Nanomedicines emerged from nanotechnology and have been introduced to bring advancements in treating multiple diseases. Nano-phytomedicines are synthesized from active phytoconstituents or plant extracts. Advancements in nanotechnology also help in the diagnosis, monitoring, control, and prevention of various diseases. The field of nanomedicine and the improvements of nanoparticles has been of keen interest in multiple industries, including pharmaceutics, diagnostics, electronics, communications, and cosmetics. In herbal medicines, these nanoparticles have several attractive properties that have brought them to the forefront in searching for novel drug delivery systems by enhancing efficacy, bioavailability, and target specificity.
- nanomaterials
- locomotor disorder
- dermal disorder
- urogenital disorder
- phytopharmaceuticals
1. Introduction
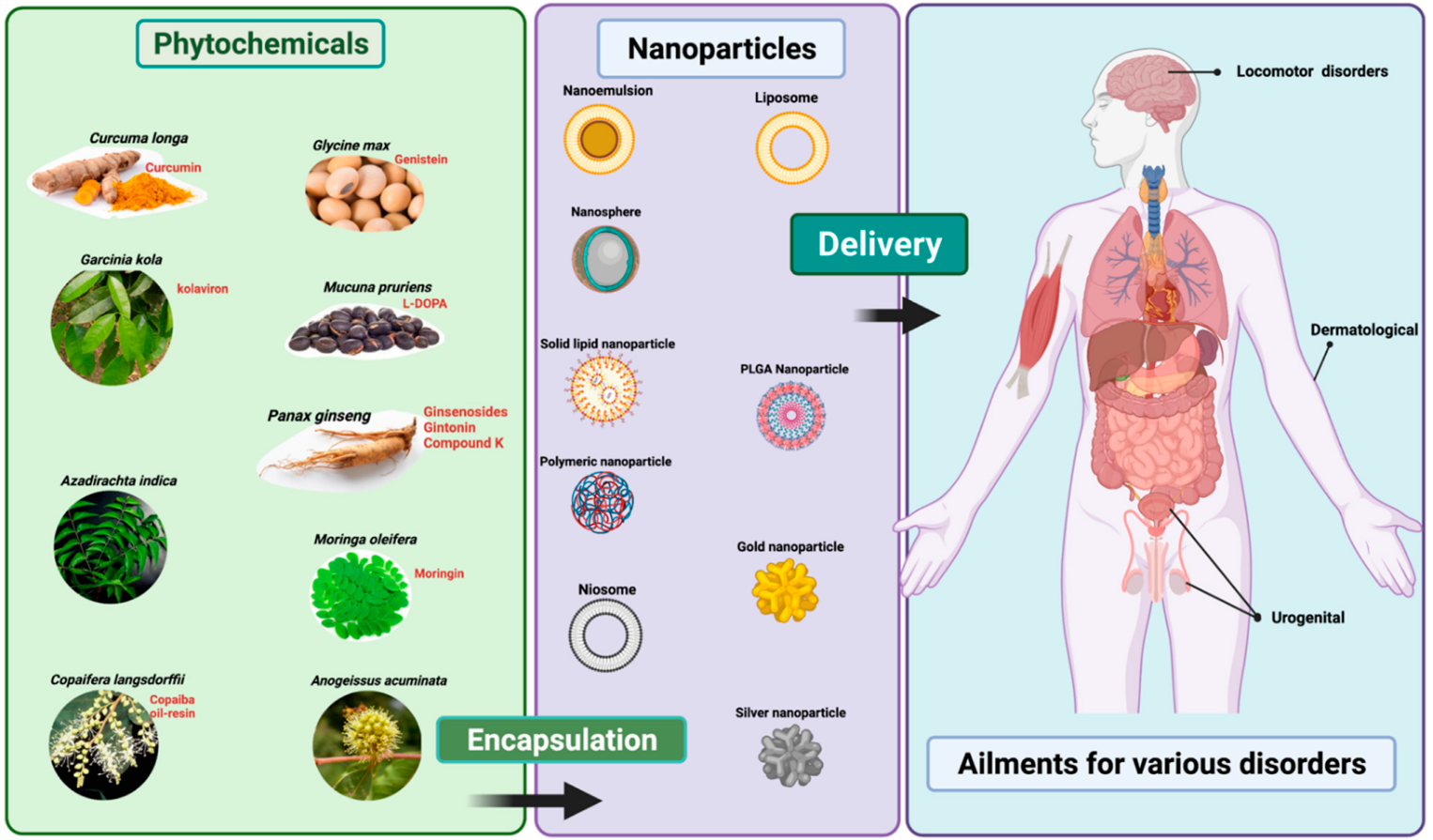
2. Therapeutic Applications of Nano-Phytopharmaceuticals
2.1. Nano-Phytopharmaceuticals in Dermal Disorders
| Plant Source | Formulation | Study Type | Action | Reference |
|---|---|---|---|---|
| Citrus fruits, onions, apples, parsley, sage, tea, and berries. | Nanoencapsulated quercetin in zein nanoparticles (NPQ) | Preclinical (rats) | NPQ improved memory and cognitive ability in rats (but no effects on locomotor activity test) |
[37,38][37][38] |
| Citrus fruits, onions, apples, parsley, sage, tea, and berries. | Quercetin nanoparticles |
Preclinical (rats) | Quercetin nanoparticles improved memory and pathological damage induced by scopolamine |
[39,40][39][40] |
| Berries, currants, grapes, red to purplish blue colored leafy vegetables, grains, roots, and tubers. | Anthocyanin-loaded poly (ethylene glycol)-gold nanoparticles (PEG-AuNPs) | Preclinical (mice) | PEG-AuNPs improved amyloid-beta (Aβ1-42) induced neuronal damage and neuroinflammation |
[41,42][41][42] |
| Curcuma longa L. (Zingiberaceae) | Nano-curcumin particles | Preclinical (mice) | Enhanced memory, motor function, contextual fear | [43] |
| Anamirtacocculus (L.) Wight and Arn. (Menispermaceae) | A.cocculus NPs in cocc 30c, in a homeopathic formulation | Preclinical | Improved attention and motor functions in sleep-deprived rats |
[44] |
| Solanum tuberosum L. (Solanaceae) | S.tuberosum Lectin NPs | Preclinical | Helped improved drug delivery enhanced memory and motor function |
[45] |
| Azadirachta indica A.Juss. (Meliaceae) | Neem oil incorporated in argan-liposomes and argan-hyalurosomes by sonicating with argan oil, soy lecithin, and water | In vitro | Protected skin cells by reducing oxidative stress | [46]. |
| Curcuma longa L. (Zingiberaceae) | Curcumin formulated with lipid-based nanoparticles such as liposomes, niosomes, solid lipid nanoparticles, and nanostructured lipid carriers | Review | Improved its penetration into skin and thus increased the solubility, stability, and therapeutic efficiencies of curcumin against various dermatological disorders such as psoriasis, dermatitis, bacterial, viral and fungal infections, burns, acne, arthritis, and skin cancer | [33,34][33][34] |
| Curcuma longa L. (Zingiberaceae) | C. longa leaves extract Silver nanoparticles (CL-AgNPs) loaded cotton fabric |
In vitro | Enhanced wound healing and antimicrobial activity on skin | [47] |
| Curcuma longa L.(Zingiberaceae) | Solid lipid nanoparticles (SLN-curcuminoids) |
Ex vivo (Sheep ear skin) | Showed good spreadability and stability on skin |
[48] |
| Curcuma longa L. (Zingiberaceae) | Curcumin nanoparticles (curc-NPs) |
Preclinical (rats) | Improved erectile response in diabetic male rats |
[49,50][49][50] |
| Panax ginseng C.A.Mey (Araliaceae) | P.ginseng nanoparticles |
Preclinical (rats) | Improved serum testosterone secretion and decrease sperm abnormalities in male rats |
[51] |
| Oxaliscorniculata L. (Oxalidaceae) | Aqueous extract of O. corniculata and its biofabricated silver nanoparticles (AgNPs) |
In vitro | Effective against urinary tract infection (UTI) causing microorganisms |
[52] |
| Anogeissusacuminata Wall.(Combretaceae) | Aqueous leaf extract of A. acuminata and its AgNPs | In vitro | Effective against multidrug resistant UTI causing bacteria | [53] |
| Passiflora caerulea L. (Passifloraceae) | Zinc oxide nanoparticles (ZnO NPs) using P. caerulea extract | In vitro | Effective against multidrug resistant UTI causing bacteria | [54] |
| Catharanthus roseus (L.) G. Don (Apocynaceae) | Sulphur nanoparticles (SNPs) produced from C. roseus leaf extract |
In vitro | Effective against multidrug resistant UTI causing bacteria |
[55] |
| Mimosa pudica L. (Fabaceae) | Sulphur nanoparticles (SNPs) produced from M. pudica alcoholic extracts |
In vitro | Antibacterial effects on uropathogenic E. coli (UPEC) and S. aureus and other UTI pathogens | [56] |
| Nigella sativa L. (Ranunculaceae) | Sulphur nanoparticles (SNPs) produced from seeds of N. sativa L. alcoholic extracts |
In vitro | Antibacterial effects on UPEC and S. aureus and other UTI pathogens | [57] |
| Rauwolfia serpentina L. (Apocynaceae) | Biologically synthe-sized gold nanopar-ticles with aqueous leaf extract of R. serpentina L. |
In vitro | Antibacterial effects on E. coli and S. aureus |
[58] |
2.2. Nano-Phytopharmaceuticals in Urogenital Disorders
References
- Kesarwani, K.; Gupta, R.; Mukerjee, A. Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266.
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207.
- Bonifácio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15.
- Hafez, D.A.; Elkhodairy, K.A.; Teleb, M.; Elzoghby, A.O. Nanomedicine-based approaches for improved delivery of phyto-therapeutics for cancer therapy. Expert Opin. Drug Deliv. 2020, 17, 279–285.
- Lim, C.L.; Raju, C.S.; Mahboob, T.; Kayesth, S.; Gupta, K.K.; Jain, G.K.; Dhobi, M.; Nawaz, M.; Wilairatana, P.; de Lourdes Pereira, M.; et al. Precision and advanced nano-phytopharmaceuticals for therapeutic applications. Nanomaterials 2022, 12, 238.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074.
- Dowling, A.; Clift, R.; Grobert, N.; Hutton, D.; Oliver, R.; O’neill, O.; Pethica, J.; Pidgeon, N.; Porritt, J.; Ryan, J. Nanoscience and nanotechnologies: Opportunities and uncertainties, lond. R. Soc. R. Acad. Eng. Rep. 2004, 46, 618.
- Mishra, V.; Kesharwani, P.; Mohd Amin, M.C.I.; Iyer, A.K. (Eds.) Preface. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Elsevier: Amsterdam, The Netherlands, 2017; pp. xix–xx.
- Sandhiya, V.; Ubaidulla, U. A review on herbal drug loaded into pharmaceutical carrier techniques and its evaluation process. Future J. Pharm. Sci. 2020, 6, 1–16.
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885.
- Sachan, A.K.; Gupta, A. A review on nanotized herbal drugs. Int. J. Pharma. Sci. Res. 2015, 6, 961.
- Morigi, V.; Tocchio, A.; Pellegrini, B.C.; Sakamoto, J.H.; Arnone, M.; Tasciotti, E. Nanotechnology in medicine: From inception to market domination. J. Drug Deliv. 2012, 2012, 389485.
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Invest. Dermatol. 2014, 134, 1527–1534.
- Barankin, B.; DeKoven, J. Psychosocial effect of common skin diseases. Can. Fam. Physician 2002, 48, 712–716.
- Hazarika, N.; Archana, M. The psychosocial impact of acne vulgaris. Ind. J. Dermatol. 2016, 61, 515–520.
- Mian, M.; Silfvast-Kaiser, A.; Paek, S.; Kivelevitch, D.; Menter, A. A review of the most common dermatologic conditions and their debilitating psychosocial impacts. Int. Arch. Int. Med. 2019, 3, 018.
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O. Human skin penetration of hyaluronic acid of different molecular weights as probed by raman spectroscopy. Skin Res. Technol. 2016, 22, 55–62.
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105.
- Kocbek, P.; Teskač, K.; Kreft, M.E.; Kristl, J. Toxicological aspects of long-term treatment of keratinocytes with ZNO and TiO2 nanoparticles. Small 2010, 6, 1908–1917.
- Schaeffer, H.E.; Krohn, D.L. Liposomes in topical drug delivery. Invest. Ophthalmol. Vis. Sci. 1982, 22, 220–227.
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics 2020, 12, 288.
- Xu, Y.; Guo, Y.; Yang, Y.; Meng, Y.; Xia, X.; Liu, Y. Stabilization of deformable nanovesicles based on insulin-phospholipid complex by freeze-drying. Pharmaceutics 2019, 11, 539.
- Chaiyana, W.; Anuchapreeda, S.; Punyoyai, C.; Neimkhum, W.; Lee, K.-H.; Lin, W.-C.; Lue, S.-C.; Viernstein, H.; Mueller, M. Ocimum sanctum linn. as a natural source of skin anti-ageing compounds. Ind. Crops Prod. 2019, 127, 217–224.
- Chaiyana, W.; Anuchapreeda, S.; Somwongin, S.; Marsup, P.; Lee, K.H.; Lin, W.C.; Lue, S.C. Dermal delivery enhancement of natural anti-ageing compounds from Ocimum sanctum linn. extract by nanostructured lipid carriers. Pharmaceutics 2020, 12, 309.
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Topical anti-inflammatory potential of quercetin in lipid-based nanosystems: In vivo and in vitro evaluation. Pharm. Res. 2014, 31, 959–968.
- Kaur, L.; Jain, S.K.; Manhas, R.K.; Sharma, D. Nanoethosomal formulation for skin targeting of amphotericin B: An in vitro and in vivo assessment. J. Liposome Res. 2015, 25, 294–307.
- Ramezanli, T.; Kilfoyle, B.E.; Zhang, Z.; Michniak-Kohn, B.B. Polymeric nanospheres for topical delivery of vitamin D3. Int. J. Pharm. 2017, 516, 196–203.
- Petit, R.G.; Cano, A.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Severino, P.; Souto, E.B.; García, M.L.; Pujol, M.; et al. Psoriasis: From pathogenesis to pharmacological and nano-technological-based therapeutics. Int. J. Mol. Sci. 2021, 22, 4983.
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 2012, 17, 5972–5987.
- Waghule, T.; Gorantla, S.; Rapalli, V.K.; Shah, P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Emerging trends in topical delivery of curcumin through lipid nanocarriers: Effectiveness in skin disorders. AAPS PharmSciTech 2020, 21, 284.
- Paul, A.K.; Jahan, R.; Paul, A.; Mahboob, T.; Bondhon, T.A.; Jannat, K.; Hasan, A.; Nissapatorn, V.; Wilairatana, P.; de Lourdes Pereira, M.; et al. The role of medicinal and aromatic plants against obesity and arthritis: A review. Nutrients 2022, 14, 985.
- Mahmood, A.; Rapalli, V.K.; Waghule, T.; Gorantla, S.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Uv spectrophotometric method for simultaneous estimation of betamethasone valerate and tazarotene with absorption factor method: Application for in-vitro and ex-vivo characterization of lipidic nanocarriers for topical delivery. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118310.
- Battaglia, L.; Gallarate, M. Lipid nanoparticle: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508.
- Moreno, L.; Puerta, E.; Suárez-Santiago, J.E.; Santos-Magalhães, N.S.; Ramirez, M.J.; Irache, J.M. Effect of the oral administration of nanoencapsulated quercetin on a mouse model of Alzheimer’s disease. Int. J. Pharm. 2017, 517, 50–57.
- Shankar, G.M.; Antony, J.; Anto, R.J. Chapter two-quercetin and tryptanthrin: Two broad spectrum anticancer agents for future chemotherapeutic interventions. In The Enzymes; Bathaie, S.Z., Tamanoi, F., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 37, pp. 43–72.
- Palle, S.; Neerati, P. Quercetin nanoparticles attenuates scopolamine induced spatial memory deficits and pathological damages in rats. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 101–106.
- de Andrade Teles, R.B.; Diniz, T.C.; Costa Pinto, T.C.; de Oliveira Júnior, R.G.; Gama, E.S.M.; de Lavor, É.M.; Fernandes, A.W.C.; de Oliveira, A.P.; de Almeida Ribeiro, F.P.R.; da Silva, A.A.M.; et al. Flavonoids as therapeutic agents in Alzheimer’s and Parkinson’s diseases: A systematic review of preclinical evidences. Oxidative Med. Cell. Longev. 2018, 2018, 7043213.
- Kim, M.J.; Rehman, S.U.; Amin, F.U.; Kim, M.O. Enhanced neuroprotection of anthocyanin-loaded peg-gold nanoparticles against aβ (1-42)-induced neuroinflammation and neurodegeneration via the NF-(k)b/JNK/GSK3β signaling pathway. Nanomedicine 2017, 13, 2533–2544.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.; Baum, L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease tg2576 mice. AAPS J. 2013, 15, 324–336.
- Zubedat, S.; Freed, Y.; Eshed, Y.; Cymerblit-Sabba, A.; Ritter, A.; Nachmani, M.; Harush, R.; Aga-Mizrachi, S.; Avital, A. Plant-derived nanoparticle treatment with cocc 30c ameliorates attention and motor abilities in sleep-deprived rats. Neuroscience 2013, 253, 1–8.
- Zhang, C.; Chen, J.; Feng, C.; Shao, X.; Liu, Q.; Zhang, Q.; Pang, Z.; Jiang, X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer’s disease. Int. J. Pharm. 2014, 461, 192–202.
- Manca, M.L.; Manconi, M.; Meloni, M.C.; Marongiu, F.; Allaw, M.; Usach, I.; Peris, J.E.; Escribano-Ferrer, E.; Tuberoso, C.I.G.; Gutierrez, G. Nanotechnology for natural medicine: Formulation of neem oil loaded phospholipid vesicles modified with argan oil as a strategy to protect the skin from oxidative stress and promote wound healing. Antioxidants 2021, 10, 670.
- Maghimaa, M.; Alharbi, S.A. Green synthesis of silver nanoparticles from Curcuma longa l. and coating on the cotton fabrics for antimicrobial applications and wound healing activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111806.
- Zamarioli, C.M.; Martins, R.M.; Carvalho, E.C.; Freitas, L.A. Nanoparticles containing curcuminoids (curcuma longa): Development of topical delivery formulation. Rev. Bras. Farmacogn. 2015, 25, 53–60.
- Masuku, N.P.; Unuofin, J.O.; Lebelo, S.L. Advances in nanoparticle delivery system for erectile dysfunction: An updated review. Sex. Med. 2021, 9, 100420.
- Draganski, A.; Tar, M.T.; Villegas, G.; Friedman, J.M.; Davies, K.P. Topically applied curcumin-loaded nanoparticles treat erectile dysfunction in a rat model of type-2 diabetes. J. Sex. Med. 2018, 15, 645–653.
- Linjawi, S.A. Evaluation of the protective effect of Panax ginseng nanoparticles against nicotine-induced reproductive disorders in male rats. Int. J. Pharma. Sci. Rev. Res. 2015, 32, 38–45.
- Das, P.; Kumar, K.; Nambiraj, A.; Awasthi, R.; Dua, K.; Malipeddi, H. Antibacterial and in vitro growth inhibition study of struvite urinary stones using Oxalis corniculata linn. leaf extract and its biofabricated silver nanoparticles. Recent Pat. Drug Deliv. Formul. 2018, 12, 170–178.
- Mishra, M.P.; Padhy, R.N. Antibacterial activity of green silver nanoparticles synthesized from Anogeissus acuminata against multidrug resistant urinary tract infecting bacteria in vitro and host-toxicity testing. J. App. Biomed. 2018, 16, 120–125.
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour. Effic. Technol. 2017, 3, 459–465.
- Paralikar, P.; Ingle, A.P.; Tiwari, V.; Golinska, P.; Dahm, H.; Rai, M. Evaluation of antibacterial efficacy of sulfur nanoparticles alone and in combination with antibiotics against multidrug-resistant uropathogenic bacteria. J. Environ. Sci. Health Part A Toxic Hazard Subst. Environ. Eng. 2019, 54, 381–390.
- Yogapiya, R.; Balakrishnaraja, R.; Gowthamraj, G. Comparative analysis and synthesis of silver nano-particles from selected parts of Mimosa pudica to treat urinary tract infection. Res. Sq. 2021; preprint.
- Ranjan, M.P.; Das, M.P.; Kumar, M.S.; Anbarasi, P.; Sindhu, S.; Sagadevan, E.; Arumugam, P. Green synthesis and characteriza-tion of silver nanoparticles from Nigella sativa and its application against UTI causing bacteria. J. Acad. Ind. Res. 2013, 2, 45–49.
- Alshahrani, M.Y.; Rafi, Z.; Alabdallah, N.M.; Shoaib, A.; Ahmad, I.; Asiri, M.; Zaman, G.S.; Wahab, S.; Saeed, M.; Khan, S. A comparative antibacterial, antioxidant, and antineoplastic potential of Rauwolfia serpentina (l.) leaf extract with its biologically synthesized gold nanoparticles (r-aunps). Plants 2021, 10, 2278.
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.; Howard, B.V.; Thomson, C.A.; LaCroix, A.Z.; et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the women’s health initiative randomized trials. JAMA 2013, 310, 1353–1368.
- Chen, M.N.; Lin, C.C.; Liu, C.F. Efficacy of phytoestrogens for menopausal symptoms: A meta-analysis and systematic review. Climacteric 2015, 18, 260–269.
- Mathur, M.; Vyas, G. Role of nanoparticles for production of smart herbal drug-An overview. Indian J. Nat. Prod. Resour. 2013, 4, 329–338.
- Tang, J.; Xu, N.; Ji, H.; Liu, H.; Wang, Z.; Wu, L. Eudragit nanoparticles containing genistein: Formulation, development, and bioavailability assessment. Int. J. Nanomed. 2011, 6, 2429.
- Bilal, I.; Chowdhury, A.; Davidson, J.; Whitehead, S. Phytoestrogens and prevention of breast cancer: The contentious debate. World J. Clin. Oncol. 2014, 5, 705–712.
- Huang, L.; Wang, Z.; Liu, G.; Wu, Y.; Yang, C.; Mei, L.; Zhang, H.; Zeng, X. Fabrication of genistein-loaded biodegradable TPGS-b-PCL nanoparticles for improved therapeutic effects in cervical cancer cells. Int. J. Nanomed. 2015, 10, 2461–2473.
- Johnson, A.; Roberts, R.L.; Elkins, G. Complementary and alternative medicine for menopause. J. Evid. Based Integr. Med. 2019, 24, 2515690X19829380.
- Ruhlen, R.L.; Sun, G.Y.; Sauter, E.R. Black cohosh: Insights into its mechanism(s) of action. Integr. Med. Insights 2008, 3, 21–32.
- Masserini, M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013, 2013, 238428.
