPs are commonly used.
Additionally, the unique properties of gold nanoparticles allow the detection of specific components of complex biological mixtures. For this purpose, Raman spectroscopy is used. The Raman effect is a by-product of photon scattering interacting with matter, leading to the obtaining of a chemical fingerprint of a molecule. AuNPs greatly enhance this effect. This phenomenon is called surface-enhanced Raman scattering (SERS) and can be applied to highly sensitive probes [50,56,57][23][24][25]. In particular, non-spherical, irregular AuNPs, which exhibit a much stronger electromagnetic field, have been investigated for use in the SERS imaging method [58][26]. It is a particularly desirable method due to the possibility of establishing the exact distributions of neoplastic cells, the multifocality of which may cause minor recurrence or metastasis.
Diagnostic approaches based on nanotechnology have also proved to be a promising alternative to conventional and low-cost effective methods of detecting miRNAs in body fluids or tissue samples [60][27]. MicroRNAs, or small regulatory RNAs, can be used as biomarkers in the early diagnosis of cancer. However, they occur in low concentrations and are difficult to detect [61][28]. Li et al. developed a colorimetric miRNA detection method based on the isothermal exponential amplification reaction (EXPAR)—AuNP-assisted amplification. EXPAR provides a linear detection range of 50 fM to 10 nM miRNA with a detection limit of 46 fM in 60 min. The method also detects single-nucleotide differences between homologous nucleic acids [62][29]. Amal et al. verified the possibility of diagnosing stomach cancer based on a breath test. The volatile organic compounds that appear in exhaled breath can be linked to disease condition. The samples from patients were analyzed using the gas chromatography linked to mass spectrometry (GCMS) method, and a nanoarray sensor composed of gold nanoparticles and single-wall carbon nanotubes (SWCNTs) covered with different ligands was used. The obtained results indicate that nanoarrays could be a tool for screening gastric cancer and related precancerous lesions, as well as for monitoring the effectiveness of therapy [63][30].
The main problem of commonly used contrast agents in magnetic resonance imaging, such as gadolinium-based contrast agents (GBCA), is the possible release of free heavy metals in vivo [64][31]. In 2006, gadolinium (Gd) was linked to a debilitating and potentially fatal condition called nephrogenic systemic fibrosis (NSF), a rare disease of unknown cause that affects patients with renal insufficiency treated with Gd as a contrast agent [65][32]. It is also possible that Gd accumulates in tissues over a long period of time [66][33]. The brain is particularly at risk of accumulation of contrast agents. Due to the less developed lymphatic system, it is difficult to wash it out. Although the clinical consequences of the accumulation of contrast agents are unclear, the search for new compounds with improved magnetic properties is ongoing [67][34]. The solution may lie in the use of gold nanoparticles. The absence of contrast agent accumulation in the lungs, liver, and spleen of mice was demonstrated by Alric et al. through the use of gadolinium chelate—functionalized gold nanoparticles [68][35].
AuNPs can serve, not only as carriers, but they can also be combined with superparamagnetic materials, such as iron oxides, e.g., Fe3O4 [69][36]. Iron oxide nanoparticles are the only NP-based contrast agents that have been approved by the Food and Drug Administration (FDA) [70][37]. They are an example of negative contrast agents, which means that they make the enhanced parts of a T2-weighted image darker, while shortening the T2 relaxation time [71][38]. Fe3O4@AuNPs combine, not only unique magnetic properties, but also their gold coating endows the material with photothermal, photodynamic, or SERS capabilities and allows functionalization with antibodies, ensuring targeted action. Iancu et al. investigated the physical and biological properties of nanoparticles of this type and obtained stable nanoparticles that produced a negative T2 signal in vivo when injected into rats, which means that they can be used as negative contrast in MRI. A reduction in cytotoxicity was also observed [67][34]. In 2019, research was carried out on the use of gold nanoparticles in the diagnosis of breast cancer. Chauhan et al. used GBCA-functionalized spherical gold NPs and a cancer-targeted DNA aptamer for MRI imaging. They showed increased contrast agent uptake with relevant breast cancer cell lines compared to non-targeted control counterparts [72][39]. Currently, however, the use of contrast agents with gold NPs is limited to imaging small animals due to autofluorescence, strong light absorption and scattering, which significantly reduces spatial resolution [68][35].
One of the obstacles in using optical methods is that the penetration depth for tissue samples is limited to several hundred µm, mostly due to strong scattering within the tissues [50][23]. Photoacoustic imaging (PAI) is a relatively new imaging method that is rapidly gaining ground in the context of biomedical imaging applications. It is a hybrid method that uses the same properties as fluorescence imaging and also combines ultrasound detection. Compared to other methods, PAI imaging allows to obtain images of deep tissues, up to 7 cm. A nanosecond pulsed laser is used here; the molecules absorb optical energy and convert it into heat, inducing a temperature change, generating acoustic waves that are detected by ultrasonic transducers [73,74,75][40][41][42]. Gold nanoparticles have been considered as excellent photoacoustic contrast agents, mainly due to their large absorption cross section tuned to the optical window (700–1000 nm), which minimizes endogenous absorption and maximizes imaging depth [76][43]. Copland et al. demonstrated the use of bioconjugated gold nanoparticles as a tool for deep imaging of breast cancer cells. The sensitivity of this assay showed that concentrations low as 109 NPs per milliliter were detectable at a depth of 6 cm [77][44].
The use of gold nanoparticles in the treatment of cancer depends on their size, and thus the ability to penetrate tissues. Size also affects nanoparticle accumulation, systemic toxicity, and half-life [78][45]. It has been shown that, the so-called ultra-small gold nanoparticles, i.e., <10 nm in size, in particular, have a high tissue penetration capacity, lower toxicity, and better renal cleansing efficiency [79,80,81][46][47][48]. Chen et al. used ultra-small gold nanoparticles as a contrast agent for PET (positron emission tomography) imaging. They obtained copper-labelled gold nanoparticles which exhibited efficient and rapid renal clearance from the kidneys. Thus, gold nanoparticles can be potentially used for the diagnosis of kidney disease [82][49].
Gold nanoparticles, in addition to being used as a diagnostic tool in the oncological environment, can also be used in ophthalmology. Many ophthalmic imaging methods are available, but are not sufficient to diagnose eye disease at the molecular level before morphological changes become visible. In particular, the optical coherence tomography (OCT) method, using gold nanoparticles, has attracted a lot of attention [83][50]. OCT is a widely used, non-destructive, and non-invasive technique that provides real-time images of tissue sections with a deep resolution. The limitation of this technique is the inability to provide information about the physiology or molecular processes of the examined tissue, which can be achieved using an exogenous contrast agent with a high optical scattering coefficient [84][51].
Genetics could also benefit from the use of gold nanoparticles, especially the detection of single nucleotide polymorphisms. Currently, the liquid biopsy method, which is used to detect DNA samples in patient’s blood, is associated with the most modern laboratory methods and professional personnel are required to perform these analyses. Nanomaterials offer an opportunity to create easy-to-use diagnostic devices that operate on the colorimetric principle, without the need to perform advanced techniques, which could speed up cancer detection [87][52]. Chengnan et al. proposed a novel and simple optical biosensor for DNA detection based on unmodified gold nanowires as signal and hybridization chain reaction (HCR) transducers. They were able to detect target DNA in the range of 0–60 nM, with a detection limit of 1.47 nM. This biosensor exhibits a wide linear range, high sensitivity, and selectivity of DNA detection [88][53].
2. Therapy
Tunable surface chemistry of gold nanoparticles, as well as their unique properties, facilitate their coating, functionalization, and integration with a host of biomolecular moieties opens the door to a wide gamut of applications in therapy [89][54]. Among various approaches, photothermal therapy (PTT), where gold NPs are applied, is known as a less invasive technique in cancer treatment [90][55]. It is based on the conversion of light energy (usually in the NIR region) into heat to induce subsequent cellular necrosis or apoptosis (Figure 42) and causes tumor ablation [91][56]. Unfortunately, healthy tissues are also exposed to damage during this process; therefore, methods with improved specificity and precise spatial–temporal selectivity of PTT are currently under investigation [92][57]. To achieve these features, AuNPs for PTT could be functionalized with specific tumor-targeting molecules [90][55]. Passive and active targeting are commonly used methods, in which poly (ethylene) glycol or selected molecule/antibody specific to tumor markers are introduced [93][58]. In fact, the FDA approved PEGylated gold NPs as the most successful NP, and are being used in ongoing human pilot studies [94][59]. PEGylated nanoparticles accumulate preferentially in tumor tissues due to the enhanced permeability and retention effect (EPR) exhibited by solid tumor [95][60]. Hirsch et al. achieved irreversible photothermal ablation of tumor tissue in mice after exposure of PEGylated gold nanoshells to NIR light [96][61]. In addition, PEGylation of nanoparticles saves them from immunorecognition and prolongs their blood circulation [94,97][59][62]. The accumulation of intratumoral PEGylated particles (after 2, 6, and 24 h) was checked in solid HSC-3 solid tumor in mice after intravenously injection via the tail vein [98][63]. High particle loading was observed following 24 h of circulation. These authors also compared the efficiency of PTT therapy with direct and intravenously administered AuNPs. The results reported dramatic tumor size decreases in mice either for directly-injected (resorption of ˃57%) or intravenously-treated (25%) tumors with AuNPs after NIR laser irradiation. The selectivity of the PTT treatment may be increased by conjugating NPs to antibodies or other disease-specific molecules. The efficiency of gold nanospheres as a photothermal agent was demonstrated by selective delivery of AuNPs to oral squamous carcinoma cells that overexpress EGFR, a clinically related cancer biomarker [99][64]. Another molecule used for PTT in cancer treatment is folic acid (FA), the tumor-targeting ligand, due to its high binding affinity to folate receptors [100][65]. These receptors are often overexpressed on the surface of tumor cells, thus, combining them with AuNPs may be useful in PTT [101][66]. Zhang et al. reported a high efficacy of targeted delivery of FA-attached nanomaterials to melanoma cells. They also showed that the photothermal killing of cancer cells depends on the temperature in PTT. An alternative promising approach to cancer treatment is photodynamic therapy (PDT), in which the activation of photosensitizer (PS) occurs in response to exogenously applied light, which in turn triggers the generation of reactive oxygen species (ROS) and cause cell death (Figure 42) [102][67]. However, due to the low solubility of PS in the physiological environment [94][59], and their limited delivery due to insufficient depth of penetration [103][68], a new strategy, the PDT/PTT dual approach, is being investigated [104][69]. Photosensitizers can be conjugated to AuNPs (nanorods, nanostars, nanoclusters, etc.) through different approaches based on self-assembly [105][70]. In this dual therapy, light-induced heating can be exploited to either induce heating to release a chemical payload or to generate reactive oxygen species to induce cell death [94][59]. Wang et al. covalently anchored Chlorin e6 (Ce6, a commonly used photosensitizer) on the surface of gold nanostars (GNS) to perform simultaneous PDT/PTT treatments of breast cancer and lung cancer models at different irradiation times, both in vitro and in vivo [106][71]. The authors proved the theranostic potential of using this nano-device. The heat-inducing effect after being applied to gold nanostructures was also obtained for radiofrequency therapy (RF) when electromagnetic waves at radio frequencies were introduced [92][57]. Cardinal et al. reported non-invasive radiofrequency technique coupled with gold nanoparticle to the thermal ablation of tissue and cancer cells, in both in vitro and in vivo systems [107][72]. PTT and/or PDT therapies may also be applicable as a therapeutic strategy for infections caused by bacteria or fungi. Zharov et al. have showed a new laser nanoparticle-based PTT for antimicrobial therapy [108][73]. By using combined techniques, AuNPs and laser, a successful killing rate of Staphylococcus aureus was achieved. Various modification of AuNPs and their conjugates with other molecules have been used for the selective destruction of microorganism by light exposure [104][69].
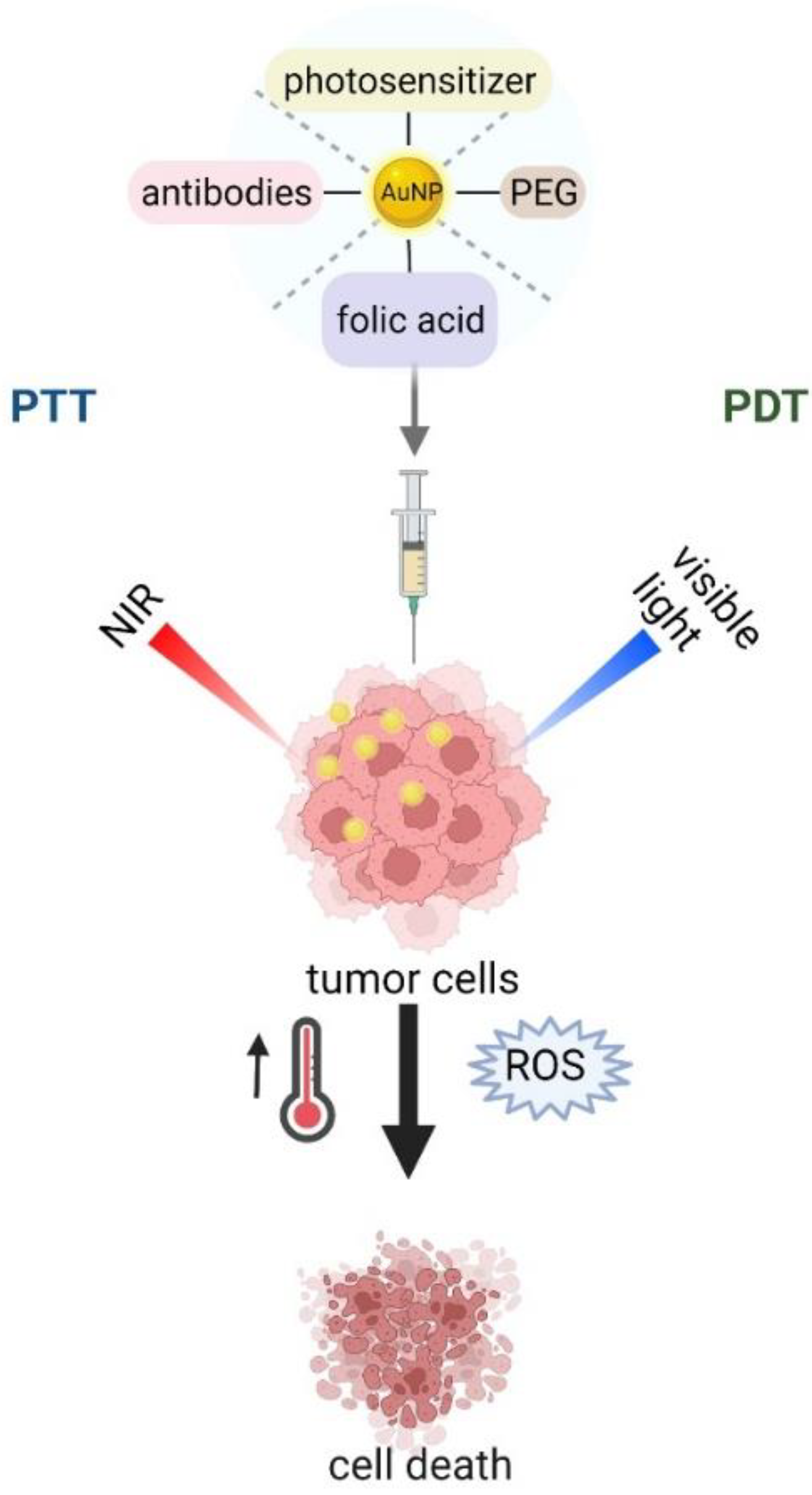
Figure 42.
Schematic illustration of phototherapy.
Gold nanoparticles have also found application in cancer immunotherapy. This type of therapy works by stimulating the natural immune system to attack cancer cells (Figure 53) [109][74]. However, immunomodulators used for this purpose have several disadvantages, including high cost, instability, limited half-life, and rapid drug clearance [110][75]. Systemic toxic reactions are an additional problem in immuno-oncological treatment [111][76]. The safety and efficacy of immunotherapy can be improved by modifying the developed biomaterials, including various therapeutic agents, increasing the targeting of specific immune responses [112][77]. AuNPs can, not only provide an ideal platform for loading immunomodulators, but are also biocompatible, which allows them to be administered intravenously, and thus have the potential to accumulate in tumor cells, which is especially useful for vaccines or adjuvants [113][78]. A vaccine consisting of AuNPs functionalized with the MUC-1 protein developed by Mocan et al. acted as a strong macrophage activator, resulting in the release of cytokines in murine peritoneal macrophages, and thus demonstrates a strong antitumor effect [114][79].
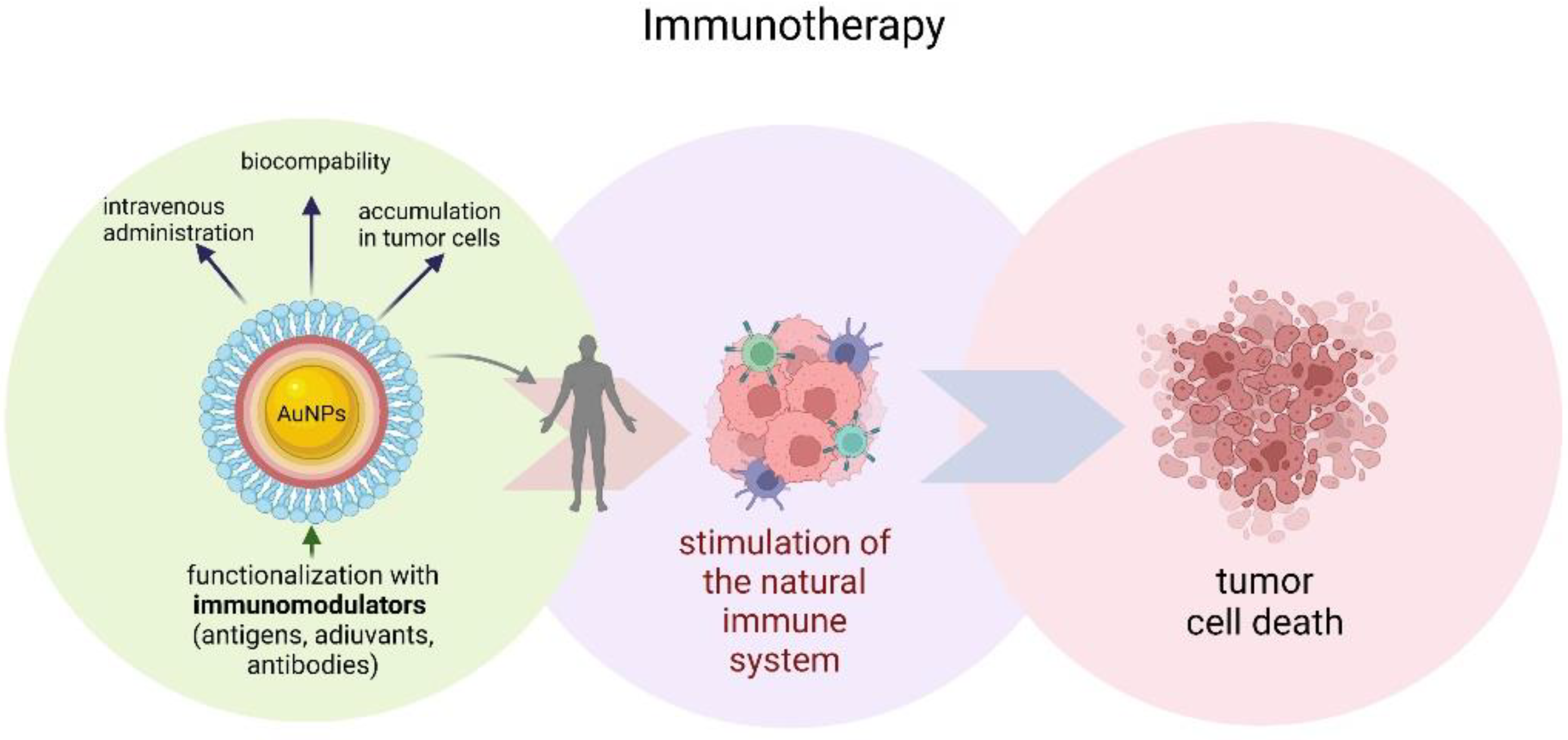
Figure 53.
Schematic illustration of gold nanoparticles immunotherapy.
AuNS are one of the most promising platforms due to their remarkable geometry, which greatly enhances light absorption and provides a high photon-to-heat conversion efficiency due to the plasmonic effect [110][75]. Liu et al. created a new nanoplatform by preparing GNS@CaCO3/Ce6 nanoparticles attached to natural killer cells (NK cells). They applied it in a lung cancer model, demonstrating a significant inhibitory effect on the growth of A549 cells in vitro and in vivo [115][80].
AuNPs facilitate delivery to the immune system, promote the therapeutic effect of antigens and adjuvants, and have an adjuvant effect themselves. This has been demonstrated in studies of the delivery of ovalbumin OVA and CpG adjuvant using gold nanoparticles in an in vivo B16-OVA tumor model. The use of AuNP, not only increased the efficacy of both agents and induced strong antigen-specific responses, but, also, the delivery of AuNP-OVA alone promotes significant antigen-specific responses. Therefore, AuNPs are effective carriers of peptide vaccines with the potential to use lower and safer doses of adjuvant during vaccination [116][81].
AuNS has also been used in a new treatment of glioblastoma. The SYMPHONY therapy (synergistic immunophotothermal nanotherapy) combines treatments using gold nanostars as photothermal inducers and laser-induced photothermal therapy with checkpoint blockade immunotherapy. Mice cured using this therapy successfully rejected reintroduction of cancer cells, which means that, thanks to the SYMPHONY treatment, they gained anticancer memory [117][82]. Liang et al. combined PPT/PDT therapy using gold nanostars fabricated with HER-2 monoclonal antibody and near-infrared region (NIR) photosensitizer indocyanine green (ICG). AuNS@ICG-Ab was loaded on CIK cells (Cytokine-induced killer cells). The nanoplatform was able to migrate into SK-BR-3 tumor cells, accumulate efficiently, activate the immune response, and, through the specific link between trastuzumab and cells, the platform could enhance the effect of PDT/PTT therapy, leading to inhibiting the progression of tumors in mice models [118][83].
References
- Smith, B.R.; Gambhir, S.S. Nanomaterials for in Vivo Imaging. Chem. Rev. 2017, 117, 901–986.
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689.
- Nune, K.C.; Montes, I.; Injeti, V.S.Y.; Somani, M.C.; Misra, R.D.K. The Determining Role of Nanoscale Mechanical Twinning on Cellular Functions of Nanostructured Materials. J. Mech. Behav. Biomed. Mater. 2018, 88, 185–195.
- Chen, P.C.; Mwakwari, S.C.; Oyelere, A.K. Gold Nanoparticles: From Nanomedicine to Nanosensing. Nanotechnol. Sci. Appl. 2008, 1, 45–66.
- Seeram, E. Computed Tomography: A Technical Review. Radiol. Technol. 2018, 89, 279CT–302CT.
- Liu, Z.; Kiessling, F.; Gätjens, J. Advanced Nanomaterials in Multimodal Imaging: Design, Functionalization, and Biomedical Applications. J. Nanomater. 2010, 2010, 894303.
- Haller, C.; Hizoh, I. The Cytotoxicity of Iodinated Radiocontrast Agents on Renal Cells In Vitro. Investig. Radiol. 2004, 39, 149–154.
- Kim, D.; Park, S.; Jae, H.L.; Yong, Y.J.; Jon, S. Antibiofouling Polymer-Coated Gold Nanoparticles as a Contrast Agent for in Vivo X-Ray Computed Tomography Imaging. J. Am. Chem. Soc. 2007, 129, 7661–7665.
- Cormode, D.P.; Naha, P.C.; Fayad, Z.A. Nanoparticle Contrast Agents for Computed Tomography: A Focus on Micelles. Contrast Media Mol. Imaging 2014, 9, 37–52.
- Popovtzer, R.; Agrawal, A.; Kotov, N.A.; Popovtzer, A.; Balter, J.; Carey, T.E.; Kopelman, R. Targeted Gold Nanoparticles Enable Molecular CT Imaging of Cancer. Nano Lett. 2008, 8, 4593–4596.
- Cai, W.; Chen, X. Nanoplatforms for Targeted Molecular Imaging in Living Subjects. Small 2007, 3, 1840–1854.
- Meir, R.; Popovtzer, R. Cell Tracking Using Gold Nanoparticles and Computed Tomography Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1480.
- Galper, M.W.; Saung, M.T.; Fuster, V.; Roessl, E.; Thran, A.; Proksa, R.; Fayad, Z.A.; Cormode, D.P. Effect of Computed Tomography Scanning Parameters on Gold Nanoparticle and Iodine Contrast. Investig. Radiol. 2012, 47, 475–481.
- Bernstein, A.L.; Dhanantwari, A.; Jurcova, M.; Cheheltani, R.; Naha, P.C.; Ivanc, T.; Shefer, E.; Cormode, D.P. Improved Sensitivity of Computed Tomography towards Iodine and Gold Nanoparticle Contrast Agents via Iterative Reconstruction Methods. Sci. Rep. 2016, 6, 26177.
- Cai, Q.-Y.; Kim, S.H.; Choi, K.S.; Kim, S.Y.; Byun, S.J.; Kim, K.W.; Park, S.H.; Juhng, S.K.; Yoon, K.-H. Colloidal Gold Nanoparticles as a Blood-Pool Contrast Agent for X-ray Computed Tomography in Mice. Investig. Radiol. 2007, 42, 797–806.
- Cole, L.E.; Ross, R.D.; Tilley, J.M.; Vargo-Gogola, T.; Roeder, R.K. Gold Nanoparticles as Contrast Agents in X-Ray Imaging and Computed Tomography. Nanomedicine 2015, 10, 321–341.
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of Noble Metal-Based Nanoparticles in Medicine. Int. J. Mol. Sci. 2018, 19, 4031.
- Hainfeld, J.F.; O’Connor, M.J.; Dilmanian, F.A.; Slatkin, D.N.; Adams, D.J.; Smilowitz, H.M. Micro-CT Enables Microlocalisation and Quantification of Her2-Targeted Gold Nanoparticles within Tumour Regions. Br. J. Radiol. 2011, 84, 526–533.
- Shilo, M.; Motiei, M.; Hana, P.; Popovtzer, R. Transport of Nanoparticles through the Blood–Brain Barrier for Imaging and Therapeutic Applications. Nanoscale 2014, 6, 2146–2152.
- Zhan, H.; Stanciauskas, R.; Stigloher, C.; Dizon, K.K.; Jospin, M.; Bessereau, J.-L.; Pinaud, F. In Vivo Single-Molecule Imaging Identifies Altered Dynamics of Calcium Channels in Dystrophin-Mutant C. Elegans. Nat. Commun. 2014, 5, 4974.
- Chan, W.C.W.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent Quantum Dots for Multiplexed Biological Detection and Imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46.
- Yguerabide, J.; Yguerabide, E.E. Resonance Light Scattering Particles as Ultrasensitive Labels for Detection of Analytes in a Wide Range of Applications. J. Cell. Biochem. 2001, 84, 71–81.
- Wu, Y.; Ali, M.R.K.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold Nanoparticles in Biological Optical Imaging. Nano Today 2019, 24, 120–140.
- Harmsen, S.; Huang, R.; Wall, M.A.; Karabeber, H.; Samii, J.M.; Spaliviero, M.; White, J.R.; Monette, S.; O’Connor, R.; Pitter, K.L.; et al. Surface-Enhanced Resonance Raman Scattering Nanostars for High Precision Cancer Imaging. Sci. Transl. Med. 2015, 7, 271ra7.
- Wu, D.; Chen, Y.; Hou, S.; Fang, W.; Duan, H. Intracellular and Cellular Detection by SERS-Active Plasmonic Nanostructures. ChemBioChem 2019, 20, 2432–2441.
- Ou, J.; Zhou, Z.; Chen, Z.; Tan, H. Optical Diagnostic Based on Functionalized Gold Nanoparticles. Int. J. Mol. Sci. 2019, 20, 4346.
- Chaudhary, V.; Jangra, S.; Yadav, N.R. Nanotechnology Based Approaches for Detection and Delivery of MicroRNA in Healthcare and Crop Protection. J. Nanobiotechnol. 2018, 16, 40.
- Coutinho, C.; Somoza, Á. MicroRNA Sensors Based on Gold Nanoparticles. Anal. Bioanal. Chem. 2018, 411, 1807–1824.
- Li, R.D.; Yin, B.C.; Ye, B.C. Ultrasensitive, Colorimetric Detection of MicroRNAs Based on Isothermal Exponential Amplification Reaction-Assisted Gold Nanoparticle Amplification. Biosens. Bioelectron. 2016, 86, 1011–1016.
- Amal, H.; Leja, M.; Funka, K.; Skapars, R.; Sivins, A.; Ancans, G.; Liepniece-Karele, I.; Kikuste, I.; Lasina, I.; Haick, H. Detection of Precancerous Gastric Lesions and Gastric Cancer through Exhaled Breath. Gut 2016, 65, 400–407.
- Runge, V.M. Safety of Approved MR Contrast Media for Intravenous Injection. J. Magn. Reson. Imaging 2000, 12, 205–213.
- Marckmann, P.; Skov, L.; Rossen, K.; Dupont, A.; Damholt, M.B.; Heaf, J.G.; Thomsen, H.S. Nephrogenic Systemic Fibrosis: Suspected Causative Role of Gadodiamide Used for Contrast-Enhanced Magnetic Resonance Imaging. J. Am. Soc. Nephrol. 2006, 17, 2359–2362.
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-Based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015, 276, 228–232.
- Iancu, S.D.; Albu, C.; Chiriac, L.; Moldovan, R.; Stefancu, A.; Moisoiu, V.; Coman, V.; Szabo, L.; Leopold, N.; Bálint, Z. Assessment of Gold-Coated Iron Oxide Nanoparticles as Negative T2 Contrast Agent in Small Animal MRI Studies. Int. J. Nanomed. 2020, 15, 4811–4824.
- Alric, C.; Taleb, J.; Le Duc, G.; Mandon, C.; Billotey, C.; Le Meur-Herland, A.; Brochard, T.; Vocanson, F.; Janier, M.; Perriat, P.; et al. Gadolinium Chelate Coated Gold Nanoparticles as Contrast Agents for Both X-ray Computed Tomography and Magnetic Resonance Imaging. J. Am. Chem. Soc. 2008, 130, 5908–5915.
- Bouché, M.; Hsu, J.C.; Dong, Y.C.; Kim, J.; Taing, K.; Cormode, D.P. Recent Advances in Molecular Imaging with Gold Nanoparticles. Bioconjug. Chem. 2020, 31, 303–314.
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic Iron Oxide Nanoparticles as MRI Contrast Agents for Non-Invasive Stem Cell Labeling and Tracking. Theranostics 2013, 3, 595–615.
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI Contrast Agents: Classification and Application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326.
- Chauhan, R.; El-Baz, N.; Keynton, R.S.; James, K.T.; Malik, D.A.; Zhu, M.; El-Baz, A.; Ng, C.K.; Bates, P.J.; Malik, M.T.; et al. Targeted Gold Nanoparticle–Oligonucleotide Contrast Agents in Combination with a New Local Voxel-Wise MRI Analysis Algorithm for In Vitro Imaging of Triple-Negative Breast Cancer. Nanomaterials 2019, 9, 709.
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.S.; Bi, R.; Ntziachristos, V.; Olivo, M. A Review of Clinical Photoacoustic Imaging: Current and Future Trends. Photoacoustics 2019, 16, 100144.
- Mahan, M.M.; Doiron, A.L. Gold Nanoparticles as X-Ray, CT, and Multimodal Imaging Contrast Agents: Formulation, Targeting, and Methodology. J. Nanomater. 2018, 2018, 5837276.
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462.
- Jain, K.K. Nanomedicine: Application of Nanobiotechnology in Medical Practice. Med. Princ. Pract. 2008, 17, 89–101.
- Copland, J.A.; Eghtedari, M.; Popov, V.L.; Kotov, N.; Mamedova, N.; Motamedi, M.; Oraevsky, A.A. Bioconjugated Gold Nanoparticles as a Molecular Based Contrast Agent: Implications for Imaging of Deep Tumors Using Optoacoustic Tomography. Mol. Imaging Biol. 2004, 6, 341–349.
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.-J.; Zhang, J. Ultrasmall Gold Nanoparticles in Cancer Diagnosis and Therapy. Theranostics 2020, 10, 4944–4957.
- Tsai, C.-Y.; Lu, S.-L.; Hu, C.-W.; Yeh, C.-S.; Lee, G.-B.; Lei, H.-Y. Size-Dependent Attenuation of TLR9 Signaling by Gold Nanoparticles in Macrophages. J. Immunol. 2012, 188, 68–76.
- Zhou, C.; Long, M.; Qin, Y.; Sun, X.; Zheng, J. Luminescent Gold Nanoparticles with Efficient Renal Clearance. Angew. Chem. Int. Ed. 2011, 50, 3168–3172.
- Huang, K.; Ma, H.; Liu, J.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-Dependent Localization and Penetration of Ultrasmall Gold Nanoparticles in Cancer Cells, Multicellular Spheroids, and Tumors in Vivo. ACS Nano 2012, 6, 4483–4493.
- Chen, F.; Goel, S.; Hernandez, R.; Graves, S.A.; Shi, S.; Nickles, R.J.; Cai, W. Dynamic Positron Emission Tomography Imaging of Renal Clearable Gold Nanoparticles. Small 2016, 12, 2775–2782.
- Chen, F.; Si, P.; de la Zerda, A.; Jokerst, J.V.; Myung, D. Gold Nanoparticles to Enhance Ophthalmic Imaging. Biomater. Sci. 2021, 9, 367–390.
- de la Zerda, A.; Prabhulkar, S.; Perez, V.L.; Ruggeri, M.; Paranjape, A.S.; Habte, F.; Gambhir, S.S.; Awdeh, R.M. Optical Coherence Contrast Imaging Using Gold Nanorods in Living Mice Eyes. Clin. Exp. Ophthalmol. 2015, 43, 358–366.
- Iglesias, M.S.; Grzelczak, M. Using Gold Nanoparticles to Detect Single-Nucleotide Polymorphisms: Toward Liquid Biopsy. Beilstein J. Nanotechnol. 2020, 11, 263–284.
- Xu, C.; Lan, L.; Yao, Y.; Ping, J.; Li, Y.; Ying, Y. An Unmodified Gold Nanorods-Based DNA Colorimetric Biosensor with Enzyme-Free Hybridization Chain Reaction Amplification. Sens. Actuators B Chem. 2018, 273, 642–648.
- Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Au Nanoparticles Target Cancer. Nano Today 2007, 2, 18–29.
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic Photothermal Therapy (PPTT) Using Gold Nanoparticles. Lasers Med. Sci. 2008, 23, 217–228.
- Ray, P.C.; Khan, S.A.; Singh, A.K.; Senapati, D.; Fan, Z. Nanomaterials for Targeted Detection and Photothermal Killing of Bacteria. Chem. Soc. Rev. 2012, 41, 3193–3209.
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23.
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167.
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979.
- Maeda, H.; Fang, J.; Inutsuka, T.; Kitamoto, Y. Vascular Permeability Enhancement in Solid Tumor: Various Factors, Mechanisms Involved and Its Implications. Int. Immunopharmacol. 2003, 3, 319–328.
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-Mediated near-Infrared Thermal Therapy of Tumors under Magnetic Resonance Guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554.
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and Pharmacokinetic Studies of CYT-6091, a Novel PEGylated Colloidal Gold-RhTNF Nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149.
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.; El-Sayed, I.H.; Chu, H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold Nanorod Assisted Near-Infrared Plasmonic Photothermal Therapy (PPTT) of Squamous Cell Carcinoma in Mice. Cancer Lett. 2008, 269, 57–66.
- Elsayed, I.; Huang, X.; Elsayed, M. Selective Laser Photo-Thermal Therapy of Epithelial Carcinoma Using Anti-EGFR Antibody Conjugated Gold Nanoparticles. Cancer Lett. 2006, 239, 129–135.
- Zhang, Y.; Zhan, X.; Xiong, J.; Peng, S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.; Li, R.; Yuan, K.; et al. Temperature-Dependent Cell Death Patterns Induced by Functionalized Gold Nanoparticle Photothermal Therapy in Melanoma Cells. Sci. Rep. 2018, 8, 8720.
- Chitgupi, U.; Qin, Y.; Lovell, J.F. Targeted Nanomaterials for Phototherapy. Nanotheranostics 2017, 1, 38–58.
- Kim, D.; Shin, K.; Kwon, S.G.; Hyeon, T. Synthesis and Biomedical Applications of Multifunctional Nanoparticles. Adv. Mater. 2018, 30, 1802309.
- Benov, L. Photodynamic Therapy: Current Status and Future Directions. Med. Princ. Pract. 2015, 24, 14–28.
- Bucharskaya, A.; Maslyakova, G.; Terentyuk, G.; Yakunin, A.; Avetisyan, Y.; Bibikova, O.; Tuchina, E.; Khlebtsov, B.; Khlebtsov, N.; Tuchin, V. Towards Effective Photothermal/Photodynamic Treatment Using Plasmonic Gold Nanoparticles. Int. J. Mol. Sci. 2016, 17, 1295.
- Calavia, P.G.; Bruce, G.; Pérez-García, L.; Russell, D.A. Photosensitiser-Gold Nanoparticle Conjugates for Photodynamic Therapy of Cancer. Photochem. Photobiol. Sci. 2018, 17, 1534–1552.
- Wang, H.; Wang, X.; Wang, P.; Zhang, K.; Yang, S.; Liu, Q. Ultrasound Enhances the Efficacy of Chlorin E6-Mediated Photodynamic Therapy in MDA-MB-231 Cells. Ultrasound Med. Biol. 2013, 39, 1713–1724.
- Cardinal, J.; Klune, J.R.; Chory, E.; Jeyabalan, G.; Kanzius, J.S.; Nalesnik, M.; Geller, D.A. Noninvasive Radiofrequency Ablation of Cancer Targeted by Gold Nanoparticles. Surgery 2008, 144, 125–132.
- Zharov, V.P.; Mercer, K.E.; Galitovskaya, E.N.; Smeltzer, M.S. Photothermal Nanotherapeutics and Nanodiagnostics for Selective Killing of Bacteria Targeted with Gold Nanoparticles. Biophys. J. 2006, 90, 619–627.
- Banstola, A.; Jeong, J.H.; Yook, S. Immunoadjuvants for Cancer Immunotherapy: A Review of Recent Developments. Acta Biomater. 2020, 114, 16–30.
- Chauhan, A.; Khan, T.; Omri, A. Design and Encapsulation of Immunomodulators onto Gold Nanoparticles in Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 8037.
- Savitsky, K.; Yu, X. Combined Strategies for Tumor Immunotherapy with Nanoparticles. Clin. Transl. Oncol. 2019, 21, 1441–1449.
- Surendran, S.P.; Moon, M.J.; Park, R.; Jeong, Y.Y. Bioactive Nanoparticles for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3877.
- He, J.; Liu, S.; Zhang, Y.; Chu, X.; Lin, Z.; Zhao, Z.; Qiu, S.; Guo, Y.; Ding, H.; Pan, Y.; et al. The Application of and Strategy for Gold Nanoparticles in Cancer Immunotherapy. Front. Pharmacol. 2021, 12, 687399.
- Mocan, T.; Matea, C.; Tabaran, F.; Iancu, C.; Orasan, R.; Mocan, L. In Vitro Administration of Gold Nanoparticles Functionalized with MUC-1 Protein Fragment Generates Anticancer Vaccine Response via Macrophage Activation and Polarization Mechanism. J. Cancer 2015, 6, 583–592.
- Liu, B.; Cao, W.; Cheng, J.; Fan, S.; Pan, S.; Wang, L.; Niu, J.; Pan, Y.; Liu, Y.; Sun, X.; et al. Human Natural Killer Cells for Targeting Delivery of Gold Nanostars and Bimodal Imaging Directed Photothermal/Photodynamic Therapy and Immunotherapy. Cancer Biol. Med. 2019, 16, 756–770.
- Almeida, J.P.M.; Lin, A.Y.; Figueroa, E.R.; Foster, A.E.; Drezek, R.A. In Vivo Gold Nanoparticle Delivery of Peptide Vaccine Induces Anti-Tumor Immune Response in Prophylactic and Therapeutic Tumor Models. Small 2015, 11, 1453–1459.
- Liu, Y.; Chongsathidkiet, P.; Crawford, B.M.; Odion, R.; Dechant, C.A.; Kemeny, H.R.; Cui, X.; Maccarini, P.F.; Lascola, C.D.; Fecci, P.E.; et al. Plasmonic Gold Nanostar-Mediated Photothermal Immunotherapy for Brain Tumor Ablation and Immunologic Memory. Immunotherapy 2019, 11, 1293–1302.
- Liang, S.; Sun, M.; Lu, Y.; Shi, S.; Yang, Y.; Lin, Y.; Feng, C.; Liu, J.; Dong, C. Cytokine-Induced Killer Cells-Assisted Tumor-Targeting Delivery of Her-2 Monoclonal Antibody-Conjugated Gold Nanostars with NIR Photosensitizer for Enhanced Therapy of Cancer. J. Mater. Chem. B 2020, 8, 8368–8382.
More
