Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Csaba Kopitkó.
Acute kidney injury (AKI), especially if recurring, represents a risk factor for future chronic kidney disease. In intensive care units, increased intra-abdominal pressure is well-recognized as a significant contributor to AKI. Renal interstitial pressure changes synchronously to renal perfusion pressure. The renal parenchyma is primarily drained by the urine conducting and renal venous system. The renal lymphatic system can also contribute to renal compartment syndrome, the functional changes in the lymphatic flow was not investigated in connection with pneumo-peritoneum.
- acute kidney injury
- intra-abdominal pressure
- oxidative stress
- renal cortical blood flow
1. The Hilar and the Cortical Route
The cortical lymphatic capillaries begin near the Bowman’s capsule and the blind ends of the medullary capillaries are observed in the sub-mucosal layer of the papilla in humans (Figure 41) [1][2]. By contrast, several publications argue against the existence of medullary lymphatics. Most authors who are in favor of their existence restrict their origin to the outer, fluid-rich medulla and reject the theory of a deeper-layer origin [1][3][4][5]. One possible explanation is that significant interspecies and inter-individual differences, e.g., lympho-venous communication, were demonstrated in many animal autopsies, but not in humans [1][6]. The main route for draining renal lymph is the hilar path under physiologic conditions (the flow is 4–8 times greater through the hilar than the renal direction). The channels perforating the capsule of the kidney bear a far lower importance.
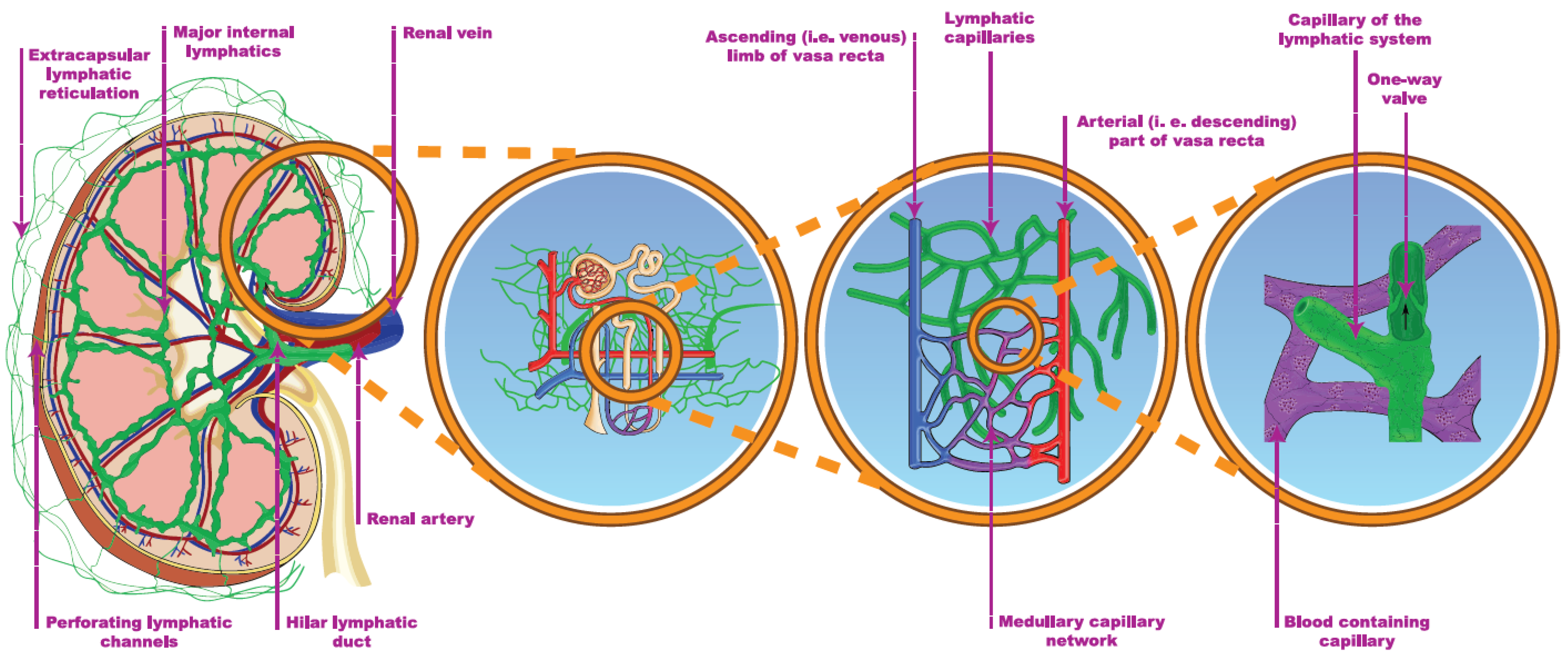
Figure 41. The renal lymphatic system (hilar and capsular). Red vessels: arteries; blue vessels: veins; purple vessels: connection vessels between arteries and veins; yellow tubes: urine conducting system; green vessels: lymphatic network.
The two systems are connected through communicating tubules (Figure 41) [1][7]. The hilar lymph can be diverged to the capsular system, e.g., after ureteric obstruction [8]. The electrolyte concentration of the hilar lymph is almost identical to the electrolyte concentration of the plasma, implying that the cortical lymph is also drained in the hilar direction [1][9]. The source of lymph may be tracked by a mixture of labeled glucose and mannitol. Glucose is reabsorbed in proximal tubules, while mannitol is filtrated only. Accordingly, a higher glucose/mannitol ratio in the renal lymph would suggest the medullary origin of the lymphatic fluid under physiologic circumstances. During donor nephrectomy, lymphatic vessels are transected, but start to regenerate within 7 days, leading to intactly functioning lymphatics 2–3 weeks later [10]. The activity and completeness of lymphangio-genesis can be associated with a lower rejection rate [10][11].
2. The Microanatomy of Lymphatic Capillaries
During lymph generation, the fluid enters the lymphatic capillaries either passively through the gaps between the “button-like” (i.e., tight connecting points certain distances apart) intercellular junctions in contrast to the “zipper-like” junctions between the endothelial cells of the blood vessels, or via the active trans-cellular uptake across the endothelial cells [1][10]. The blunt openings of the vessels are tethered to the surrounding matrix of the tissue, with this anchoring preventing the collapse of the ducts [1][12].
3. Lymph Moving Forces
Unlike amphibians, which possess so-called lymphatic hearts (up to 15 pairs), in mammals lymph is mostly propelled by the movement of surrounding tissues such as muscle contractions and bowel peristalsis or by passive forces, with pressure differences, e.g., derived from the respiratory cycle [5]. The one-way valves found in lymphatic vessels ensure a unidirectional flow, which is generated by the active contractions of smooth muscles in their wall [8]. These valves are formed from the overlapping junctions of endothelial cells at the beginning of the ducts, but in the larger lymphatic canals true traditional valves can also be observed [12].
Renal vein compression increases lymphatic pressure within five minutes [1][13][14]. In contrast, the occlusion of ureters exerts a more gradual effect, whereas the lymphatic drainage system can cope with the inflow to the renal compartment only within a certain range (Figure 52). As Rohn et al. nicely demonstrated in dogs, when the venous pressure rose to 20–25 mmHg, lymphatic resistance decreased, lymphatic driving pressure increased and lymphatic flow was augmented [14]. The new steady state was reached in about 30–45 min. The lymphatic pressure-flow curve was shifted to the right when renal venous pressure was elevated, a phenomenon which could be special for the kidneys. This mechanism can serve as a safety release valve to escape from parenchymal pressure elevations.
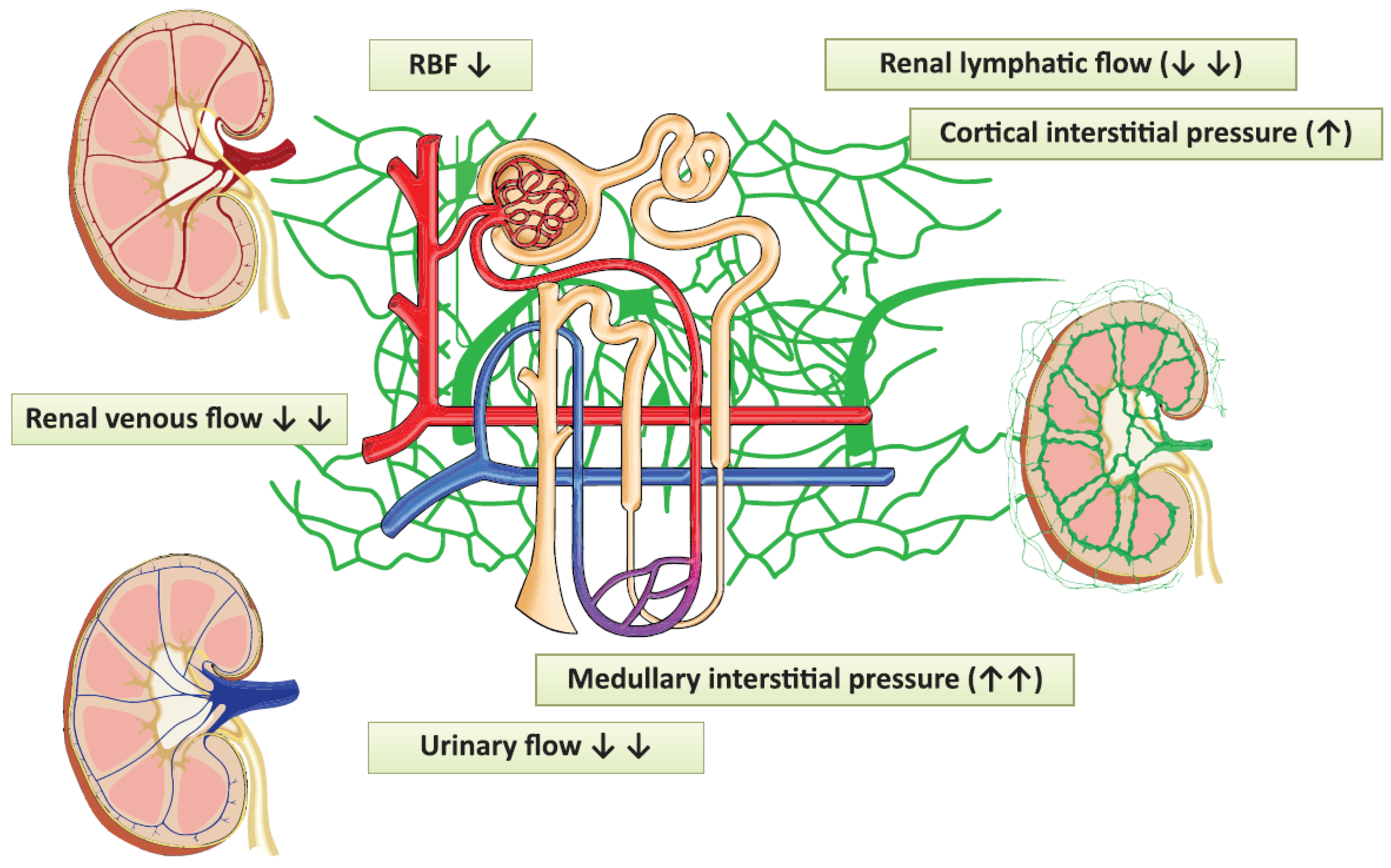
Figure 52. The pathophysiological changes evoked by laparoscopy, which can contribute to the development of acute kidney injury. Red vessels: arteries; blue vessels: veins; purple vessels: connection vessels between arteries and veins; yellow tubes: urine conducting system; green vessels: lymphatic network; RBF: renal blood flow.
There were individual differences detected in lymphatic flow rates and the renal pressures at which the lymphatic flow started to decline. Inter-individual variations in the development of the perforating system or medullary drainage can explain the different consequences of the elevated renal venous pressure in dogs mentioned above [15]. Further possible compensatory processes are the diversion of lymph from hilar to capsular vessels, which was observed in dogs after three days of ureteric compression, and lymphangio-genesis taking place in a larger time-frame [1][8][12][16][17][18][19].
4. The Turnover of Albumin, the Key Element of the Interstitium
5–25% of the total peri-tubular vascular endothelial surface is occupied by large (0.04–0.05 µm) pores [20]. Albumin is a flexible molecule, 3.8 nm in diameter and 15 nm in length, but the split-size of a rectangular pore has been reported as small as 35 Å (3.5 nm) in cross-section [21]. It is this mobility that makes albumin capable of transmission easily through capillary pores, while the larger size globulins are retained intra-luminarly. The protein concentration of renal lymphatic fluid is 43% of plasma in sheep, but the proportion of albumin is higher than in serum (albumin/globulin ratio is 1.3 vs. 0.69) [1][20][22]. The extravascular albumin pool is important for the maintenance of oncotic pressure in the interstitium: as mentioned earlier, its concentration is at least twofold in plasma at the tip of the papilla as compared to peripheral blood [20].
After intravascular injection, fluorescein-labelled albumin can be detected as early as 40 s in the extravascular space of rat kidneys [20]. The transmission of radioactively labelled albumin into the renal lymph takes about 2 h, but 85% of the equilibrium is realized within 3 min in the papilla and 1 min in the cortex [20]. This fact can be explained by a significant reuptake of albumin on the venous side. Albumin exchanges with a short turnover time in kidneys, but about 30% resides in the slower exchange compartment, supposedly in the extravascular space of the medulla [1][20].
References
- Russell, P.S.; Hong, J.; Windsor, J.A.; Itkin, M.; Phillips, A.R.J. Renal Lymphatics: Anatomy, Physiology, and Clinical Implications. Front. Physiol. 2019, 10, 251.
- Rawson, A.J. Distribution of the lymphatics of the human kidney as shown in a case of carcinomatous permeation. Arch. Pathol. 1949, 47, 283–292.
- Cockett, A.T. Lymphatic network of kidney I. anatomic and physiologic considerations. Urology 1977, 9, 125–129.
- Cuttino, J.T.; Jennette, J.C.; Clark, R.L.; Kwock, L. Renal medullary lymphatics: Microradiographic, light, and electron microscopic studies in pigs. Lymphology 1985, 18, 24–30.
- Rusznyak, I.; Foldi, M.; Szabo, G.; Pierre, R.L.S. Lymphatics and lymph circulation—Physiology and pathology. Am. J. Med. Sci. 1968, 255, 339.
- Karmali, R.J.; Suami, H.; Wood, C.G.; Karam, J.A. Lymphatic drainage in renal cell carcinoma: Back to the basics. Br. J. Urol. 2014, 114, 806–817.
- Holmes, M.J.; O’Morchoe, P.J.; O’Morchoe, C.C.C. Morphology of the intrarenal lymphatic system. Capsular and hilar communications. Am. J. Anat. 1977, 149, 333–351.
- Ranghino, A.; Segoloni, G.P.; Lasaponara, F.; Biancone, L. Lymphatic disorders after renal transplantation: New insights for an old complication. Clin. Kidney J. 2015, 8, 615–622.
- Keyl, M.J.; Scott, J.B.; Dabney, J.M.; Haddy, F.J.; Harvey, R.B.; Bell, R.D.; Ginn, H.E. Composition of canine renal hilar lymph. Am. J. Physiol. Content 1965, 209, 1031–1033.
- Seeger, H.; Bonani, M.; Segerer, S. The role of lymphatics in renal inflammation. Nephrol. Dial. Transpl. 2012, 27, 2634–2641.
- Stuht, S.; Gwinner, W.; Franz, I.; Schwarz, A.; Jonigk, D.; Kreipe, H.; Kerjaschki, D.; Haller, H.; Mengel, M. Lymphatic Neoangiogenesis in Human Renal Allografts: Results from Sequential Protocol Biopsies. Am. J. Transplant. 2007, 7, 377–384.
- Yazdani, S.; Navis, G.; Hillebrands, J.-L.; Van Goor, H.; van den Born, J. Lymphangiogenesis in renal diseases: Passive bystander or active participant? Expert Rev. Mol. Med. 2014, 16, e15.
- Gabriel Navar, L. Regulation of renal hemodynamics. Am. J. Physiol. 1998, 275 Pt 2, 221–235.
- Rohn, D.A.; Stewart, R.H.; Elk, J.R.; Laine, G.A.; Drake, R.E. Renal lymphatic function following venous pressure elevation. Lymphology 1996, 29, 67–75.
- Blake, W.D.; Wégria, R.; Keating, R.P.; Ward, H.P. Effect of increased renal venous pressure on renal function. Am. J. Physiol. Content 1949, 157, 1–13.
- Ishikawa, Y.; Akasaka, Y.; Kiguchi, H.; Akishima-Fukasawa, Y.; Hasegawa, T.; Ito, K.; Kimura-Matsumoto, M.; Ishiguro, S.; Morita, H.; Sato, S.; et al. The human renal lymphatics under normal and pathological conditions. Histopathology 2006, 49, 265–273.
- Cuttino, J.; Clark, R.; Fried, F.; Stevens, P. Microradiographic demonstration of pyelolymphatic backflow in the porcine kidney. Am. J. Roentgenol. 1978, 131, 501–505.
- Bidgood, W.D.; Cuttino, J.T.; Clark, R.L.; Volberg, F.M. Pyelovenous and Pyelolymphatic Backflow During Retrograde Pyelography in Renal Vein Thrombosis. Investig. Radiol. 1981, 16, 13–19.
- Mobison, D.M. Routes of absorption in hydronephrosis; Experimentation with dyes in the totally obstructed ureter. Br. J. Urol. 1929, 1, 30–45.
- Slotkoff, L.M.; Lilienfield, L.S. Extravascular renal albumin. Am. J. Physiol. Content 1967, 212, 400–406.
- Tojo, A.; Kinugasa, S. Mechanisms of Glomerular Albumin Filtration and Tubular Reabsorption. Int. J. Nephrol. 2012, 2012, 481520.
- McIntosh, G.H.; Morris, B. The lymphatics of the kidney and the formation of renal lymph. J. Physiol. 1971, 214, 365–376.
More
