Definition: The type 2 dopamine receptor D2 (D2-R), member of the G protein-coupled receptor (GPCR) superfamily, exists in two isoforms, short (D2S-R) and long (D2L-R). They differ by an additional 29 amino acids (AA) in the third cytoplasmic loop (ICL3) of the D2L-R. These isoforms differ in their intracellular localization and trafficking functionality, as D2L-R possesses a larger intracellular pool, mostly in the endoplasmic reticulum (ER). This paper is by authors Blagotinšek Cokan, K.; Mavri, M.; Rutland, C.S.; Glišić, S.; Senćanski, M.; Vrecl, M.; and Kubale, V.
- Dopamine
- Endoplasmic reticulum
- Parkinson’s disease
- schizophrenia
- Huntington’s
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
Definition: The type 2 dopamine receptor D2 (D2-R), member of the G protein-coupled receptor (GPCR) superfamily, exists in two isoforms, short (D2S-R) and long (D2L-R). They differ by an additional 29 amino acids (AA) in the third cytoplasmic loop (ICL3) of the D2L-R. These isoforms differ in their intracellular localization and trafficking functionality, as D2L-R possesses a larger intracellular pool, mostly in the endoplasmic reticulum (ER).
1. Dopamine Receptors
G-protein-coupled receptors (GPCRs), also termed seven-transmembrane receptors (7TMRs) are by far the largest family of membrane-bound receptors, which are involved in the regulation of the neurotransmitter dopamine effects as one of their targets [1]. Based on functional, structural, and pharmacological properties, five types of dopamine receptors have been described, that belong to the D1- or D2-like subfamily of receptors (D1-R and D2-R respectively), with differing abilities of stimulation or inhibition of adenylyl cyclase (AC), respectively.
The D1-R subfamily is comprised of D1 and D5 receptors (D1-R and D5-R), and the D2 subfamily includes D2, D3 and D4 receptors (D2-R, D3-R, and D4-R). Members of the D1-R subfamily have a short third cytoplasmic loop (ICL3) and a very long C-terminal cytoplasmic end. In contrast, D2-Rs have a very long ICL3 and a short C-terminal end and include the receptor variants generated by alternative splicing (D2 and D3) or polymorphic variation (D4) (reviewed by Beaulieu et al.) [2]. The D2-R subgroup has a long ICL3 whose structure is common to the receptor interaction with the heterotrimeric protein Gαi [1]. In D1-R, with its characteristically short ICL3, coupling with Gαs proteins occurs [1][3][1,3]. The C-terminal end is approximately seven times longer in D1-Rs than in D2-Rs. Both the ICL3 and the C-terminal end are thought to serve as possible communication points for interaction with intracellular proteins. The N-terminal tail has a similar number of amino acids in all receptor subtypes and contains sites for N-glycosylation. D1-R and D5-R have two glycosylation sites, located at the N-terminal end and in extracellular loop 2 (ECL2). D2-R has three potential N-linked glycosylation sites, all in the N-terminus: N5, N17, and N23, D3-R has four potential glycosylation sites: N12 and N19 in the N-terminus, N97 in the first extracellular loop (ECL1), and N173 in the second extracellular loop (ECL2) [4] [4] and D4-R only one in the N-terminus [5]. Cysteines located in the first and second extracellular loops (ECL1 and ECL2) are linked by a disulfide bond that stabilizes the receptor structure [6]. The endogenous ligand for the dopamine receptors is the neurotransmitter dopamine. After dopamine binds to the D1-R, the signaling pathway is canonically activated via the heterotrimeric protein Gαs and Golf G-proteins, leading to adenylate cyclase (AC) activation and cyclic adenosine monophosphate (cAMP) formation in the cell. Diversity in functional outcomes may also be achieved via selective binding to Gαi and Gαo proteins. Previous work has shown that D2-R can be stabilized by an agonist, which affect the selectivity and amount of coupling with Gαi and Gαo [7][8][7,8]. Although previous work had indicated that Gαi2 was selective for D2L-R [9][10][9,10], experimental data has indicated that selectivity regulation of Gαi is driven by the agonist-activated conformation of D2-R. R(+)-3-PPP hydrochloride stimulation of D2-R resulted in reduced coupling with Gαi1 or Gαi2 and preferential coupling with Gαi3 [11]. The movement magnitude of the sixth transmembrane helix of the activated receptor was predicted to be the primary modulator of the selectivity of the G-protein subtypes [12].
Using cryo-electron microscopy, the structure of an agonist-bound activated D2–Gαi complex reconstituted into a phospholipid membrane has been demonstrated recently [13], both as the first experimental model of a GPCR complex embedded in a phospholipid bilayer, as well as the first model of activated D2-R. The models revealed interactions that are unique to the membrane-embedded complex, such as conformational changes in ECL2, TM5, TM6 and TM7, propagating to the opening of the intracellular Gαi-binding site and helix 8 burial in the inner leaflet, ordered lysine and arginine side chains in the membrane interfacial regions, and lipid anchoring of the G-protein in the membrane [13].
Although all D-Rs recognize the same ligand, they have a differential tissue distribution and are involved in different functions in vivo [3][14][3,14]. By binding to various types of D-Rs, dopamine controls locomotor system functions, cognition, emotion, hunger, satiety, and endocrine secretion [3][5][3,5]. Impaired D2-R signaling is associated with the pathophysiology of many psychiatric and neurological diseases or states, including Parkinson’s disease, schizophrenia, Tourette’s syndrome, Huntington’s disease, bipolar disorder, depression, dementia, as well as others, such as restless leg syndrome and sexual dysfunction. D-Rs are an essential target for currently available modern drugs, including the dopamine precursor levodopa [3][5][3,5] for Parkinson’s disease, where dopaminergic neurons are damaged and a dopamine deficiency leads to a combination of movement and psychiatric pathologies. Thus, D-Rs are targets for motor deficits, cognitive, and motivational deficits in neuropsychiatric disorders [15]. In schizophrenia and psychosis inhibitors of D2-R are used to reduce increased dopaminergic signaling [16].
2. Dopamine Receptor Type 2 (D2-R)
The D2-R is a key component of the dopamine system that is present in two alternatively spliced transcripts of the Drd2 gene and classified as short (D2S-R) and long (D2L-R) receptor isoforms. The long isoform differs from the short one only by the presence of an additional 29 amino acids (AA) encoded by exon 5 in the ICL3 of the D2L-R [17][18][19][17,18,19]. The inclusion is interspersed between the AA lysine (K241) and glutamic acid (E271). D2S-R in mice and rats are made up of 415 AA and D2L-R is made up of 444 AAs. Human D2S-R and D2L-R are shorter than murine and rat equivalents by one AA, consisting of 414 and 443 AAs, respectively. The isoleucine is missing between lysine (K331) and aspartic acid (D332). This region might have an essential role in the functional differences between both D2-R isoforms such as interactions related to G-proteins [20][21][22][20,21,22], post-translation modification and cell localization [11][23] [11,23]. D2-R isoforms also indicate different in vivo functions, whereby D2L-R primarily acts at postsynaptic and D2S-R in presynaptic dopaminergic transmissions [24][25][24,25]. Data acquired on genetically engineered D2-R mouse model indicates additional evidence for different roles of two isoforms in cognitive and motor functions [24], responsiveness to cocaine exposure [26], and therapeutic effects of antipsychotic drugs [27]. Furthermore, they are expressed in the same cell types with more abundant expression of the D2L-R isoform over D2S-R, but with differences in their intracellular localization. While D2S-R is primarily localized on the plasma membrane (PM), a substantial fraction of D2L-R is located intracellularly, especially in the perinuclear compartments around the Golgi apparatus (GA) [14] and endoplasmic reticulum (ER) [23].
The D2-R is the most commonly studied dopamine receptor subtype since the majority of antipsychotic drugs act as D2-R antagonists in the mesolimbic dopaminergic system [28]. As a primary target for atypical and typical antipsychotic drugs and treatment of the Parkinson’s disease, many of those agents can cause potentially life-threatening and severe side effects due to the promiscuous activities against related D2-Rs [29]. Precisely because of this reason, it is necessary to be familiar with the details of the dopamine receptor’s complex structure and functions.
3. D2-R Interaction Proteins (DRIPs)
More than 20 dopamine receptor-interacting membrane-associated or cytoplasmic D2-R interaction proteins (DRIPs) are known and several of them bind the ICL3 of the D2-R [30][79]. Using the informational spectrum method (ISM), a virtual spectroscopy method for investigating protein-protein interactions, the analysis of known interaction partners of IC3 of D2-R [30][79] was performed as previously described [31][32] [80,81] and obtained the results presented in Table 2. ISM analysis of the IC3 D2-R interaction with protein partners corroborates with published data (reviewed in[30] [79]) (Table 2, Figure 1). However, in addition to previously identified protein partners it has also been suggested that there are some new potential interaction partners.
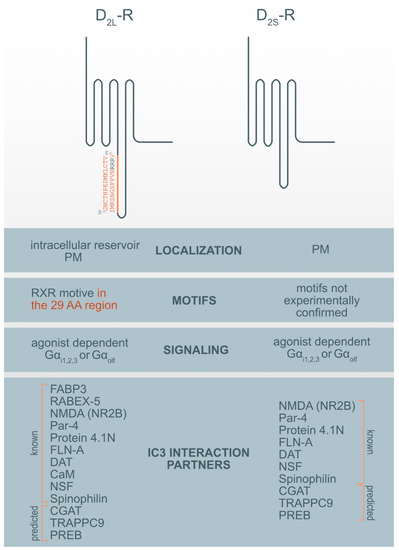
Figure 1. Motif and interaction partners’ differences between D2L-R and D2S-R. CaM—Ca2+-binding protein calmodulin; CGAT—Chromaffin granule amine transporter; DAT—dopamine transporter; FABP3-Fatty acid binding protein 3; FLN-A—filamin A; NMDA (NR2B)—NR2B subunit of the NMDA glutamate (N-methyl-D-aspartate); NSF—N-ethylmaleimide-sensitive factor; Par-4—Prostate apoptosis response-4; PM-plasma membrane; PREB—prolactin regulatory element-binding protein; Rabex-5-Rabaptin-5 interacting protein; TRAPPC9—Trafficking protein particle complex subunit 9.
Among previously described interaction partners, the highest affinity for the interaction with the D2-R was ascribed to N-methyl-D-aspartate (NMDA) receptor NR2B subunits. It was shown that a distinct region within the first 32 AA of the D2-R ICL3 interacts with the NR2B and disrupts the association of Ca2+/calmodulin-dependent protein kinase II (CaMKII) with NR2B, reduces NR2B phosphorylation at a CaMKII-sensitive site (Ser1303), and inhibits NMDA receptor-mediated currents in medium-sized striatal neurons. The D2-R-NR2B interaction is therefore critical for modulating NMDA receptor-mediated currents and behavioral responsiveness to cocaine [33][82]. The second highest propensity for interaction with the D2-R was observed for prostate apoptosis response-4 (Par-4). Par-4 is a protein expressed in the nervous system, where it is known to be a regulatory component in dopaminergic signaling. It is a mediator of neuronal degeneration, and is associated with the pathogenesis of Alzheimer’s disease [34][83]. Par-4 directly interacts with the D2-R via the calmodulin-binding motif in the ICL3. Furthermore, Par-4 constitutes a molecular link between impaired dopaminergic signaling and depression [35][84]. The N-terminal segment of the D2-Rs and D3-R was also shown to interact with neuronally enriched 4.1N protein; an interaction that contributes to the localization and stability of D2-Rs at the neuronal PM [36][85]. Similarly, filamin-A (FLN-A) also interacts with the N-terminal segment of the ICL3 of the D2-R and D3-R, and connects D-Rs with some other GPCRs, such as rhodopsin and, metabotropic glutamate receptors to the cytoskeleton, and therefore participate in their final subcellular localization [37][86]. The dopamine transporter (DAT) is a membrane-spanning protein that facilitate the reuptake of extracellular dopamine to the cytosol and is therefore, an essential target for cocaine, amphetamine, and some other drugs of abuse. One study showed a direct interaction between the DAT and the ICL3 (I340-Q373) of both D2-R isoforms. However, D2L-R is more capable of physically interacting with the DAT [38][87].
The Ca2+-binding protein calmodulin (CaM) binds to the N-terminal portion of the ICL3 of the D2L-R, within an Arg-rich epitope (VLRRRRKRVN) that is also involved in the binding to Gi/o proteins and the adenosine A2A receptor, with the formation of A2A-D2-R heteromers[39][40] [88,89]. N-ethylmaleimide-sensitive factor (NSF) is an ATPase and an essential part of the protein network responsible for different membrane fusion events, including transport through the GA and exocytosis [41][90]. Using immunoprecipitation and in vitro binding assays, it has been shown that NSF binds to the ICL3 of D-R (F341-Q373) and has a putative role in the interaction of D2-R and the Glu2 AMPA receptor [42][91]. Agonist stimulation of D2-R promotes the formation of direct protein-protein interactions between the ICL3 of the D2-R and the ATPase N-ethylmaleimide-sensitive factor (NSF). Spinophilin is F-actin and protein phosphatase-1-binding protein with a single PDZ domain that was identified as a protein associated with the ICL3 region of the D2-R. It is hypothesized to be necessary for establishing signaling complexes for dopaminergic neurotransmission through D2-Rs by linking receptors to downstream signaling molecules and the actin cytoskeleton [43][92].
Three additional hypothetical ICL3 D2-R interaction partners were suggested by ISM: prolactin regulatory element-binding protein (PREB), chromaffin granule amine transporter (CGAT) and trafficking protein particle complex subunit 9 (TRAPPC9). Among prospective partners, CGAT displayed the highest affinity for interacting with the ICL3 D2-R, followed by TRAPPC9 and PREB. For all three prospective interaction partners we were unable to find experimental evidence for the direct interaction with the ICL3 of the D2-R but only some indirect indication for their involvement in dopamine synthesis, transport, or D2-R binding. PREB is an ubiquitously expressed protein and, a member of the WD-repeat protein family, that acts as a transcriptional regulator and suppresses the expression of the adiponectin gene [44][93], regulates prolactin (PRL) gene expression [45][94] and functions as a transcriptional regulator of PRL promoter activity, and therefore might be involved in thyrotropin-releasing hormone (TRH)-induced PRL gene transcription [46][95]. PRL gene expression and secretion are regulated by various hormones and growth factors, including dopamine, epidermal growth factor, and thyrotropin-releasing hormone (TRH) [46][95]. PREB is highly expressed in the anterior pituitary. Prolactinomas are the most common pituitary tumors and are treated with the selective dopamine D2-R agonist cabergoline [47][96]. Mutation of the PREB-binding site within the promoter abrogated the ability of cabergoline to inhibit PRL promoter activity. The chromaffin granule amine transporter (CGAT), also named the vesicular monoamine transporter 1 (VMAT1), is involved in the transport of biogenic monoamines, such as serotonin, from the cytoplasm into the secretory vesicles of neuroendocrine and endocrine cells. It has a positive impact on dopamine synthesis, secretion, and transport to storage vesicles, which releases neurotransmitters into synapses as chemical messages to postsynaptic neurons [48][97]. The pharmaceutical industry also targets VMATs for treating hypertension, drug addiction, psychiatric disorders, Parkinson’s disease, and other neurological disorders. The trafficking protein particle complex subunit 9 (TRAPPC9), also known as NIBP, belongs to the TRAPPII multiprotein complex. TRAPPC9 is involved in vesicular trafficking from the ER to the GA and promotes the activation of NFκB signaling. It is highly expressed in the postmitotic neurons of the cerebral cortex [49][98].
To the best of our knowledge, only two proteins have been identified that specifically interact only with the D2L-R i.e., 29 AA within its ICL3. These proteins are fatty acid-binding protein 3 (FABP3) [50][99] and Rabaptin-5 interacting protein (Rabex-5) [23]. Fatty acid-binding protein 3 (FABP3), also named the heart-type FABP (H-FAB), is one of the novel 29 AA insert binding protein on the position (G242-V270), which also alters D2L-R function [50][99]. D2L-R, when activated with a ligand, is known to activate the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) pathways, which are enhanced by FABP3 in FABP3-overexpressed cells, showing that FABP3 enhances D2L-R signaling [14]. A co-expression study of D2L-R and D2S-R with this protein in NG108-15 cells shows overexpression and colocalization of endogenous FABP only with the D2L-R in the GA and ER but not in the PM [51][100]. Dysfunction of FABP3 protein binding to D2L-R was shown in FABP3 KO mice [51][100], which affects emotional behavior, and is characteristic of neurodegenerative diseases such as schizophrenia and Alzheimer’s disorder. These KO mice, which showed altered sensory, motor, and emotional behaviors, also exhibited decreased methamphetamine-induced sensitization and enhanced haloperidol-induced catalepsy due to D2-R dysfunction. Impaired FABP brain function was observed as an essential factor in the perturbation of D2-R signaling [52][101]. Rabaptin-5 interacting protein (Rabex-5) was identified in mouse brain lysates as another protein binding the 29 AA of D2L-R and has been shown to promote the early-endosome formation and Rab5 activation [55][71]. Both proteins are essential for prolonged D2L-R mediated ERK signaling.
DRIPs have the propensity to bind to conserved motifs in receptors. For D1-R it was shown that the ER-membrane-associated protein DRiP78 binds to a FXXXFXXXF motif in the C-terminus of D1-R and other GPCRs. Overexpression or down-modulation of this putative two-TM domain protein leads to ER retention of D1-Rs, reduced ligand binding, and impaired kinetics of receptor glycosylation [56][48]. This mechanism acts as a chaperone and may control PM receptor targeting without traveling to the cell surface.
Some of the DRIPs are also possible “private” chaperones with other functions, escorting proteins for D2L-R or proteins of the quality-control machinery involved in its retention within intracellular compartments [57] [37] and facilitating receptor cell surface expression by enabling their trafficking to the PM. Pools of intracellular D1-R exist in renal tubular cells, and receptor recruitment to the PM is independent of agonist activation elicited by the activation of cell surface receptors and via atrial natriuretic peptide-dependent heterologous activation [53][54][102,103].
Table 2. The bioinformatics approach-informational spectrum method (ISM) analysis of interaction partners of the third cytoplasmic loop (ICL3) of the D2-R. A lower signal to noise S/N ratio suggests a lower interaction affinity between tested protein partners.
| Interaction Partner | S/N Ratio | Function | Reference |
|---|---|---|---|
| Glutamate, NMDA (NR2B) | 62.39 | ionotropic glutamate receptor | Liu, X.Y. et al. (2006) [33][82] |
| Par-4 | 48.63 | regulatory component in dopamine signaling | Guo, Q. et al. (1998) [34][83] Park, S.K. et al. (2005) [35][84] |
| Protein 4.1N | 38.61 | membrane-cytoskeleton adaptor | Binda, A.V. et al. (2002) [36][85] |
| FLN-A | 26.65 | actin binding protein | Lin, R. et al. (2001) [37][86] |
| DAT | 20.29 | facilitating reuptake of extracellular dopamine back in the cytosol | Lee, F.J. et al. (2007) [38][87] |
| Gα i/z/o | 17.85 | binding GPCRs | |
| CaM | 13.36 | intermediate calcium-binding messenger | Navarro, G. et al. (2009) [39][88] |
| NSF | 13.03 | ATPase | Hanson, P.I. et. al. (1995) [40][90] Zou S. et al. (2005) [42][91] |
| Spinophilin | 12.14 | F-actin and protein phosphatase-1-binding protein | Smith, F.D. et al. (1999) [43][92] |
| Predicted Interaction Partner | 12.14 | ||
| CGAT | 19.90 | involved in the transport of biogenic monoamines | |
| TRAPPC9 | 19.73 | involved in vesicular trafficking from ER to GA | |
| PREB | 18.78 | transcriptional regulator |
Legend: Glutamate, NMDA (NR2B)—NR2B subunit of the NMDA glutamate receptor (N-methyl-D-aspartate); FLN-A—filamin-A; Par-4—prostate apoptosis response-4; DAT—dopamine transporter; CGAT—chromaffin granule amine transporter; TRAPPC9—trafficking protein particle complex subunit 9; PREB—prolactin regulatory element-binding protein; NSF—N-ethylmaleimide-sensitive factor; CaM—Ca2+-binding protein calmodulin.
