You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Luca Ferretto and Version 2 by Catherine Yang.
A promising cell treatment in wound healing is the local injection of peripheral blood mononuclear cells (PBMNCs). The treatment is aimed to induce angiogenesis as well to switch inflammatory macrophages, called the M1 phenotype, into anti-inflammatory macrophages, called M2, a phenotype devoted to tissue repair. This mechanism is called polarization and is a critical step for the healing of all human tissues. PBMNCs are the mononuclear cells of the blood: lymphocytes (T cells, B cells and NK cells), monocytes and a small fraction of the endothelial progenitor cells (EPCs) .
- macrophages
- peripheral blood mononuclear cells
- critical limb ischemia
1. PBMNCs and Angiogenesis
One of the most studied PBMNCs mechanisms of action is angiogenesis.
The term angiogenesis represents the creation of new blood vessels from pre-existing ones. This phenomenon is distinct from vasculogenesis, which happens during embryogenesis, and is the formation of blood vessels from EPCs and/or angioblasts [1][19].
EPCs are derived from the hemangioblast in BM, which is the precursor of both hematopoietic stem cells and EPCs. The latter could be isolated in BM, in peripheral blood, in adipose tissue and in the umbilical cord, and they constitute 0.001% of the total stem cell population [1][19]. Their final differentiation is endothelial cells (ECs), contributing to form the inner lining of new blood vessels. Their most common markers are CD34+/VEGFR2+, VEGFR2+/CD 133+ and DiI-Ac-LDL-positive cells [1][19].
CD34+ is a marker expressed by the HSCs, and flk-1 is a receptor for VEGF expressed by HSCs and EPCs. Both these markers are lost during hematopoietic differentiation and, secondly, at the maturation stage, they are divided into two sub-populations: early and late EPCs. Early EPCs have a more paracrine action, whereas the late EPCs lose CD14+ and are capable of forming colonies of endothelial cells with a high proliferative capacity and, thus, with a high vasculogenic involvement, differentiating into mature ECs and incorporating into new blood vessels [1][19].
Cells isolated with anti-CD34 or anti-Flk-1 differentiate into ECs in vitro, suggesting that they can contribute, in vivo, to angiogenesis [2][20].
In diabetes vascular wound, EPCs are dysfunctional because of the hyperglycemia and the higher oxidative stress; in fact, there was found an inverse proportion between HbA1c levels and circulating EPCs [3][21].
Another proposed mechanism of action of PBMNCs (especially reported in BM mononuclear cells studies) is the stimulation of pericyte differentiation. Pericytes are cells presenting along the capillaries and postcapillary venule walls. Their markers of expression are the platelet growth factor (PDGF) receptor and the proteoglycan NG2 (a coreceptor for PDGF). Usually, they surround arterioles, venules and capillaries in different shapes according to the different kind of smooth muscle fibers. Their principal role is to control blood flow thanks to the intrinsic contractile power of their smooth muscle fibers around capillaries, permitting to adjust the diameter of arterioles and venules [4][22]. This capacity, whether improved, could potentiate the tissue vascularization.
Moryia et al. retrospectively studied patients with CLI treated with PBMNCs, noticing that, clinically, ischemic rest pain ameliorates after this kind of treatment, but they also noticed a wide range of clinical responses. They investigated the peak plasma level of VEGF after PBMNC injection, discovering a significantly higher expression in responder patients than in non-responders. In particular, the majority of non-responders were patients with multiple comorbidities, especially chronic renal insufficiency in hemodialysis treatment [5][23].
It has been demonstrated that monocytes and macrophages maintain their angiogenic potency in diabetic patients, while HSCs showed a reduced angiogenic power [6][24]. In addition, Spaltro et al. tested PBMNC injection into a mouse model of hind limb ischemia, finding an induced tissue neo-vascularization by an increasing number of capillaries, arterioles and regenerative fibers [7][25]. These data were confirmed by De Angelis et al. after PBMNC implantation in NO-CLI patients including a subset of diabetic patients [7][25]. The histological data confirmed the formation of dermal granulation tissue, an increased monocytes tissue concentration and newly formed microvessels, whereas dermal inflammation and monocyte infiltration were reduced [8][26].
Moreover, PBMNC implantation promotes other significant changes in the diabetic foot tissues such as the inhibition of HIF-1, NF-KB and TNF-alpha; an increase of VEGF; the appearance of newly formed capillaries [9][27].
2. Macrophages Polarization from the M1 Phenotype to M2
2.1. Wound Healing and the Paradigm of M1–M2 Macrophages
Physiologically, adult wound healing is a process characterized by three independent overlapping phases: inflammation, granulation and remodeling.
During inflammation, polymorphonuclear neutrophils are the first cells infiltrating the damaged tissue, followed by circulating monocytes that, once achieved at the wound site, differentiate into macrophages [10][28].
Macrophages represent the most important protagonist during wound repair. They are extremely plastic and dynamic, and they are capable of changing their phenotype according to different external stimuli [11][12][29,30]. In particular, the microbial components lipopolysaccharide (LPS), Toll-like receptor (TLR) ligands and interferon-gamma (IFN-γ) usually activate M1 macrophages, whereas IL4/IL-13, immune complexes and TLR, IL-1 receptor ligands and IL-10 stimulate the alternative M2 activation. This phenomenon is the so-called macrophage polarization [13][14][31,32].
During inflammation, the M1 macrophages support microbicidal and cytotoxic host defense functions by releasing high concentrations of pro-inflammatory cytokines such as tumor necrosis factor α (TNFα), interleukin (IL)-1β, IL-6 and IL-12 and also reactive oxygen species (ROS) [15][16][33,34]. The accumulation of apoptotic cells stimulates the switch from the M1 to the M2 phenotype. From this moment on, the anti-inflammatory or granulation one phases begin, characterized by anti-inflammatory cytokines (IL-10); multiple growth factors such as transforming growth factor (TGF)-β, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF); the deposition of extracellular matrix (ECM) directed by fibroblasts and myofibroblasts. In parallel, M2 macrophages and activated fibroblasts also release proangiogenic factors, recruiting EPCs and improving new vessels formation [17][18][35,36].
The origin of macrophages has long been debated over the last years [19][37]. Nowadays, it is well known that the majority of tissue macrophages are almost already present in the target tissues, even before the definitive hematopoiesis is performed. Monocytes derived from a common progenitor, called a macrophage dendritic cell precursor (MDP), are able to differentiate both inflammatory macrophages and dendritic cells [20][38]. MDPs can also differentiate into other hematopoietic lineages.
Pathology is frequently associated with dynamic changes in macrophages activation. Classically, activation of M1 or M1-like cells is implicated in initiating and sustaining inflammation, and the activation of M2 or M2-like cells is associated with resolution or smoldering of chronic inflammation [21][39].
Plasticity is one of the major characteristics of monocytes and macrophages and the M1–M2 polarization is not yet totally defined. It has also been hypothesized the existence of a third macrophages phenotype: M3 or switching phenotype which could be capable to direct the M1/M2 polarization by inducing the secretion of M1/M2 activators [22][40].
An M1/M2 discriminating system is critically needed to improve the knowledge about the macrophages phenotype and their potential in diagnostic and therapeutic use [23][24][41,42]. Unfortunately, it is quite difficult detect specific M1/M2 markers, both in vivo and in vitro, and no pure M1–M2 macrophage marker has yet been found [22][40]. It seems that M1 macrophages express M2 markers and vice versa. Moreover, in some inflammatory cases, M1-like or M2-like phenotypes could have specific markers expressed by both phenotypes.
Historically, the first studies on M1/M2 markers were practiced in mice with the help of complementary DNA (cDNA) subtraction [21][23][39,41] followed by human macrophage transcriptome profiling [25][26][27][28][43,44,45,46].
Jablonski et al. studied M1/M2 gene expression during their different activations using murine in vitro samples. Specifically, they performed different transcriptional messenger RNA (mRNA) and applied them to undifferentiated (M0), M1 and M2 murine macrophages, showing that M1 and M2 macrophages co-express many genes such as the transcription factors (TFs): Kruppel-like factor (Klf) 4 and activating transcription factor (Atf) 4. However, they also identified M1 and M2 specific genes: CD38, G protein-coupled receptor 18 (Gpr18) and formyl peptide receptor 2 (Fpr2) expressed by the M1 population and early-growth response gene 2 (Egr2) and c-myelocytomatosis oncogene product (c-Myc) expressed by the M2 macrophages [29][47].
Figure 1 shows macrophage gene expression comparing M1 vs. M0 (Figure 1a) and M2 vs. M0 (Figure 1b). The M0 macrophage population are quiescent nonactivated cells that, according to the environmental stimuli, can change their phenotype into M1 or M2 macrophages.
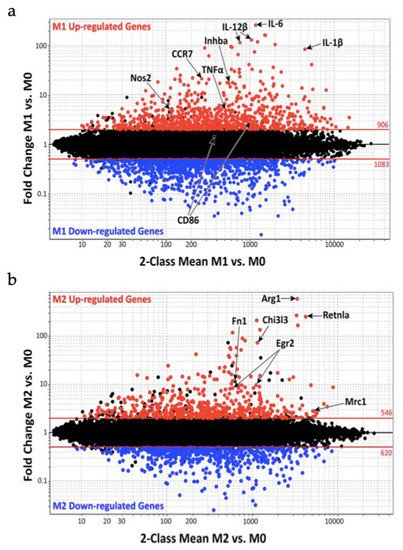
Figure 1. Macrophage gene expression during the classically activated M1 and alternatively activated M2 phenotypes: (a) genes up and down regulation during M1 activation; (b) genes up and down regulation during M2 activation.
Jablonski et al. noticed during M1 activation an increased expression of 629 genes and a decreased expression of 732 genes, whereas M2 activation was characterized by 388 upregulation genes. Furthermore, the study group compared their results with the already known mouse and human M1 markers finding 21 M1 common markers such as nitric oxide synthase 2 (Nos2), IL-1β, IL-6, IL-12β, CC chemokine Receptor 7 (CCR7), inhibin beta A (Inhba) and tumor necrosis factor α (TNF-α). The same study was made on M2 markers finding the same markers in Arginase 1 (Arg1), Chitinase 3-like-3 (Chi3l3/Ym1), Resistin-like molecule alpha (Retnla/Fizz1), Egr2, fibronectin 1 (Fn1) and mannose receptor C-type 1 (Mrc1/CD206) [29][47].
Figure 2 shows genes up- and/or downregulated by both M1 and M2 macrophages (upper-right and bottom-left quadrants). Distinct activated M1 or M2 genes are reported in the bottom-right and upper-left quadrants. These two sets of genes provide a promising group of M1 and M2 macrophage specific markers that may be used to distinguish these two populations during clinical practice [29][30][31][32][47,48,49,50].
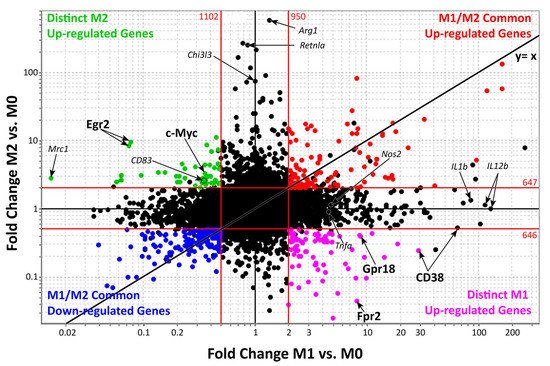
Figure 2.
Genes expressed during M1 and M2 macrophages activation.
2.2. PBMNCs and Macrophages Polarization
In PAD and, especially, in diabetic PAD patients, macrophages are mostly stopped into the M1 phenotype. It seems that in diabetic chronic wounds, the persistent activation of M1 macrophages induces a higher expression of pro-inflammatory cytokines, inducible NO synthase and metalloproteinase 9, perpetuating an inflammatory state and impairing new granulation tissue formation. The macrophages’ deficiency to switch from the M1 to M2 phenotype could be attributed to their impaired phagocytosis of apoptotic cells in the diabetic microenvironment [33][51].
Furthermore, macrophages found in chronic wounds showed a reduce ability to eliminate dead neutrophils. This process could cause the creation of a highly inflammatory state with an excess of inflammatory molecules such as TNF-α and IL-1β [34][35][52,53].
The consequent cellular and biochemical adaptations after PBMNC implantation favor the establishment of conditions similar to the physiological ones and progressively support the regeneration of damaged tissues and wound healing. This phenomenon has been measured biochemically as the inhibition of HIF, NF-KB and TNF-alpha; in the progressive polarization of M1 into M2 macrophages; the increase in VEGF and newly formed capillaries [9][27].
Moreover, by means of the histological examination of incisional biopsies after PBMNC treatment, it has been noticed that the perilesional area of diabetic nonhealing wounds had a more powerful polarization of M1 macrophages into the M2 phenotype [33][51].
Masuda et al. studied a method to obtain a quality- and quantity-control (QQ) culture of EPCs, assuming the paucity of stem cells in PBMNCs. The polarization of monocytes/macrophages from the M1 phenotype to M2 is usually marked by an increase in CD 206+ cells and a decrease in CCR2+ cells. In particular, they noticed that monocyte/macrophages in the QQ cultures have a higher tendency to the angiogenic and anti-inflammatory phenotype, improving the regenerative process during ischemia [34][52].
They also focalized their studies on the lymphocyte lineage cells during regeneration, noticing that B lymphocytes, NK cells and cytotoxic T cells significantly decrease or disappear during the regenerative phase, whereas helper T cells are the last disappearing lymphocyte population [36][54].
Over the last years, the interaction between monocytes/macrophages and T lymphocytes has been studied, discovering that IFN-c, produced by Th1 lymphocytes, induces the M1 phenotype, whereas IL-4, IL-13 and IL-10, produced by Th2 and regulatory T lymphocytes, induce the alternative activation of M2 macrophages. Moreover, M1 macrophages activate Th1 lymphocytes, secreting IL-12 and IL-6, whereas M2 macrophages improve Th2 and regulatory T-lymphocyte functions, producing IL-10 and TGF-b [36][54]. Finally, the interactions between M2 macrophages, Th2 lymphocytes and regulatory T cells improve and accelerate the angiogenic and anti-inflammatory phases in the QQ cultures [36][54].
3. PBMNCs and Paracrine Stimulation
PBMNCs release pleiotropic paracrine factors stimulating tissue regeneration, and maybe this is one of their strongest ways of contributing to angiogenesis [37][55].
Thum et al. proposed the idea that the anti-inflammatory response to stem cells depends on the fact that the 5–25% of the injected stem cells are apoptotic and, physiologically, apoptotic neutrophils switch the inflammatory response to the anti-inflammatory one, secreting TGF-β, PGE2 and inhibiting inflammatory mediators. It was demonstrated that the injection of paracrine factors released by apoptotic PBMNCs eases the myocardial damage and contributes the preservation of the myocardial function and microvascular perfusion [38][39][15,56].
The injection of PBMNCs is strongly associated with the secretion of paracrine and pro-angiogenic factors such as b-FGF, VEGF, HGF and angiopoietin-1 [19][37].
Rehman et al. analyzed EPC proliferation and surface marker expression by means of flow cytometry, finding that cultured EPCs upregulated monocyte activation and macrophage differentiation markers and secreted numerous growth factors such as VEGF, HGF, GCSF and GMCSF [40][57].
These results express the powerful way of PMNBCs and, especially, of monocytes to contribute to angiogenesis and wound regeneration thanks to their paracrine stimulation. Specifically, it was noticed that, especially in diabetic CLI patients, the CD14+ monocytes seemed to react much more to the hypoxic stimuli and had better paracrine action to stimulate angiogenesis in respect to CD34+ cells [41][58].
Recently, a study on the secretome of stressed PBMNCs was published. It was found that they improve short- and long-term cardiac performance in a porcine infarction model. The transcriptional analyses of the nonperfused and perfused heart 24 h after myocardial infarction showed a highly tissue-specific effect of the secretome and, except for the transition zone, a uniform downregulation of pro-inflammatory factors and pathways [40][57]. Simultaneously, the secretome strongly promoted the expression of genes that are essential for heart functioning in the nonperfused area [42][59].
Many studies on the secretome of γ-irradiated PBMCs have found that they are able to attenuate the hypoxia-induced cell damage in AMI and CLI, accelerating wound healing in a diabetic mouse model [43][60].
Moreover, it was discovered that the use of particular filtration systems allows PBMNCs to migrate in response to a certain gradient of VEGF and stromal-derived factor 1 “SDF-1”. Interestingly, filtration preserves and optimizes the release of paracrine factors, whereas it is significantly reduced when the cell concentrate is produced by centrifugation [7][25].
