Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Joanna Gadzinowska.
The brain and gut, through the microbiota, can influence each other’s functions via neuroendocrine, neuroimmune and sensory-neural molecular pathways. Moreover, both hypothalamic-pituitary-adrenal (HPA) axis and gut peptides might be involved in this communication system.
- microbiome-brain-gut axis
- gut-brain axis
- microbiota
1. What Is the Gut Microbiota?
1. Gut Microbiota
Gut microbiota is a complex and highly diverse community of trillions of microorganisms that live in the digestive tracts of humans and animals, including insects [11,12][1][2]. Microbiota are ten times more abundant than our the somatic and germ line cells of the body [13][3]. The human gut microbiota consists of several types of microbes including bacteria, archaea, eukarya, viruses and parasites [14][4] that weigh approximately 1 kg and represents the first protection system of the gastrointestinal (GI) apparatus. The microenvironment of the gut favors the growth of bacteria from seven predominant divisions (Firmicutes, Bacteroidetes, Actinobacteria, Fusobacteria, Proteobacteria, Verrucomicrobia and Cyanobacteria) [15][5]. Among these, more than 90% of the total population is made up of the Bacteroidetes and Firmicutes [13][3]. The presence of the microbiota differs within the parts of the GI tract, from few micro-organisms in the stomach and small intestine, up to a concentration of approximately 1012 bacteria in the colon [16,17][6][7]. In humans, the gut microbiota has the biggest quantities of microorganisms, and the greatest number of species compared to other parts of the body [18][8]. Microbiota acquired at birth develop in parallel with the host and maintains its temporal stability and diversity through adulthood until death [19][9]. The gut microbiota forms an integral part of the human body [13][3] and plays a significant role in its normal functioning [11][1]. Though the gut microbiota is dynamic, it performs some basic immunological, metabolic, structural and neurological functions [13][3]. The metabolic role consists of the conversion of dietary elements into bioactive food components [8][10]. The gut microbiota scavenge about 10–30% of energy from the dietary fibers in the colon and the rest is excreted as feces [20][11]. Gut microbes possess an array of enzymes enabling the utilization of carbohydrates resistant to digestion by host digestive enzymes such as lignin, non-starch polysaccharides, resistant starch and oligosaccharides. Gut microbiota of the lower intestines ferments all of the dietary fibers, which results in the release of gases, short chain fatty acids (SCFAs), organic acids, and alcohols. SCFAs, the most prevalent being acetate, propionate and butyrate, meet about 10% of caloric demand of the host [21][12] and their main producers are Roseburia spp., Eubacterium rectale, Faecalibacterium prausnitzii and Clostridium spp. [22][13]. SCFAs also have promising anti-inflammatory and chemo-preventive properties [23][14]. According to the MEROPS database, gut microbiota have various peptidase and protease enzymes. Clostridium spp., Bacteroides spp. and Lactobacillus spp. are of special importance because of the diversity of possessed enzymes [24][15]. Some gut bacteria take part in the transformation of bile acids—their bile salt hydrolase deconjugates the unabsorbed bile salt and produces deoxycholate, ursodeoxycholate and lithocholate [8][10]. Gut microbiota executes its protective role by occupying intestinal surfaces and preventing the invasion of pathogenic microorganisms through creating a stable system. The epithelial cells of the mucosal barrier use the SCFAs as an energy source. Gut microbiota exhibit a significant impact on bone growth and development though SCFAs, regulation of calcium and phosphorus absorption from the diet and immunoregulation by the Lactobacillus spp. of the osteoclast-osteoblast mediated bone remodeling process [8][10]. Gut microbiota can control both the central and enteric nervous system through various mechanisms such as the production and expression of neurotransmitters and neurotrophic factors, modulating the enteric sensory afferents, metabolite production, immunoregulation of mucosa and maintaining the integrity of the intestinal barrier and tight junctions. There are several factors that can change the gut microbiota composition and function. Numerous studies have indicated that host genetics influences the composition of the gut microbiome [25][16]. Pattern recognition receptors modulate microbiome composition and the diseases associated with it. After birth, gut microbiota is shaped mainly by diet as the microbiome adjust to absorbed nutrients. Firstly, it is enriched in genes involved in the metabolism of breast milk’s oligosaccharides, whereas later in the ones associated with the digestion of polysaccharides and vitamins [26][17]. The method of feeding the newborn significantly influences the microbiome composition—breast-fed infants exhibit an overgrowth of Actinobacteria and an inhibition of Firmicutes and Proteobacteria, whereas formula-fed infants experience an increase of Clostridium, Streptococci, Bacteroides and Enterobacteriaceae [27][18]. Vegetarians exhibit the dominance of Firmicutes and Bacteroidetes [8][10]. The abundance of bile-tolerant species (Bacteroides, Bilophila and Alistipes) and suppression of Firmicutes have been correlated with a diet rich in protein and fats. Another factors significantly affecting the microbiota composition is age. The first year of age is considered to be the most important period of development. Taxonomic diversity is low at birth, but increases over time. Firmicutes and Bacteroidetes are dominating the adult gut microbiota, while the elderly exhibit a decrease in Bacteroidetes to Firmicutes ratio, a reduction in Bifidobacterium, amylolytic activity and SCFAs production and an abundance of Enterobacteriaceae [28][19]. Exercise increases the diversity of microflora by both internal and external factors such as overall healthy lifestyle, intrinsic adaptation to training, lower levels of inflammation, reduced morbidity and improved metabolic markers. Greater amounts of Firmicutes and a lower amount of Bacteroidetes were found in athletes as compared to non-athletes [8][10]. What is more, the antibiotics destroy both pathogenic and beneficial microbes causing dysbiosis—a disturbance of gut microbiota [29][20]. The kind of antibiotic and the length of the treatment are associated with the effect on gut microbiota. Moreover, studies have demonstrated an impact of smoking on microbiota composition, with the most significant impact observed in the oral cavity [30][21]. The disturbance of the gut microbiota population associated with the alteration of the microbial composition was proven to be related with diverse pathological conditions, i.e., inflammatory bowel diseases (IBD) [31][22], obesity and diabetes [32][23], allergy [33][24], autoimmune diseases [34][25] and cardiovascular disease [35][26]. Examples of changes in the composition of gut microbiota correlated with various diseases are shown in Table 1.Table 1. Correlation of diseases with the changes in gut microbiota composition.
| Disease | Paper | Increase | Decrease | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irritable bowel syndrome | Jeffery et al. (2012) [36] | Jeffery et al. (2012) [27] | Firmicutes especially | Clostridium | , | Ruminococcus | and | Dorea | a | Ruminococcus albus | , | Bacteroides fragilis | , | Bacteroides vulgatus | and | Ruminococcus callidus | a | ||
| Inflammatory bowel disease (IBD) | 107] | -1 [42][43] | Nishida et al. (2018) [37] | Nishida et al. (2018) [28] | Mucolytic bacteria ( | L-cells | aRuminococcus gnavas, Ruminococcus torques | ), sulfate-reducing bacteria ( | Desulfovibrio | ), pathogenic bacteria (adhesion/invasive | Escherichia coli | ) | Firmicutes, SCFA-producing bacteria ( | Clostridium | food intake cluster IV, XIVa, XVII and | Faecalibacterium prausnitzzi | ) | ||
| stimulation of insulin release and inhibition of glucagon secretion | modulation of the HPA | axis and response to | stress |
Obesity | |||||||||||||||
| CCK [108,109] | [44][45] | Le Chatelier et al. (2013) [38] | Le Chatelier et al. (2013) [29] | Porphyromonas, Campylobacter, Bacteroides, Staphylococcus, Parabacteroides, Dialister | and | Ruminococcus | Lactobacillus, Bifidobacterium, Faecalibacterium | I-cells | a | , | Akkermansia | , | Methanobrevibacter | and | Coprococcus | ||||
| food intake | suppression of appetite, gastric emptying, gallbladder contraction, | pancreatic enzymes release | increased anxiety-like behavior |
Insulin resistance and Diabetes mellitus type 2 | Munoz-Garach et al. (2016) [39] | Munoz-Garach et al. (2016) [30] | Firmicutes, | Lactobacillus gasseri, Streptococcus mutans, Escherichia coli | Bacteroidetes, | Roseburia | , | Eubacterium halli, Faecalibacterium prauznitzi | |||||||
| Hypertension | Dan et al. (2019) [40] | Dan et al. (2019) [31] | Acetobacteroides, Alistipes, Bacteroides, Christensenella, Clostridium | sensu stricto, | Desulfovibrio, Parabacteroides | Acetobacteroides, Clostridium, Coprobacter, Enterococcus, Enterorhabdus, Lachnospiracea, Lactobacillus, Paraprevotella, Prevotella Romboutsia, Ruminococcus, Veillonella | |||||||||||||
| Asthma | O’Connor et al. (2018) [41] | O’Connor et al. (2018) [32] | Bifidobacterium adolescentis | Staphylococcus aureus, Faecalibacterium prausnitzii | and | Clostridium | |||||||||||||
| Autistic spectrum disorder | Strati et al. (2017) [42] | Strati et al. (2017) [33] | Collinsella, Corynebacterium, Dorea | and | Lactobacillus | Alistipes, Bilophila, Dialister, Parabacteroides | and | Veillonella |
2. Relationship Between Alterations in Gut Microbiota and Depression?
The association between gut microbiota and depressive disorder has been the subject of many studies conducted in recent years. The complex mechanism, which might allow this bidirectional communication between intestines and the brain, is explained via the microbiota-gut-brain axis [99][34]. This pathway includes the immune, endocrine and autonomic system as well as molecules originating from the microbiota that take part in the regulation of these interactions (Figure 2). Alterations in gut microbiota are not considered to be the main factor that leads to depression. However, they are an important part.
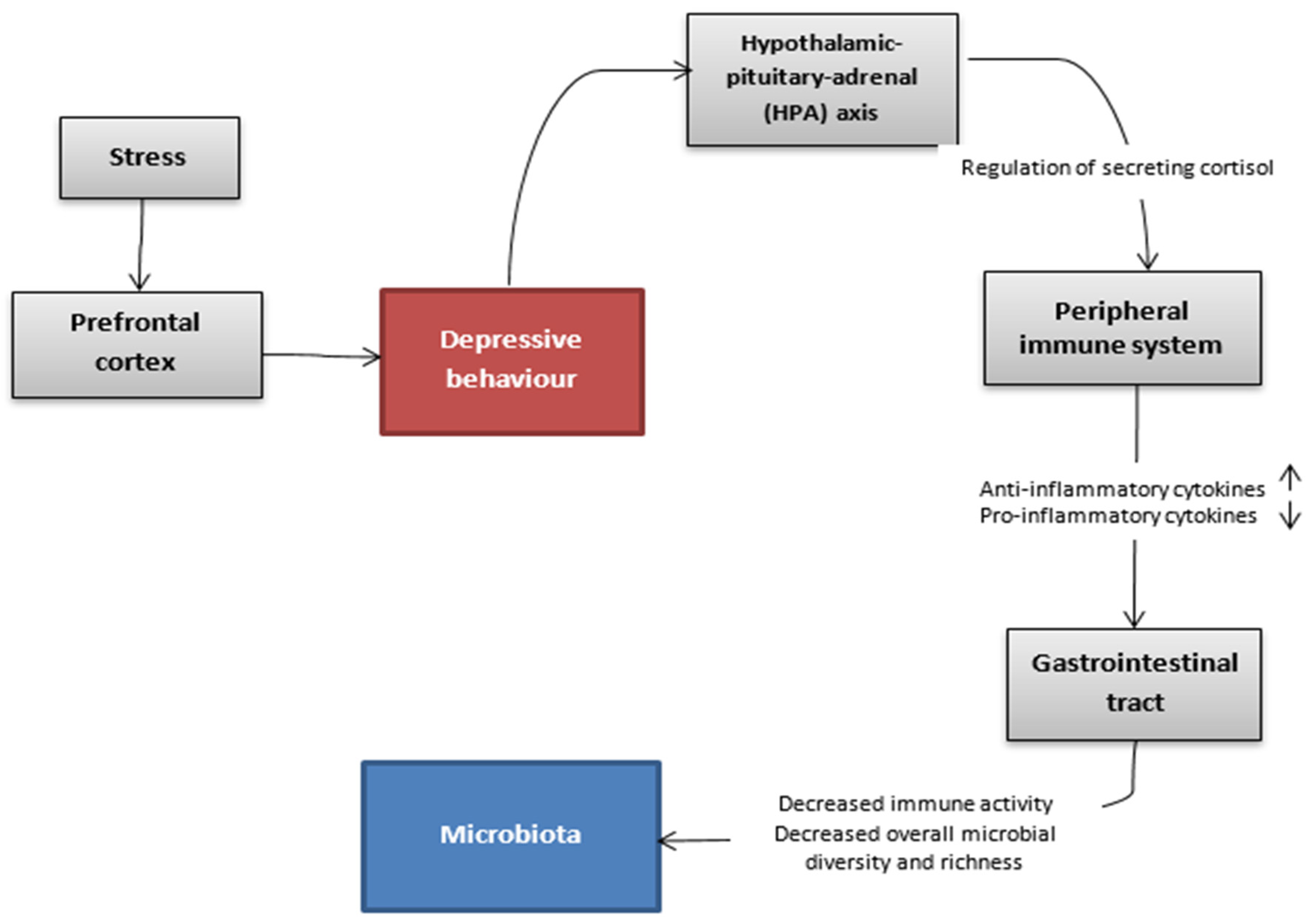
Figure 2. Hypothetic communication pathways between the gut microbiota and the brain in the depressive state.
What wresearchers know is that rodents suffering from depression have a changed ratio in profitable bacteria such as Bacteroides and Firmicutes compared to healthy ones. According to the meta-analysis, bacteria from the families Veillonellaceae, Prevotellaceae and Sutterellaceae were less numerous in patients with MDD than in healthy controls, although Actinomycetaceae were more abundant in patients with MDD than in healthy controls. In addition, the same meta-analysis showed that patients with MDD had decreased levels of genus Coprococcus, Faecalibacterium, Ruminococcus, Bifidobacterium and Escherichia and increased levels of Paraprevotella [100][35].
Interestingly, study observations showed that probiotics including combination therapy with antidepressants had a large effect on depressive symptoms compared with the control group [101][36]. Lactobacillus-only trials showed that Lactobacillus have no clinical effects on depression based on the finding that Lactobacillus-only trials had a small, non-significant pooled effect in contrast to the significantly larger effects for other probiotic trials for depression [102][37].
Furthermore, the composition of intestinal microbiota might have an impact on the secretion of gut peptides and therefore regulate the endocrine activity of these molecules in the whole organism. As wresearchers already know, gut peptides regulate the endocrine activity and can communicate with the central nervous system. Their job is not only connected with food intake, but also with stress behaviors and reactions to such situations. The composition of intestinal microbiota might have an impact on intestinal barrier permeability and thus secreted gut peptides do not enter the braincells in the same way and efficacy what can implicate in different action of peptides in organism. That difference can result in altered behavior of decreased mood pursuing to depressive-like behavior [7][38].
2.1. What Are the Gut Peptides?
The group of gut peptides consists of over 20 molecules secreted by enteroendocrine cells (EECs) that perform many different signaling functions including endocrine and metabolic activity, and moreover present the ability to communicate with the central nervous system (CNS). The most important gut peptides include peptide YY (PYY), glucagon-like peptide (GLP-1), cholecystokinin (CCK), corticotropin-releasing factor (CRF), ghrelin and oxytocin (Table 32) [103][39].
Table 32. The most important gut peptides and their characteristics (HPA—hypothalamic-pituitary-adrenal).
| Gut Peptide | Producing Cells | Releasing Factor | Peripheral Function | Central Function | ||
|---|---|---|---|---|---|---|
| PYY [104,105] | [40][41] | L-cells | a | food intake | inhibition of gastric emptying and intestinal motor activity | modulation of anxiety and stress-related disorders |
| GLP-1 [106, | ||||||
| CRF [110,111,112,113] | [46][47][48][49] | effector neurons of hypothalamus and enterochromaffin cells of the colon | stress | inhibition of gastric emptying, stimulation of colonic motility and impairment of the intestinal epithelial barrier | increased anxiety and depressive disorder | |
| ghrelin [114,115] | [50][51] | A-cells | a | starvation | increase of appetite and adipogenesis | modulation of stress response, anxiety and depressive disorder |
| oxytocin [116] | [52] | magnocellular neurons in hypothalamus | stress | facilitation of parturition and stimulation of lactation | reduced anxiety-like behavior and antidepressant effect |
a—enteroendocrine cells present in the small intestine.
2.2. How Does the Microbiota Interact with the Secretion of Gut Peptides?
Firstly, the changes in the composition of gut microbiota might lead to alterations in the intestinal barrier permeability through the interaction with endothelial tight junctions (TJs). It might cause an imbalance in the amount of gut peptides absorbed to the circulation and, furthermore, influence their function on the braincells [7,117][38][53]. Moreover, some Gram-negative bacterial genres present in the gut microbiota secrete an endotoxin, lipopolysaccharide (LPS), which promotes the activation of immune cascades and the production of pro-inflammatory cytokines. The pro-inflammatory phenotype associated with gut dysbiosis might be a trigger factor for the stress-induced inappropriate secretion of gut peptides [117][53]. Apart from the gut peptides, there are also specific molecules secreted by microorganisms, including metabolites and neurotransmitters (e.g., GABA, serotonin, tryptophan metabolites, catecholamines) that might penetrate to the bloodstream and act directly on receptors in the brain [99][34].
2.3. What Changes in the Composition of Gut Microbiota Might Cause Depressive Disorder?
The studies conducted both on human and animal subjects have suggested that there might be some differences in the composition of gut microbiota between healthy and depressed individuals. The strongest association refers to the Firmicutes/Bacteroidetes ratio [118,119][54][55]. Rodents with a higher amount of Bacteroidetes and a lower share of Firmicutes in their intestines had a tendency toward depressive-like behavior [119][55]. Moreover, mice subjected to chronic stress had decreased populations of Bacteroides and increased ones of Clostridium [94][56]. The association between fecal microbiota transplants from depressed subjects to healthy ones has also been the subject of research in different studies. For instance, the results showed that rats colonized with the microbiota from depressive-like individuals developed the symptoms of depressive behavior. However, no specific changes in the composition of microbiota that might be the cause of this phenomenon have been found [59][57]. Furthermore, several metanalyses have taken into consideration the effect of probiotic usage on mood [120,121,122][58][59][60]. Some of them proved that patients with symptoms of depression might benefit from this kind of supportive treatment (mostly using probiotics containing Lactobacillus and Bifidobacterium species) [123][61]. However, no profiling of gut microbiota was conducted on the participants before and after the use of probiotics and probiotics with different compositions of bacterial species that were used in the studies. Some of the studies did not find any effect of probiotics on depression [124][62]. These are the reasons why the use of probiotics in the treatment of depression still requires further research.
References
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2020, 113, 2019–2040.
- Passos, M.d.C.F.; Moraes-Filho, J.P. Microbiota intestinal nas doenças digestivas. Arq. Gastroenterol. 2017, 54, 255–262.
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493.
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230.
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920.
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut microbiota in autism and mood disorders. World J. Gastroenterol. 2016, 22, 361–368.
- Sartor, R.B. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 2008, 134, 577–594.
- Quigley, E.M.M.; Eamonn, D.; Quigley, M.M. Gut Bacteria in Health and Disease. Gastroenterol. Hepatol. 2013, 9, 560–569.
- Mändar, R.; Mikelsaar, M. Transmission of mother’s microflora to the newborn at birth. Biol. Neonat. 1996, 69, 30–35.
- Limbana, T.; Khan, F.; Eskander, N. Gut Microbiome and Depression: How Microbes Affect the Way We Think. Cureus 2020, 12, e9966.
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590.
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced Dietary Intake of Carbohydrates by Obese Subjects Results in Decreased Concentrations of Butyrate and Butyrate-Producing Bacteria in Feces. Appl. Environ. Microbiology 2007, 73, 1073–1078.
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8.
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200.
- Portune, K.J.; Beaumont, M.; Davila, A.M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci. Technol. 2016, 57, 213–232.
- Chen, C.; Huang, X.; Fang, S.; Yang, H.; He, M.; Zhao, Y.; Huang, L. Contribution of Host Genetics to the Variation of Microbial Composition of Cecum Lumen and Feces in Pigs. Front. Microbiol. 2018, 9, 2626.
- Thompson, A.L.; Monteagudo-Mera, A.; Cadenas, M.B.; Lampl, M.L.; Azcarate-Peril, M.A. Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front. Cell. Infect. Microbiol. 2015, 5, 3.
- Lee, S.A.; Lim, J.Y.; Kim, B.S.; Cho, S.J.; Kim, N.Y.; Kim, O.B.; Kim, Y. Comparison of the gut microbiota profile in breast-fed and formula-fed Korean infants using pyrosequencing. Nutr. Res. Pract. 2015, 9, 242–248.
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.D.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123.
- Klingensmith, N.J.; Coopersmith, C.M. The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness. Crit. Care Clin. 2016, 32, 203–212.
- Biedermann, L.; Brülisauer, K.; Zeitz, J.; Frei, P.; Scharl, M.; Vavricka, S.R.; Fried, M.; Loessner, M.J.; Rogler, G.; Schuppler, M. Smoking cessation alters intestinal microbiota: Insights from quantitative investigations on human fecal samples using FISH. Inflamm. Bowel. Dis. 2014, 20, 1496–1501.
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 2018, 53, 95–106.
- Tokarek, J.; Gadzinowska, J.; Młynarska, E.; Franczyk, B.; Rysz, J. What Is the Role of Gut Microbiota in Obesity Prevalence? A Few Words about Gut Microbiota and Its Association with Obesity and Related Diseases. Microorganisms 2021, 10, 52.
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.; Sampson, H.; Sicherer, S.; et al. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130.
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326.
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 1–11.
- Jeffery, I.B.; O’Toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.; Simrén, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012, 61, 997–1006.
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10.
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546.
- Muñoz-Garach, A.; Diaz-Perdigones, C.; Tinahones, F.J. Microbiota y diabetes mellitus tipo 2′. Endocrinol. Y Nutr. 2016, 63, 560–568.
- Dan, X.; Mushi, Z.; Baili, W.; Han, L.; Enqi, W.; Huanhu, Z.; Shuchun, L. Differential Analysis of Hypertension-Associated Intestinal Microbiota. Int. J. Med. Sci. 2019, 16, 872–881.
- O’Connor, G.T.; Lynch, S.V.; Bloomberg, G.R.; Kattan, M.; Wood, R.A.; Gergen, P.J.; Jaffee, K.F.; Calatroni, A.; Bacharier, L.B.; Beigelman, A. Early-life home environment and risk of asthma among inner-city children. J. Allerg. Clin. Immunol. 2018, 141, 1468–1475.
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24.
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938.
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13.
- Nadeem, I.; Rahman, M.Z.; Ad-Dab’bagh, Y.; Akhtar, M. Effect of probiotic interventions on depressive symptoms: A narrative review evaluating systematic reviews. Psychiatry Clin. Neurosci. 2019, 73, 154–162.
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23.
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59.
- Dockray, G.J. Gastrointestinal hormones and the dialogue between gut and brain. J. Physiol. 2014, 592, 2927–2941.
- Reichmann, F.; Holzer, P. Neuropeptide Y: A stressful review. Neuropeptides 2016, 55, 99–109.
- Farzi, A.; Reichmann, F.; Holzer, P. The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour. Acta Physiol. 2015, 213, 603–627.
- Zietek, T.; Rath, E. Inflammation Meets Metabolic Disease: Gut Feeling Mediated by GLP-1′. Front. Immunol. 2016, 7, 154.
- Ghosal, S.; Myers, B.; Herman, J.P. Role of central glucagon-like peptide-1 in stress regulation. Physiol. Behav. 2013, 122, 201–207.
- del Boca, C.; Lutz, P.E.; le Merrer, J.; Koebel, P.; Kieffer, B.L. Cholecystokinin knock-down in the basolateral amygdala has anxiolytic and antidepressant-like effects in mice. Neuroscience 2012, 218, 185–195.
- Wang, H.; Wong, P.T.H.; Spiess, J.; Zhu, Y.Z. Cholecystokinin-2 (CCK2) receptor-mediated anxiety-like behaviors in rats. Neurosci. Biobehav. Rev. 2005, 29, 1361–1373.
- Turnbull, A.V.; Rivier, C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc. Soc. Exp. Biol. Med. 1997, 215, 1–10.
- Kawahito, Y.; Sano, H.; Kawata, M.; Yuri, K.; Mukai, S.; Yamamura, Y.; Kato, H.; Chrousos, G.P.; Wilder, R.L.; Kondo, M. Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology 1994, 106, 859–865.
- Rodiño-Janeiro, B.K.; Alonso-Cotoner, C.; Pigrau, M.; Lobo, B.; Vicario, M.; Santos, J. Role of Corticotropin-releasing Factor in Gastrointestinal Permeability. J. Neurogastroenterol. Motil. 2015, 21, 33–50.
- Sanders, J.; Nemeroff, C. The CRF System as a Therapeutic Target for Neuropsychiatric Disorders. Trends Pharmacol. Sci. 2016, 37, 1045–1054.
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660.
- Schaeffer, M.; Langlet, F.; Lafont, C.; Molino, F.; Hodson, D.J.; Roux, T.; Lamarque, L.; Verdié, P.; Bourrier, E.; Dehouck, B.; et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 1512–1517.
- Cohen, H.; Kaplan, Z.; Kozlovsky, N.; Gidron, Y.; Matar, M.A.; Zohar, J. Hippocampal microinfusion of oxytocin attenuates the behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. J. Neuroendocrinol. 2010, 22, 889–904.
- Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Gut microbiota: A new path to treat obesity. Int. J. Obes. Supp. 2019, 9, 10–19.
- Labus, J.S.; Hollister, E.B.; Jacobs, J.; Kirbach, K.; Oezguen, N.; Gupta, A.; Acosta, J.; Luna, R.A.; Aagaard, K.; Versalovic, J.; et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome 2017, 5, 49.
- Yu, M. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharm. Biomed. Anal. 2017, 138, 231–239.
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a Social Stressor Alters the Structure of the Intestinal Microbiota: Implications for Stressor-Induced Immunomodulation. Brain Behav. Immun. 2011, 25, 397–407.
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118.
- Pirbaglou, M.; Katz, J.; de Souza, R.J.; Stearns, J.C.; Motamed, M.; Ritvo, P. Probiotic supplementation can positively affect anxiety and depressive symptoms: A systematic review of randomized controlled trials. Nutr. Res. 2016, 36, 889–898.
- Wallace, C.J.K.; Milev, R. The effects of probiotics on depressive symptoms in humans: A systematic review. Annu. Gen. Psychiatry 2017, 16, 14.
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018, 228, 13–19.
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320.
- Pinto-Sanchez, M.I.; Hall, G.B.C.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.; Cominetti, O.; Welsh, C.; Rieder, A.D. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8.
More
