SARS-CoV-2 virus can not only damage the respiratory system but may also pose a threat to other organs, such as the heart or vessels. This review focuses on cardiovascular complications of COVID-19, including acute cardiac injury, arrhythmias, biomarkers, accompanying comorbidities and outcomes in patients diagnosed with SARS-CoV-2 infection. The results show that cardiac injury is present in about 1 in 4 patients with COVID-19 disease, and it is an independent risk factor, which multiplies the death rate several times in comparison to infected patients without myocardial injury. New-onset cardiac injury occurs in nearly every 10th patient of the COVID-19-suffering population. Comorbidities (such as hypertension, cardiovascular disease and diabetes) severely deteriorate the outcome. Therefore, patients with SARS-CoV-2 infection should be carefully assessed in terms of cardiac medical history and possible cardiological complications.
- coronavirus disease 2019
- cardiac injury
- troponin
- cardiac complications
- prognosis
1. Cardiac Injury
1.1. Frequency and Characteristic
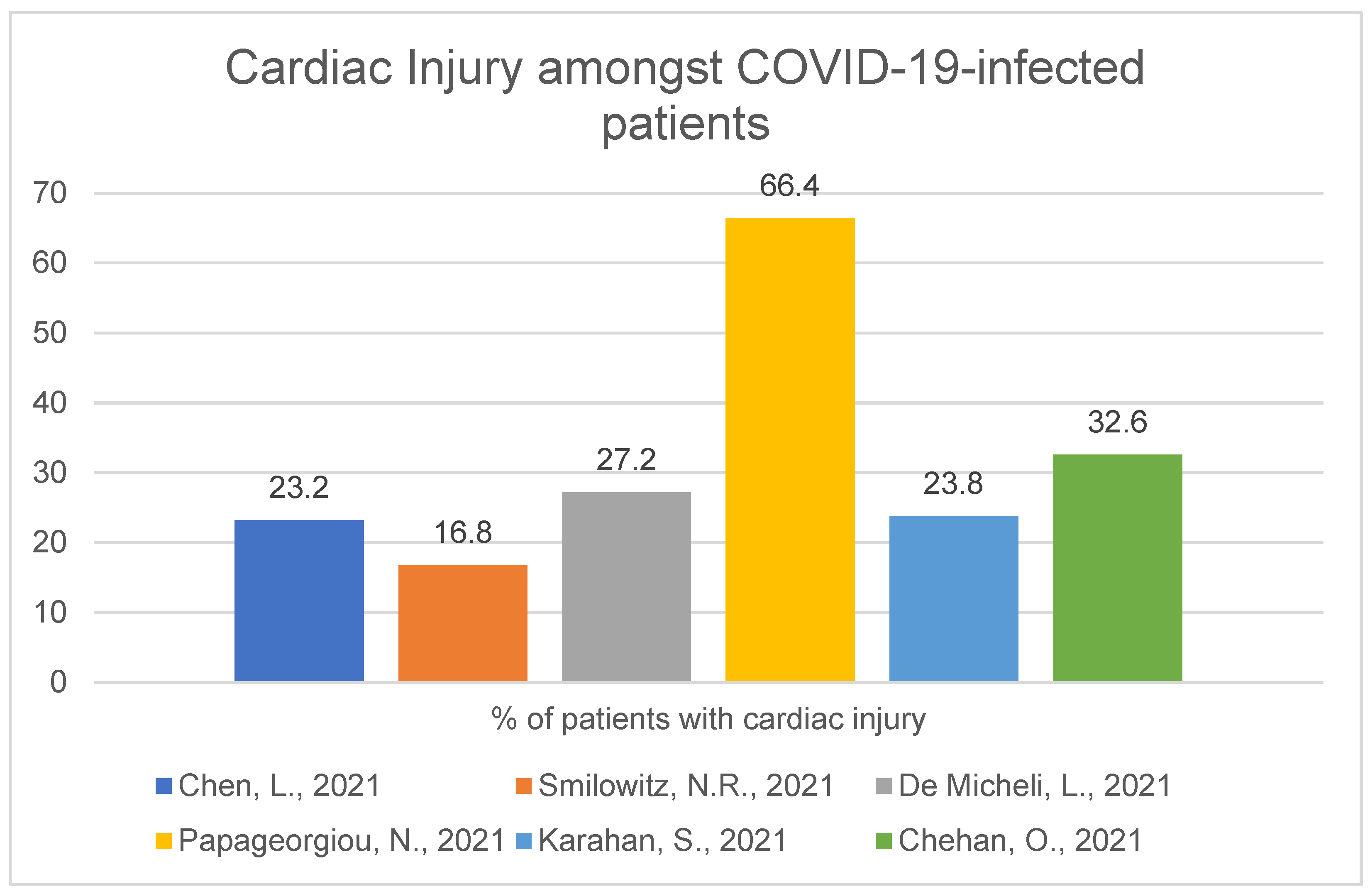
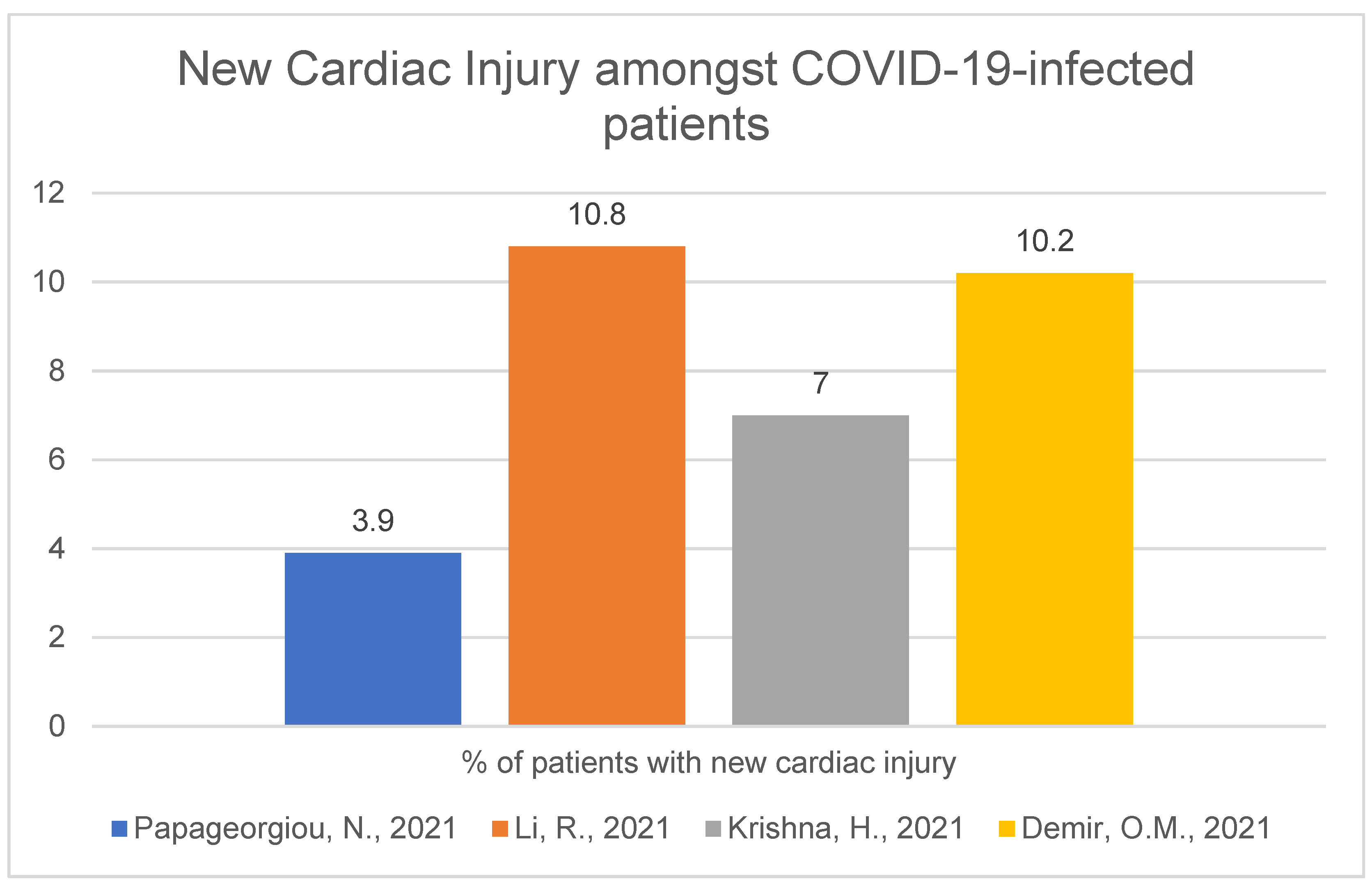
1.2. Hospitalisation and Outcome
1.1.3. Myocardial Infarction
1.3. Myocardial Infarction
1.1.4. Myocarditis
1.4. Myocarditis
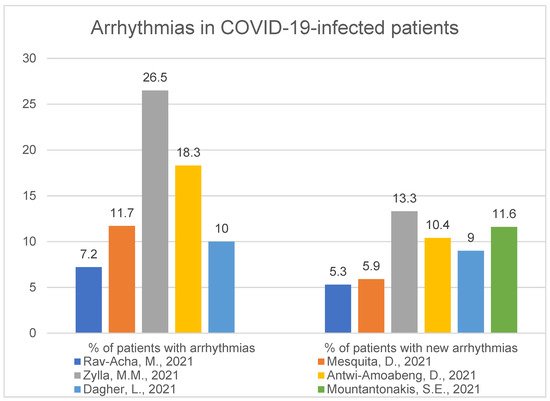
2. Arrhythmias
2.1. Hospitalisation and Outcome
2.1. Hospitalisation and Outcome
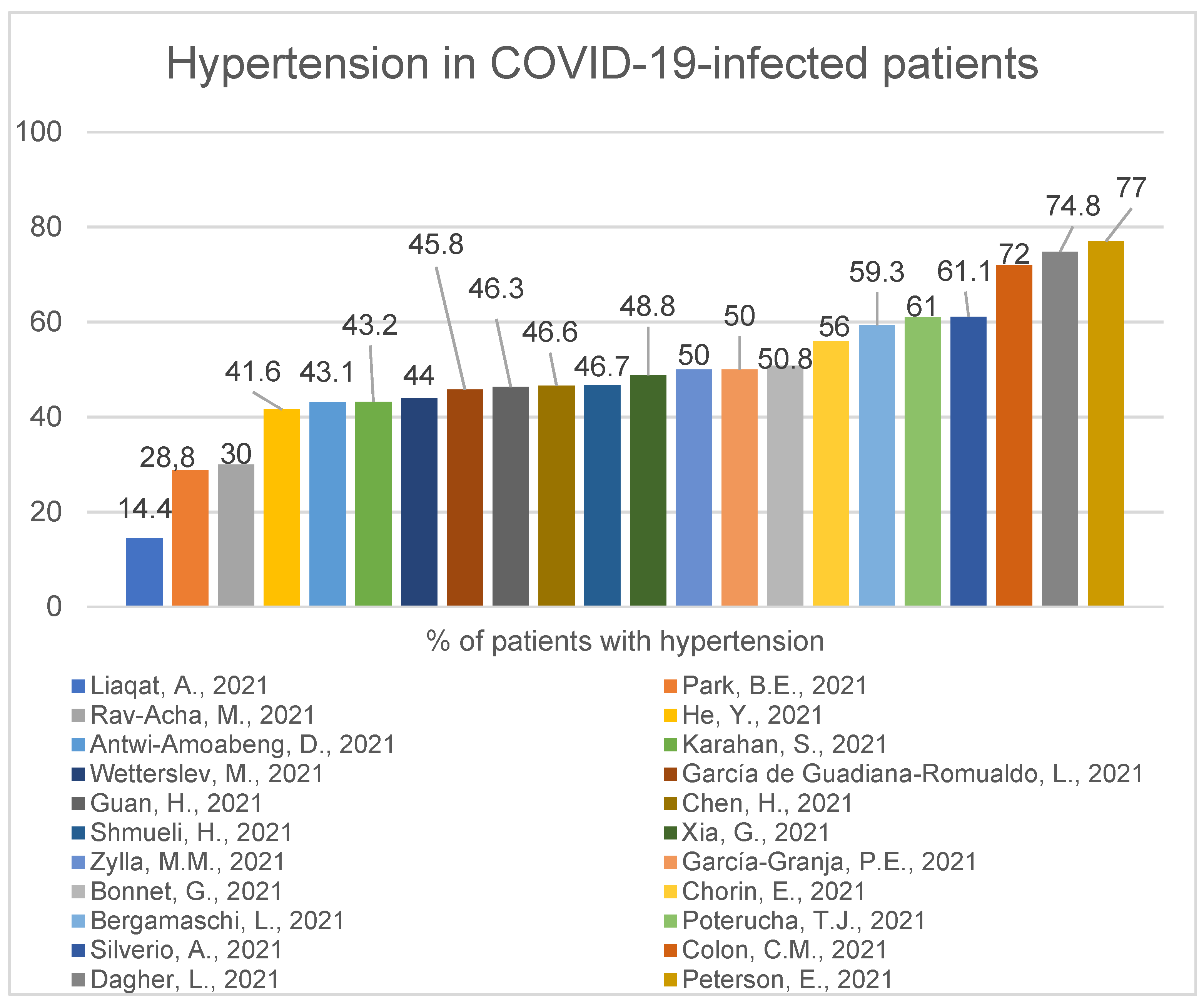
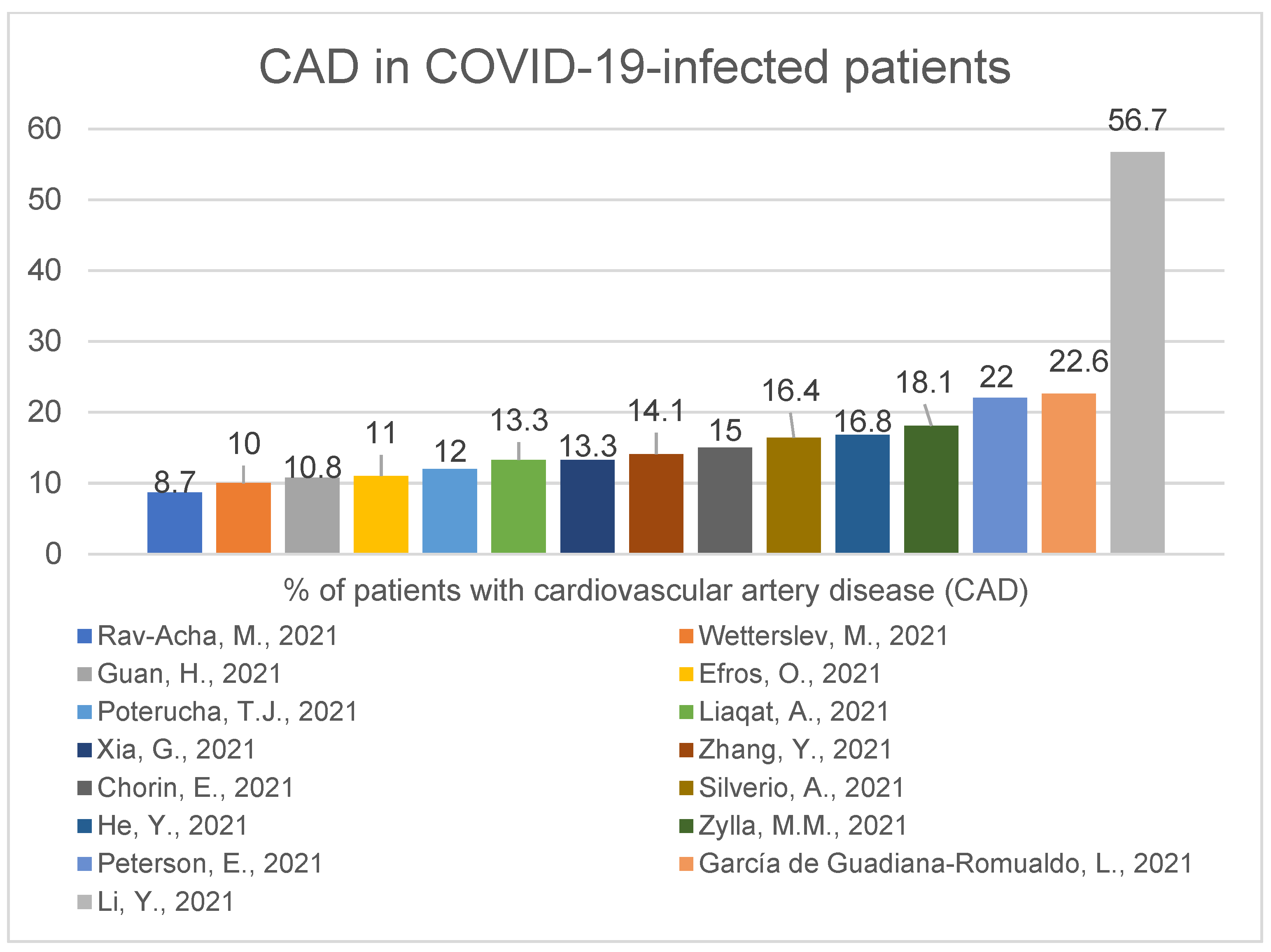
2.2. Accompanying Comorbidities

References
- Chen, L.; Hu, W.; Guo, X.; Zhao, P.; Tang, J.; Gu, Y.; Huang, N.; Wang, C.; Cui, A.; Zhang, D.; et al. Association of coagulation dysfunction with cardiac injury among hospitalized patients with COVID-19. Sci. Rep. 2021, 11, 4432.
- Smilowitz, N.R.; Nguy, V.; Aphinyanaphongs, Y.; Newman, J.D.; Xia, Y.; Reynolds, H.R.; Hochman, J.S.; Fishman, G.I.; Berger, J.S. Multiple Biomarker Approach to Risk Stratification in COVID-19. Circulation 2021, 143, 1338–1340.
- De Michieli, L.; Babuin, L.; Vigolo, S.; De Marinis, G.B.; Lunardon, A.; Favretto, F.; Lobo, R.; Sandoval, Y.; Bryant, S.C.; Donato, D.; et al. Using high sensitivity cardiac troponin values in patients with SARS-CoV-2 infection (COVID-19): The Padova experience. Clin Biochem. 2021, 90, 8–14.
- Papageorgiou, N.; Sohrabi, C.; Merino, D.P.; Tyrlis, A.; Atieh, A.E.; Saberwal, B.; Lim, W.-Y.; Creta, A.; Khanji, M.; Rusinova, R.; et al. High sensitivity troponin and COVID-19 outcomes. Acta Cardiol. 2021, 8, 1–8.
- Karahan, S.; Katkat, F.; Ozcan, S.; Sahin, I.; Okuyan, E. Impact of acute myocardial injury on prognosis in patients with COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2425–2434.
- Chehab, O.; El Zein, S.; Kanj, A.; Moghrabi, A.; Sebastian, J.; Halboni, A.; Alkassis, S.; El-Hor, N.; Briasoulis, A.; Liberman, R.; et al. SARS-CoV-2 Viral Load and Cardiac Injury are Independent and Incremental Predictors of Adverse Outcome. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 891–897.
- Liaqat, A.; Ali-Khan, R.S.; Asad, M.; Rafique, Z. Evaluation of myocardial injury patterns and ST changes among critical and non-critical patients with coronavirus-19 disease. Sci Rep. 2021, 11, 4828.
- Xia, G.; Fan, D.; Ma, C.; He, Y.; Wang, M.; Zhu, Y.; Zheng, Q. Hyper-Inflammatory Response Involves in Cardiac Injury Among Patients With Coronavirus Disease 2019. Am. J. Med. Sci. 2021, 361, 718–724.
- Metkus, T.S.; Sokoll, L.J.; Barth, A.S.; Czarny, M.J.; Hays, A.G.; Lowenstein, C.J.; Michos, E.D.; Nolley, E.P.; Post, W.S.; Resar, J.R.; et al. Myocardial Injury in Severe COVID-19 Compared with Non-COVID-19 Acute Respiratory Distress Syndrome. Circulation 2021, 143, 553–565.
- Bonnet, G.; Weizman, O.; Trimaille, A.; Pommier, T.; Cellier, J.; Geneste, L.; Panagides, V.; Marsou, W.; Deney, A.; Attou, S.; et al. Characteristics and outcomes of patients hospitalized for COVID-19 in France: The Critical COVID-19 France (CCF) study. Arch. Cardiovasc. Dis. 2021, 114, 352–363.
- Li, R.; Wang, H.; Ma, F.; Cui, G.-L.; Peng, L.-Y.; Li, C.-Z.; Zeng, H.-S.; Marian, A.J.; Wang, D.-W. Widespread myocardial dysfunction in COVID-19 patients detected by myocardial strain imaging using 2-D speckle-tracking echocardiography. Acta Pharmacol. Sin. 2021, 28, 1–8.
- Krishna, H.; Ryu, A.J.; Scott, C.G.; Mandale, D.R.; Naqvi, T.Z.; Pellikka, P.A. Cardiac Abnormalities in COVID-19 and Relationship to Outcome. Mayo Clin. Proc. 2021, 96, 932–942.
- Demir, O.M.; Ryan, M.; Cirillo, C.; Desai, N.; Pericao, A.; Sinclair, H.; Stylianidis, V.; Victor, K.; Alaour, B.; Jones, A.; et al. Impact and Determinants of High-Sensitivity Cardiac Troponin-T Concentration in Patients with COVID-19 Admitted to Critical Care. Am. J. Cardiol. 2021, 147, 129–136.
- Hendrickson, B.S.; Stephens, R.E.; Chang, J.V.; Amburn, J.M.; Pierotti, L.L.; Johnson, J.L.; Hyden, J.C.; Johnson, J.N.; Philip, R.R. Cardiovascular Evaluation After COVID-19 in 137 Collegiate Athletes: Results of an Algorithm-Guided Screening. Circulation 2021, 143, 1926–1928.
- Xie, Y.; Wang, L.; Li, M.; Li, H.; Zhu, S.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; et al. Biventricular Longitudinal Strain Predict Mortality in COVID-19 Patients. Front. Cardiovasc. Med. 2021, 7, 632434.
- He, J.; Zhang, B.; Zhou, Q.; Yang, W.; Xu, J.; Liu, T.; Zhang, H.; Wu, Z.; Li, D.; Zhou, Q.; et al. The Prognostic Value of Myocardial Injury in COVID-19 Patients and Associated Characteristics. Immun. Inflamm. Dis. 2021, 9, 1358–1369.
- Zhang, Y.; Sun, W.; Wu, C.; Zhang, Y.; Cui, L.; Xie, Y.; Wang, B.; He, L.; Yuan, H.; Zhang, Y.; et al. Prognostic Value of Right Ventricular Ejection Fraction Assessed by 3D Echocardiography in COVID-19 Patients. Front. Cardiovasc. Med. 2021, 8, 641088.
- Silverio, A.; Di Maio, M.; Scudiero, F.; Russo, V.; Esposito, L.; Attena, E.; Pezzullo, S.; Parodi, G.; D’Andrea, A.; Damato, A.; et al. Clinical conditions and echocardiographic parameters associated with mortality in COVID-19. Eur. J. Clin. Investig. 2021, 51, e13638.
- Zhou, Z.; Ryan, J.; Ernst, M.E.; Zoungas, S.; Tonkin, A.M.; Woods, R.L.; McNeil, J.J.; Reid, C.M.; Curtis, A.J.; Wolfe, R.; et al. Effect of Statin Therapy on Cognitive Decline and Incident Dementia in Older Adults. J. Am. Coll. Cardiol. 2021, 77, 3145–3156.
- Huang, J.; Wang, W.-J.; Yu, H.; Xu, J.; Wu, H.; Wang, C.; Gu, C.-H.; Li, H.-J.; Li, M.; Liu, C.; et al. Pre-existing Health Conditions and Epicardial Adipose Tissue Volume: Potential Risk Factors for Myocardial Injury in COVID-19 Patients. Front. Cardiovasc. Med. 2021, 7, 585220.
- Chen, H.; Li, X.; Marmar, T.; Xu, Q.; Tu, J.; Li, T.; Han, J.; Xu, D.; Shen, T. Cardiac Troponin I association with critical illness and death risk in 726 seriously ill COVID-19 patients: A retrospective cohort study. Int. J. Med. Sci. 2021, 18, 1474–1483.
- García de Guadiana-Romualdo, L.; Morell-García, D.; Morales-Indiano, C.; Bauça, J.M.; José Alcaide Martín, M.; Del Valle, C.E.; Gutiérrez Revilla, J.I.; Urrechaga, E.; Álamo, J.M.; Hernando Holgado, A.M.; et al. Characteristics and laboratory findings on admission to the emergency department among 2873 hospitalized patients with COVID-19: The impact of adjusted laboratory tests in multicenter studies. A multicenter study in Spain (BIOCOVID-Spain study). Scand. J. Clin. Lab. Investig. 2021, 81, 187–193.
- Wang, Y.; Shu, H.; Liu, H.; Li, X.; Zhou, X.; Zou, X.; Pan, S.; Xu, J.; Xu, D.; Zhao, X.; et al. The peak levels of highly sensitive troponin I predicts in-hospital mortality in COVID-19 patients with cardiac injury: A retrospective study. Eur. Heart J. Acute Cardiovasc. Care. 2021, 10, 6–15.
- He, Y.; Zheng, X.; Li, X.; Jiang, X. Key factors leading to fatal outcomes in COVID-19 patients with cardiac injury. Sci. Rep. 2021, 11, 4144.
- Peiró, Ó.M.; Carrasquer, A.; Sánchez-Gimenez, R.; Lal-Trehan, N.; Del-Moral-Ronda, V.; Bonet, G.; Fort-Gallifa, I.; Picó-Plana, E.; Bastón-Paz, N.; Gutiérrez, C.; et al. Biomarkers and short-term prognosis in COVID-19. Biomarkers 2021, 26, 119–126.
- Efros, O.; Barda, N.; Meisel, E.; Leibowitz, A.; Fardman, A.; Rahav, G.; Klempfner, R.; Grossmanet, E. Myocardial injury in hospitalized patients with COVID-19 infection-Risk factors and outcomes. PLoS ONE 2021, 16, e0247800.
- Poterucha, T.J.; Elias, P.; Jain, S.S.; Sayer, G.; Redfors, B.; Burkhoff, D.; Rosenblum, H.; DeFilippis, E.M.; Gupta, A.; Lawlor, M.; et al. Admission Cardiac Diagnostic Testing with Electrocardiography and Troponin Measurement Prognosticates Increased 30-Day Mortality in COVID-19. J. Am. Heart Assoc. 2021, 10, e018476.
- Briscoe, M.; Sykes, R.; Krystofiak, T.; Peck, O.; Mangion, K.; Berry, C. Clinical significance of coronavirus disease 2019 in hospitalized patients with myocardial injury. Clin. Cardiol. 2021, 44, 332–339.
- Laganà, N.; Cei, M.; Evangelista, I.; Cerutti, S.; Colombo, A.; Conte, L.; Mormina, E.; Rotiroti, G.; Giovanni Versace, A.; Porta, C.; et al. Suspected myocarditis in patients with COVID-19: A multicenter case series. Medicine 2021, 100, e24552.
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021, 42, 1866–1878.
- Martinez, M.W.; Tucker, A.M.; Bloom, O.J.; Green, G.; DiFiori, J.P.; Solomon, G.; Phelan, D.; Kim, J.H.; Meeuwisse, W.; Sills, A.K.; et al. Prevalence of Inflammatory Heart Disease Among Professional Athletes with Prior COVID-19 Infection Who Received Systematic Return-to-Play Cardiac Screening. JAMA Cardiol. 2021, 6, 745–752.
- Clark, D.E.; Parikh, A.; Dendy, J.M.; Diamond, A.B.; George-Durrett, K.; Fish, F.A.; Slaughter, J.C.; Fitch, W.; Hughes, S.G.; Soslowet, J.H.; et al. COVID-19 Myocardial Pathology Evaluation in Athletes with Cardiac Magnetic Resonance (COMPETE CMR). Circulation 2021, 143, 609–612.
- Rav-Acha, M.; Orlev, A.; Itzhaki, I.; Zimmerman, S.F.; Fteiha, B.; Bohm, D.; Kurd, R.; Samuel, T.Y.; Asher, E.; Helviz, Y.; et al. Cardiac arrhythmias amongst hospitalised Coronavirus 2019 (COVID-19) patients: Prevalence, characterisation, and clinical algorithm to classify arrhythmic risk. Int. J. Clin. Pract. 2021, 75, e13788.
- Mesquita, D.; Carmo, P.; Cabanelas, N.; Santos, N.; Martins, V.; Sanfins, V.; Costa, H.C.; Fontes, J.P.; Fonseca, P.; Parreira, L.; et al. Cardiac arrhythmias in patients presenting with COVID-19 treated in Portuguese hospitals: A national registry from the Portuguese Association of Arrhythmology, Pacing and Electrophysiology. Rev. Port. Cardiol. 2021, 40, 573–580.
- Zylla, M.M.; Merle, U.; Vey, J.A.; Korosoglou, G.; Hofmann, E.; Müller, M.; Herth, F.; Schmidt, W.; Blessing, E.; Göggelmann, C.; et al. Predictors and Prognostic Implications of Cardiac Arrhythmias in Patients Hospitalized for COVID-19. J. Clin. Med. 2021, 10, 133.
- Antwi-Amoabeng, D.; Beutler, B.D.; Singh, S.; Taha, M.; Ghuman, J.; Hanfy, A.; Manasewitsch, N.T.; Ulanja, M.B.; Ghuman, J.; Awad, M.; et al. Association between electrocardiographic features and mortality in COVID-19 patients. Ann. Noninvasive Electrocardiol. 2021, 26, e12833.
- Dagher, L.; Shi, H.; Zhao, Y.; Wetherbie, A.; Johnsen, E.; Sangani, D.; Nedunchezhian, S.; Brown, M.; Miller, P.; Denson, J.; et al. New-onset atrial arrhythmias associated with mortality in black and white patients hospitalized with COVID-19. Pacing Clin. Electrophysiol. 2021, 44, 856–864.
- Northwell COVID-19 Research Consortium; Mountantonakis, S.E.; Saleh, M.; Fishbein, J.; Gandomi, A.; Lesser, M.; Chelico, J.; Gabriels, J.; Qiu, M.; Epstein, L.M. Atrial fibrillation is an independent predictor for in-hospital mortality in patients admitted with SARS-CoV-2 infection. Heart Rhythm 2021, 18, 501–507.
- García-Granja, P.E.; Veras, C.; Aparisi, Á.; Amat Santos, I.J.; Catalá, P.; Marcos, M.; Cabezón, G.; Candela, J.; Gil, J.F.; Uribarri, A.; et al. Atrial fibrillation in patients with SARS-CoV-2 infection. Med. Clin. 2021, 157, 58–63.
- Pardo Sanz, A.; Salido Tahoces, L.; Ortega Pérez, R.; González Ferrer, E.; Sánchez Recalde, A.; Zamorano Gómez, J.L. New-onset atrial fibrillation during COVID-19 infection predicts poor prognosis. Cardiol. J. 2021, 28, 34–40.
- Guan, H.; Liu, J.; Ding, J.; Liu, W.; Feng, Y.; Bao, Y.; Li, H.; Wang, X.; Zhou, Z.; Chen, Z. Arrhythmias in patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: Incidences and implications. J. Electrocardiol. 2021, 65, 96–101.
- Yarmohammadi, H.; Morrow, J.P.; Dizon, J.; Biviano, A.; Ehlert, F.; Saluja, D.; Waase, M.; Elias, P.; Poterucha, T.J.; Berman, J.; et al. Frequency of Atrial Arrhythmia in Hospitalized Patients With COVID-19. Am. J. Cardiol. 2021, 147, 52–57.
- Bergamaschi, L.; D’Angelo, E.C.; Paolisso, P.; Toniolo, S.; Fabrizio, M.; Angeli, F.; Donati, F.; Magnani, I.; Rinaldi, A.; Bartoli, L.; et al. The value of ECG changes in risk stratification of COVID-19 patients. Ann. Noninvasive Electrocardiol. 2021, 26, e12815.
- Han, K.-Y.; Qiao, Q.; Zhu, Y.-Q.; Chen, X.-G.; Kang, X.-X.; Zhang, G.-F.; Cai, X.-C.; Du, Y.; Jin, J.; Di, R.-M.; et al. Atrial Arrhythmias in Patients with Severe COVID-19. Cardiol. Res. Pract. 2021, 2021, 8874450.
- Park, B.E.; Lee, J.H.; Park, H.K.; Kim, H.N.; Jang, S.Y.; Bae, M.H.; Yang, D.H.; Park, H.S.; Cho, Y.; Lee, B.Y.; et al. Impact of Cardiovascular Risk Factors and Cardiovascular Diseases on Outcomes in Patients Hospitalized with COVID-19 in Daegu Metropolitan City. J. Korean Med. Sci. 2021, 36, e15.
- Peterson, E.; Lo, K.B.; DeJoy, R.; Salacup, G.; Pelayo, J.; Bhargav, R.; Gul, F.; Albano, J.; Azmaiparashvili, Z.; Amanullah, A.; et al. The relationship between coronary artery disease and clinical outcomes in COVID-19: A single-center retrospective analysis. Coron. Artery Dis. 2021, 32, 367–371.
- Bhatt, A.S.; Jering, K.S.; Vaduganathan, M.; Claggett, B.L.; Cunningham, J.W.; Rosenthal, N.; Signorovitch, J.; Thune, J.J.; Vardeny, O.; Solomon, S.D. Clinical Outcomes in Patients with Heart Failure Hospitalized with COVID-19. JACC Heart Fail. 2021, 9, 65–73.
- Li, Y.; Fang, L.; Zhu, S.; Xie, Y.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; Wu, C.; et al. Echocardiographic Characteristics and Outcome in Patients With COVID-19 Infection and Underlying Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 642973.
- Tuo, H.; Li, W.; Tang, L.; He, B.; Yao, B.; Mao, P.; Tang, Q. Cardiac Biomarker Abnormalities Are Closely Related to Prognosis in Patients with COVID-19. Int. Heart J. 2021, 62, 148–152.
- Chorin, E.; Dai, M.; Kogan, E.; Wadhwani, L.; Shulman, E.; Nadeau-Routhier, C.; Knotts, R.; Bar-Cohen, R.; Barbhaiya, C.; Aizer, A.; et al. Electrocardiographic Risk Stratification in COVID-19 Patients. Front. Cardiovasc. Med. 2021, 8, 636073.
- Shmueli, H.; Shah, M.; Ebinger, J.E.; Nguyen, L.C.; Chernomordik, F.; Flint, N.; Botting, P.; Siegel, R.J. Left ventricular global longitudinal strain in identifying subclinical myocardial dysfunction among patients hospitalized with COVID-19. Int. J. Cardiol. Heart Vasc. 2021, 32, 100719.
- Wetterslev, M.; Jacobsen, P.K.; Hassager, C.; Jøns, C.; Risum, N.; Pehrson, S.; Bastiansen, A.; Andreasen, A.S.; Kristiansen, K.T.; Bestle, M.H.; et al. Cardiac arrhythmias in critically ill patients with coronavirus disease 2019: A retrospective population-based cohort study. Acta Anaesthesiol. Scand. 2021, 65, 770–777.
- Colon, C.M.; Barrios, J.G.; Chiles, J.W.; Brown, T.M.; Pogwizd, S.M.; McElwee, S.K.; Gandotra, S.; Russell, D.W.; McElderry, H.T.; Maddox, W.R. Atrial arrhythmia related outcomes in critically ill COVID-19 patients. Pacing Clin. Electrophysiol. 2021, 44, 814–823.