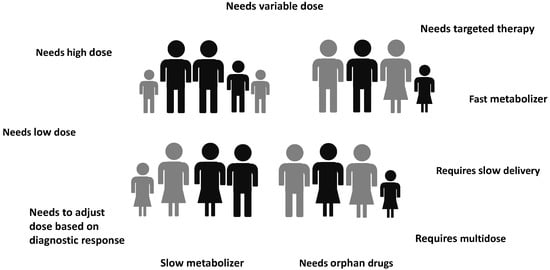
Novel additive manufacturing (AM) techniques and particularly 3D printing (3DP) have achieved a decade of success in pharmaceutical and biomedical fields. Highly innovative personalized therapeutical solutions may be designed and manufactured through a layer-by-layer approach starting from a digital model realized according to the needs of a specific patient or a patient group. The combination of patient-tailored drug dose, dosage, or diagnostic form (shape and size) and drug release adjustment has the potential to ensure the optimal patient therapy. This document provides an overview on different 3DP techniques to produce personalized medicines and medical devices, highlighting, for each method, the critical printing process parameters, the main starting materials, as well as advantages and limitations.
- additive manufacturing
- 3D-Printing
- rapid prototyping
- personalized medicines
- personalized medical devices
1. Introduction
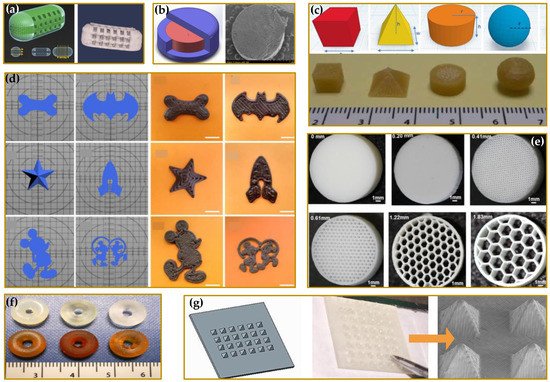
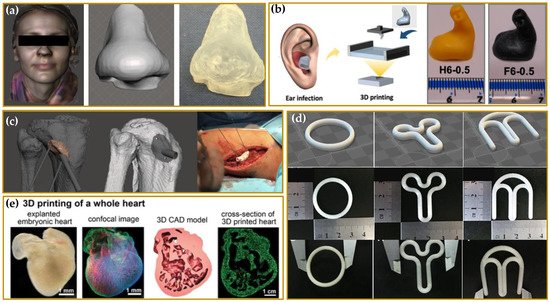
2. Driving Force in the Developing of 3DP Medicines and Medical Devices
-
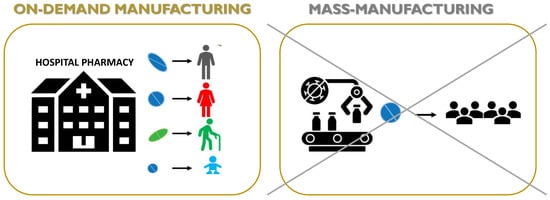
Reduced length and cost of transport and storage [81].
-
Quick and real-time responses to patient and market needs due to the possibility to rapidly produce small batches of complex formulations with unique geometries and, furthermore, the concept of digital dispensing in hard-to-reach areas or developing countries [82].
-
Reduced waste and hence reduced costs of developing and dosing due to a precise spatial control over the deposition of materials, limiting the amounts of API (active pharmaceutical ingredient) and excipients in comparison to conventional technologies [83].
3. 3D Printing: technical aspects
-
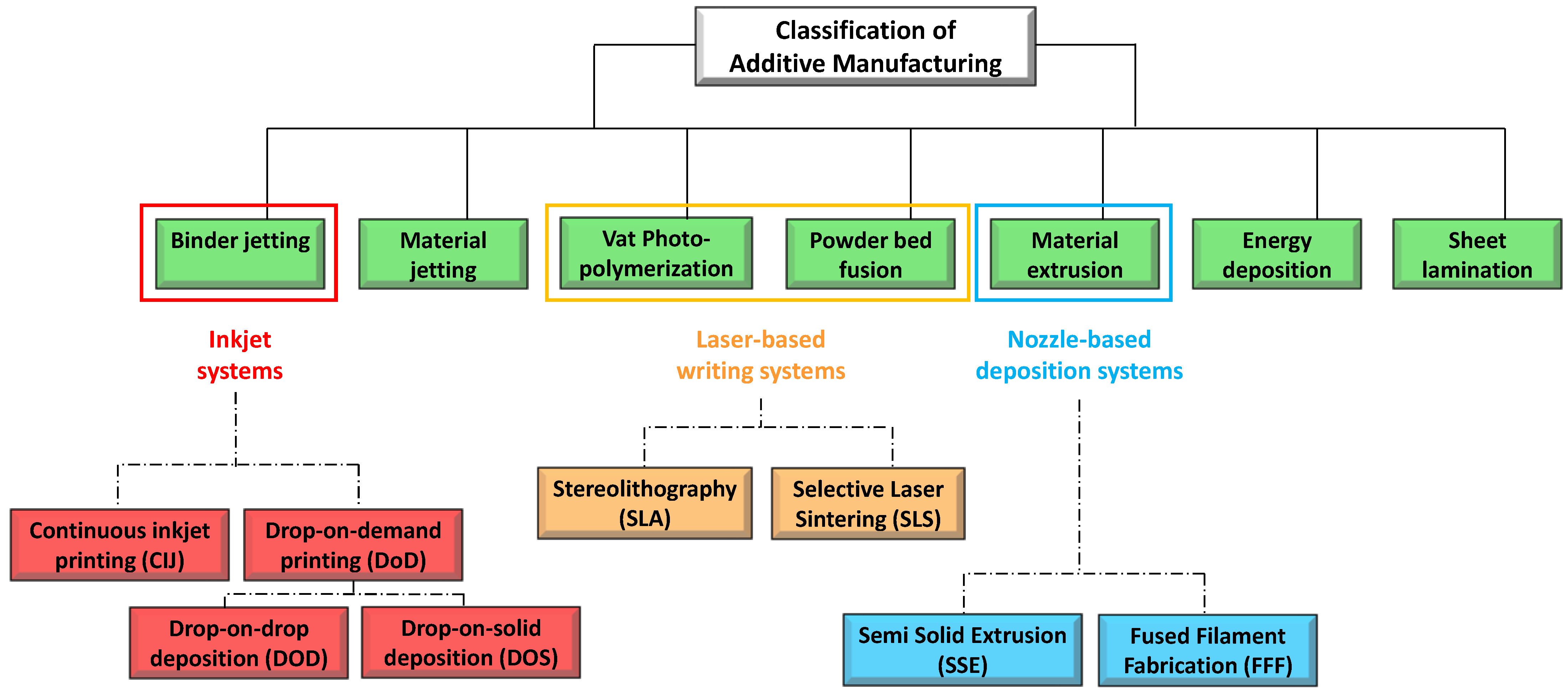
Printing based ink-jet systems
-
Laser-based writing systems,
Ink-Jet Based 3D-Printing Technologies
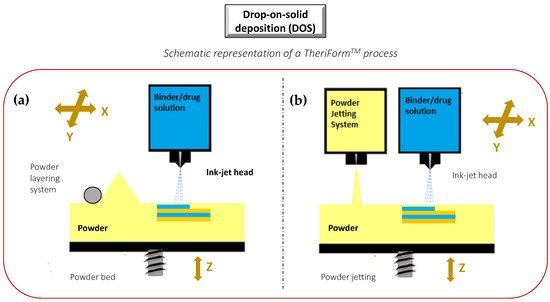
The idea of “ink-jet” systems originated from computer-operated ink-jet printing, which recreates digital images by propelling ink droplets onto paper. This was adapted for pharmaceutical application by the replacement of the ink with liquid solutions containing APIs and excipients, and normal paper with edible sheets known as substrates. In this case, the major challenge, often underestimated, is the formulation of an API-containing ink with appropriate properties. Specifically, during a generic inkjet-based 3DP process, the ink must be sprayed at a set speed, and through specific motions, into droplets with precise sizes. Operative conditions must be well established to facilitate reliable jetting and homogeneous droplet formation with minimal satellites [88][92]. In addition, the choice of the solvent for the ink formulation, as well as the ink drying rate, could influence the solid state of the loaded API after deposition, and hence its bioavailability [93]. As illustrated in Figure 6, the ink-jet based 3DP technologies can be divided into two types: continuous (CIJ) and drop-on-demand (DoD) inkjet printing [56]. In the first case, the ink is sprayed, mainly through piezoelectric crystals, in a continuous flow. On the contrary, during a DoD process, the ink flow, either provided by a thermal or a piezoelectric device, is provided only as needed. This latter process can be also defined as drop-on-drop deposition (DOD) if the drops are allowed to deposit on each other to form a bed, and drop-on-powder deposition (drop-on-solid, DOS) if the droplets are allowed to deposit on the powder bed; this approach is also known as the TERIFORM® process. During a drop-on-drop deposition process, droplets of ink are sprayed from the thermal or piezoelectric print head, deposited on the thin layers, and then cured by cooling air or in the presence of high energy light (Figure 7b). In this case, to create support for overhang geometries, it is necessary to use additional material acting as support. The most common materials used for DOD are waxes and ceramics, and this low selection of materials certainly represents a disadvantage. By contrast, the main advantages of DOD are the instantaneous solidification, the efficacy, and the cost-effectiveness.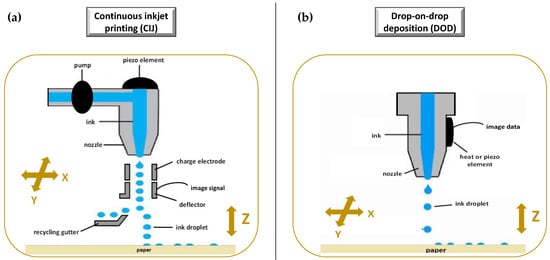
Laser Based 3D-Printing Technologies
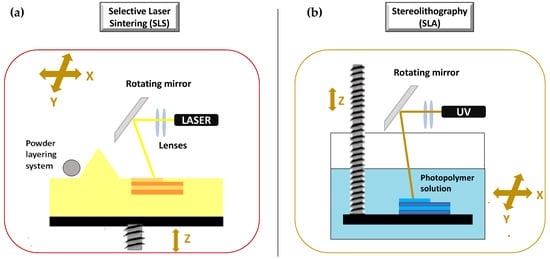
The second set of 3DP technologies is that laser-based 3DP technologies. This group includes selective laser sintering (SLS), or selective laser melting (SLM), whose constructive assumptions are similar to the DOS method, and stereolithography (SLA), for which the object is built by the solidification of photosensitive liquids.
SLS is a laser based 3DP technique based on powder solidification by applying a high-energy beam
. As illustrated in
Figure 9
a, a layer of free powder is applied by roller and each layer is formed by sintering via laser beam that is able to heat just below melting temperature
[1]
. This technique can be applied to ceramic powders as well as to thermoplastic or metal powders. In this latter case, laser beam must melt the powdered bed and the specific technique is referred to as selective laser melting (SLM)
[103]
.
Nozzle Based 3D-Printing Technologies
The third group of 3D-printing technologies is represented by nozzle-based deposition systems allowing direct writing through extrusion. Such systems deposit ink direct through a nozzle to create a 3D pattern layer-by-layer with controlled composition and architecture [119]. They can be basically divided into processes based on material melting, such as fused filament fabrication (FFF), also referred to as fused deposition modelling (FDM), and processes without material melting, such as semi solid extrusion (SSE), also known as pressure-assisted microsyringe (PAM). Nozzle based 3DP technologies have been highly investigated due to their great versatility, reproducibility, and high scalability potential. A lot of papers in the literature have focused on the application of such techniques to develop pharmaceutical as well as biomedical products. FFF is a really very investigated 3DP technique because it is cheap, easy to use, and readily available [119][120][121]. Its increasing popularity is mainly due to the progressive availability of compact sized and relatively inexpensive equipment [1][56]. During a FFF process, a thermoplastic polymeric material (mainly in form of filament) is extruded through a warmed-up nozzle and printed layer-by-layer (Figure 10a). Nozzle diameter varies from 0.2 to 0.4 mm, and it has an impact on the final resolution of 3D printed product. Generally, the width of the printed path corresponds to the nozzle diameter, while its height is equal to the half of the width. However, properties of the selected starting material as well as printer settings may induce modifications. During the process, the paths are arranged in layers until the formation of the final object, the resolution of which depends on layer height. Differently, the mechanical characteristics of the printed product are related to a number of outlines that build the external wall of the object and infill pattern (e.g., linear, or hexagonal).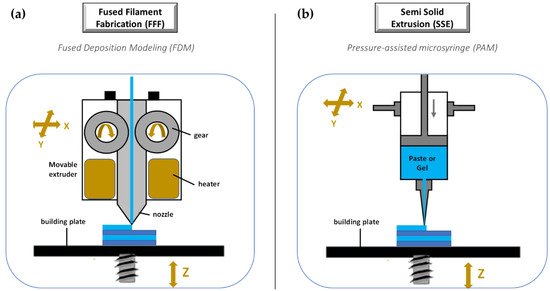
4. Conclusions
The technology evolution pathway of 3DP from 2012 to date is very notable. It is a fact that at present there is a contribution of 3D-printing to many aspects of healthcare. However, the full range of application remains to be explored in depth. In the last few years, the healthcare needs of the population have changed, also thanks to the adoption and enhancement of omics technologies in healthcare. There is an increasing demand of patient-tailored treatments to improve efficacy, safety, patient compliance, therapeutic adherence, as well as cost-efficiency. This has strongly moved the attention towards 3D printing technology, which offers innovative, digitally designed solutions able to overcome the issues of the currently marketed traditional products. Problems impacting 3DP application are mainly four-fold: the strict requirements for excipients, the development of printing software and equipment, the optimization of mechanical properties of products, and the regulatory framework. As regards excipients for pharmaceutical 3D printing, they are relatively restricted, compared to conventional manufacturing processes, mainly for technologies using heat. Much research has updated this field. However, further studies concerning biocompatible, biodegradable, and stable excipients peculiar for 3DP are required. As the complexity of product structure increases, the modeling and slicing software used to design and drive its manufacture as well as equipment, operative procedures, and control system must be constantly refreshed. From a regulatory point of view, there are still several open questions surrounding how 3D-printed healthcare products can be monitored and evaluated for quality. Although the FDA authorized in 2015 the first 3D-printed tablets, no regulations or guidelines regarding 3D-printed medicines are currently available. Progress has been made for 3D printed medical devices, for which the FDA released in 2017 guidance detailing some technical considerations. However, there is no possibility to give universal guidelines for all 3D-printed technologies and medical devices. A separate assessment of safety and effectiveness may be required for each technology and product, especially for those personalized. Furthermore, when products are customized to the patient, the question of whether 3D printing is classed as a manufacturing or compounding process also has a great impact on regulatory requirements.
References
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications-Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176.
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The status, challenges, and future of additive manufacturing in engineering. Comput.-Aided Des. 2015, 69, 65–89.
- Di Prima, M.; Coburn, J.; Hwang, D.; Kelly, J.; Khairuzzaman, A.; Ricles, L. Additively manufactured medical product–the FDA perspective. 3d Print. Med. 2016, 2, 1.
- West, T.G.; Bradbury, T.J. 3D Printing: A Case of ZipDose® Technology–World’s First 3D Printing Platform to Obtain FDA Approval for a Pharmaceutical Product. In 3D and 4D Printing in Biomedical Applications: Process Engineering and Additive Manufacturing; Wiley- VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 53–79.
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50.
- Eshkalak, S.K.; Ghomi, E.R.; Dai, Y.; Choudhury, D.; Ramakrishna, S. The role of three-dimensional printing in healthcare and medicine. Mater. Des. 2020, 194, 108940.
- Jose, P.A.; GV, P.C. 3D printing of pharmaceuticals–a potential technology in developing personalized medicine. Asian J. Pharm. Res. Dev. 2018, 6, 46–54.
- Ong, J.J.; Awad, A.; Martorana, A.; Gaisford, S.; Stoyanov, E.; Basit, A.W.; Goyanes, A. 3D printed opioid medicines with alcohol-resistant and abuse-deterrent properties. Int. J. Pharm. 2020, 579, 119169.
- Hamed, R.; Mohamed, E.M.; Rahman, Z.; Khan, M.A. 3D-printing of lopinavir printlets by selective laser sintering and quantification of crystalline fraction by XRPD-chemometric models. Int. J. Pharm. 2021, 592, 120059.
- Mohamed, E.M.; Ali, S.F.B.; Rahman, Z.; Dharani, S.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A. Formulation optimization of selective laser sintering 3D-printed tablets of clindamycin palmitate hydrochloride by response surface methodology. AAPS PharmSciTech 2020, 21, 1–15.
- Fang, D.; Yang, Y.; Cui, M.; Pan, H.; Wang, L.; Li, P.; Wu, W.; Qiao, S.; Pan, W. Three-Dimensional (3D)–Printed Zero-Order Released Platform: A Novel Method of Personalized Dosage Form Design and Manufacturing. AAPS PharmSciTech 2021, 22, 1–14.
- Cui, M.; Pan, H.; Fang, D.; Qiao, S.; Wang, S.; Pan, W. Fabrication of high drug loading levetiracetam tablets using semi-solid extrusion 3D printing. J. Drug Deliv. Sci. Technol. 2020, 57, 101683.
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162.
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T. Formulation of 3D printed tablet for rapid drug release by fused deposition modeling: Screening polymers for drug release, drug-polymer miscibility and printability. J. Pharm. Sci. 2018, 107, 390–401.
- El Aita, I.; Breitkreutz, J.; Quodbach, J. On-demand manufacturing of immediate release levetiracetam tablets using pressure-assisted microsyringe printing. Eur. J. Pharm. Biopharm. 2019, 134, 29–36.
- Khaled, S.A.; Alexander, M.R.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int. J. Pharm. 2018, 538, 223–230.
- Matijašić, G.; Gretić, M.; Vinčić, J.; Poropat, A.; Cuculić, L.; Rahelić, T. Design and 3D printing of multi-compartmental PVA capsules for drug delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 677–686.
- Azizi Machekposhti, S.; Mohaved, S.; Narayan, R.J. Inkjet dispensing technologies: Recent advances for novel drug discovery. Expert Opin. Drug Discov. 2019, 14, 101–113.
- Yan, T.-T.; Lv, Z.-F.; Tian, P.; Lin, M.-M.; Lin, W.; Huang, S.-Y.; Chen, Y.-Z. Semi-solid extrusion 3D printing ODFs: An individual drug delivery system for small scale pharmacy. Drug Dev. Ind. Pharm. 2020, 46, 531–538.
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Mohammed, G.K.; Franzé, S.; Minghetti, P.; Cilurzo, F. Personalized orodispersible films by hot melt ram extrusion 3D printing. Int. J. Pharm. 2018, 551, 52–59.
- Jamróz, W.; Kurek, M.; Łyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrek, K.; Paluch, M.; Jachowicz, R. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm 2017, 533, 413–420.
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883.
- Tian, Y.; Orlu, M.; Woerdenbag, H.J.; Scarpa, M.; Kiefer, O.; Kottke, D.; Sjöholm, E.; Öblom, H.; Sandler, N.; Hinrichs, W.L.J.; et al. Oromucosal films: From patient centricity to production by printing techniques. Expert Opin. Drug Deliv. 2019, 16, 981–993.
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123.
- Tiboni, M.; Campana, R.; Frangipani, E.; Casettari, L. 3D printed clotrimazole intravaginal ring for the treatment of recurrent vaginal candidiasis. Int. J. Pharm. 2021, 596.
- Holländer, J.; Hakala, R.; Suominen, J.; Moritz, N.; Yliruusi, J.; Sandler, N. 3D printed UV light cured polydimethylsiloxane devices for drug delivery. Int. J. Pharm. 2018, 544, 433–442.
- Naseri, E.; Cartmell, C.; Saab, M.; Kerr, R.G.; Ahmadi, A. Development of 3D printed drug-eluting scaffolds for preventing piercing infection. Pharmaceutics 2020, 12, 901.
- Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Kondiah, P.P.; Pillay, V. 3D-printing and the effect on medical costs: A new era? Expert Rev. Pharm. Outcomes Res. 2016, 16, 23–32.
- Choi, W.J.; Hwang, K.S.; Kwon, H.J.; Lee, C.; Kim, C.H.; Kim, T.H.; Heo, S.W.; Kim, J.-H.; Lee, J.-Y. Rapid development of dual porous poly(lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold application. Mater. Sci. Eng. C 2020, 110, 110693.
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping drug development using 3D printing. Drug Discov. Today 2018, 23, 1547–1555.
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. 3D Printing of Pharmaceuticals and Drug Delivery Devices; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020.
- Report, G. 3D Printed Drugs Market Research Report by Technology, by Region–Global Forecast to 2025–Cumulative Impact of COVID-19; Research and Markets, Guinness Centre, Taylors LANE: Dublin, Ireland, 2022; p. 207.
- Everett, H. Triastek Receives FDA IND Clearance for 3D Printed Drug to Treat Rheumatoid Arthritis; 3D Printing Industry Ltd.: London, UK, 2021.
- Adnkronos Triastek Closes US$ 50 Million Series B Financing, Co-led by Matrix Partners China and CPE. Available online: https://www.adnkronos.com/triastek-closes-us-50-million-series-b-financing-co-led-by-matrix-partners-china-and-cpe_3q9ZwFgfwSifRAQVbQn6Qi (accessed on 10 January 2022).
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 2018, 269, 355–363.
- Li, Q.; Wen, H.; Jia, D.; Guan, X.; Pan, H.; Yang, Y.; Yu, S.; Zhu, Z.; Xiang, R.; Pan, W. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int. J. Pharm. 2017, 525, 5–11.
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663.
- Karavasili, C.; Gkaragkounis, A.; Moschakis, T.; Ritzoulis, C.; Fatouros, D.G. Pediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3D printing. Eur. J. Pharm. Sci. 2020, 147.
- Kyobula, M.; Adedeji, A.; Alexander, M.R.; Saleh, E.; Wildman, R.; Ashcroft, I.; Gellert, P.R.; Roberts, C.J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control. Release 2017, 261, 207–215.
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212.
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432.
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48.
- Vivero-Lopez, M.; Xu, X.; Muras, A.; Otero, A.; Concheiro, A.; Gaisford, S.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Anti-biofilm multi drug-loaded 3D printed hearing aids. Mater. Sci. Eng. C 2021, 119.
- Andrés-Cano, P.; Calvo-Haro, J.A.; Fillat-Gomà, F.; Andrés-Cano, I.; Perez-Mañanes, R. Role of the orthopaedic surgeon in 3D printing: Current applications and legal issues for a personalized medicine. Rev. Española De Cirugía Ortopédica Y Traumatol. (Engl. Ed. ) 2021, 65, 138–151.
- Fu, J.; Yu, X.; Jin, Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82.
- Jung, J.P.; Bhuiyan, D.B.; Ogle, B.M. Solid organ fabrication: Comparison of decellularization to 3D bioprinting. Biomater. Res. 2016, 20, 27.
- Sonova. Available online: https://www.sonova.com/en (accessed on 10 February 2022).
- Beer, N.; Hegger, I.; Kaae, S.; De Bruin, M.L.; Genina, N.; Alves, T.L.; Hoebert, J.; Kälvemark Sporrong, S. Scenarios for 3D printing of personalized medicines-A case study. Explor. Res. Clin. Soc. Pharm. 2021, 4, 100073.
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681.
- Flores, M.; Glusman, G.; Brogaard, K.; Price, N.D.; Hood, L. P4 medicine: How systems medicine will transform the healthcare sector and society. Per Med. 2013, 10, 565–576.
- Mathur, S.; Sutton, J. Personalized medicine could transform healthcare. Biomed. Rep. 2017, 7, 3–5.
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm 2016, 499, 376–394.
- Afsana; Jain, V.; Haider, N.; Jain, K. 3D Printing in Personalized Drug Delivery. Curr Pharm Des. 2018, 24, 5062–5071.
- Vaz, V.M.; Kumar, L. 3D printing as a promising tool in personalized medicine. AAPS PharmSciTech 2021, 22, 1–20.
- Arefin, A.M.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D printing review: Materials, process, and design strategies for medical applications. Polymers 2021, 13, 1499.
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics 2019, 11, 274.
- Lafeber, I.; Ruijgrok, E.J.; Guchelaar, H.-J.; Schimmel, K.J.M. 3D Printing of Pediatric Medication: The End of Bad Tasting Oral Liquids?—A Scoping Review. Pharmaceutics 2022, 14, 416.
- Öblom, H.; Sjöholm, E.; Rautamo, M.; Sandler, N. Towards Printed Pediatric Medicines in Hospital Pharmacies: Comparison of 2D and 3D-Printed Orodispersible Warfarin Films with Conventional Oral Powders in Unit Dose Sachets. Pharmaceutics 2019, 11, 334.
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317.
- Varghese, R.; Salvi, S.; Sood, P.; Karsiya, J.; Kumar, D. 3D printed medicine for the management of chronic diseases: The road less travelled. Ann. 3d Print. Med. 2022, 5, 100043.
- Leite, M.; Soares, B.; Lopes, V.; Santos, S.; Silva, M.T. Design for personalized medicine in orthotics and prosthetics. Procedia CIRP 2019, 84, 457–461.
- van der Stelt, M.; Grobusch, M.P.; Koroma, A.R.; Papenburg, M.; Kebbie, I.; Slump, C.H.; Maal, T.J.J.; Brouwers, L. Pioneering low-cost 3D-printed transtibial prosthetics to serve a rural population in Sierra Leone—An observational cohort study. EClinicalMedicine 2021, 35, 100874.
- Fan, H.; Fu, J.; Li, X.; Pei, Y.; Li, X.; Pei, G.; Guo, Z. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: A case series and review of the literature. World J. Surg. Oncol. 2015, 13, 308.
- Zhu, Y.; Liu, K.; Deng, J.; Ye, J.; Ai, F.; Ouyang, H.; Wu, T.; Jia, J.; Cheng, X.; Wang, X. 3D printed zirconia ceramic hip joint. with precise structure and broad-spectrum antibacterial properties. Int. J. Nanomed. 2019, 14, 5977–5987.
- Sheela, U.B.; Usha, P.G.; Joseph, M.M.; Melo, J.S.; Thankappan Nair, S.T.; Tripathi, A. 7-3D printing in dental implants. In 3D Printing in Medicine and Surgery; Thomas, D.J., Singh, D., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 83–104.
- Prakash, D.; Davis, R.; Sharma, A.K. Design and Fabrication of Dental Implant Prototypes Using Additive Manufacturing. In First International Conference on Materials Science and Manufacturing Technology; Hotel Aloft, Coimbatore, Tamil Nadu, India, 12–13 April 2019, IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019.
- Calvo-Haro, J.A.; Pascau, J.; Mediavilla-Santos, L.; Sanz-Ruiz, P.; Sánchez-Pérez, C.; Vaquero-Martín, J.; Perez-Mañanes, R. Conceptual evolution of 3D printing in orthopedic surgery and traumatology: From “do it yourself” to “point of care manufacturing”. BMC Musculoskelet. Disord. 2021, 22, 1–10.
- Wixted, C.M.; Peterson, J.R.; Kadakia, R.J.; Adams, S.B. Three-dimensional Printing in Orthopaedic Surgery: Current Applications and Future Developments. JAAOS Glob. Res. Rev. 2021, 5, e20.00230–11.
- Wu, Y.; Kennedy, P.; Bonazza, N.; Yu, Y.; Dhawan, A.; Ozbolat, I. Three-Dimensional Bioprinting of Articular Cartilage: A Systematic Review. Cartilage 2021, 12, 76–92.
- Xiongfa, J.; Hao, Z.; Liming, Z.; Jun, X. Recent advances in 3D bioprinting for the regeneration of functional cartilage. Regen. Med. 2018, 13, 73–87.
- Agarwal, S.; Saha, S.; Balla, V.K.; Pal, A.; Barui, A.; Bodhak, S. Current Developments in 3D Bioprinting for Tissue and Organ Regeneration–a Review. Front. Mech. Eng. 2020, 6, 90.
- Weisman, J.A.; Ballard, D.H.; Jammalamadaka, U.; Tappa, K.; Sumerel, J.; D’Agostino, H.B.; Mills, D.K.; Woodard, P.K. 3D Printed Antibiotic and Chemotherapeutic Eluting Catheters for Potential Use in Interventional Radiology: In Vitro Proof of Concept Study. Acad. Radiol. 2019, 26, 270–274.
- Kim, T.H.; Lee, J.-H.; Ahn, C.B.; Hong, J.H.; Son, K.H.; Lee, J.W. Development of a 3D-Printed Drug-Eluting Stent for Treating Obstructive Salivary Gland Disease. ACS Biomater. Sci. Eng. 2019, 5, 3572–3581.
- Tappa, K.; Jammalamadaka, U.; Weisman, J.A.; Ballard, D.H.; Wolford, D.D.; Pascual-Garrido, C.; Wolford, L.M.; Woodard, P.K.; Mills, D.K. 3D printing custom bioactive and absorbable surgical screws, pins, and bone plates for localized drug delivery. J. Funct. Biomater. 2019, 10, 17.
- Domsta, V.; Seidlitz, A. 3D-Printing of Drug-Eluting Implants: An Overview of the Current Developments Described in the Literature. Molecules 2021, 26, 4066.
- Wang, Z.; Yang, Y. Application of 3D Printing in Implantable Medical Devices. BioMed Res. Int. 2021, 2021, 6653967.
- Mohamdeen, Y.M.G.; Tabriz, A.G.; Tighsazzadeh, M.; Nandi, U.; Khalaj, R.; Andreadis, I.; Boateng, J.S.; Douroumis, D. Development of 3D printed drug-eluting contact lenses. J. Pharm Pharm. 2021, 20, 1–10.
- Beitler, B.G.; Abraham, P.F.; Glennon, A.R.; Tommasini, S.M.; Lattanza, L.L.; Morris, J.M.; Wiznia, D.H. Interpretation of regulatory factors for 3D printing at hospitals and medical centers, or at the point of care. 3d Print. Med. 2022, 8, 1–7.
- ASME 3D Printing Medical Devices at the Point of Care: Webinar Series. Available online: https://resources.asme.org/poc3dp-events (accessed on 5 February 2022).
- GOV.UK Consultation on Point of Care Manufacturing. Available online: https://www.gov.uk/government/consultations/point-of-care-consultation/consultation-on-point-of-care-manufacturing (accessed on 7 February 2022).
- Boon, W.; van Wee, B. Influence of 3D printing on transport: A theory and experts judgment based conceptual model. Transp. Rev. 2018, 38, 556–575.
- Manners-Bell, J.; Lyon, K. The implications of 3D printing for the global logistics industry. Transp. Intell. 2012, 10, 1–5.
- Campbell, T.; Williams, C.; Ivanova, O.; Garrett, B. Could 3D printing change the world. In Technologies, Potential, and Implications of Additive Manufacturing; Atlantic Council: Washington, DC, USA, 2011; p. 3.
- Zhou, H.; Bhaduri, S.B. 12-3D printing in the research and development of medical devices. In Biomaterials in Translational Medicine; Yang, L., Bhaduri, S.B., Webster, T.J., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 269–289.
- Durfee, W.K.; Iaizzo, P.A. Chapter 21-Medical Applications of 3D Printing. In Engineering in Medicine; Iaizzo, P.A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 527–543.
- Morrison, R.J.; Kashlan, K.N.; Flanangan, C.L.; Wright, J.K.; Green, G.E.; Hollister, S.J.; Weatherwax, K.J. Regulatory Considerations in the Design and Manufacturing of ImplanTable 3D-Printed Medical Devices. Clin. Transl. Sci. 2015, 8, 594–600.
- FDA. Technical Considerations for Additive Manufactured Medical Devices. In FDA Center for Devices and Radiological Health; FDA: Silver Spring, MD, USA, 2017.
- Ian Gibson, I.G. Additive Manufacturing Technologies 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing; Springer: Berlin/Heidelberg, Germany, 2015.
- ISO/ASTM 52900:2021 (EN); Additive Manufacturing—General Principles—Fundamentals and Vocabulary. ISO, International Organization for Standardization: London, UK, 2021.
- Saleh Alghamdi, S.; John, S.; Roy Choudhury, N.; Dutta, N.K. Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges. Polymers 2021, 13, 753.
- Mohapatra, S.; Kar, R.K.; Biswal, P.K.; Bindhani, S. Approaches of 3D printing in current drug delivery. Sens. Int. 2022, 3.
- Barui, S. 3D inkjet printing of biomaterials: Principles and applications. Med. Devices Sens. 2021, 4, e10143.
- Hsiao, W.-K.; Lorber, B.; Reitsamer, H.; Khinast, J. 3D printing of oral drugs: A new reality or hype? Expert Opin. Drug Deliv. 2018, 15, 1–4.
- Karalia, D.; Siamidi, A.; Karalis, V.; Vlachou, M. 3D-Printed oral dosage forms: Mechanical properties, computational approaches and applications. Pharmaceutics 2021, 13, 1401.
- van den Heuvel, K.A.; de Wit, M.T.W.; Dickhoff, B.H.J. Evaluation of lactose based 3D powder bed printed pharmaceutical drug product tablets. Powder Technol. 2021, 390, 97–102.
- Wilts, E.M.; Ma, D.; Bai, Y.; Williams, C.B.; Long, T.E. Comparison of linear and 4-arm star poly (vinyl pyrrolidone) for aqueous binder jetting additive manufacturing of personalized dosage tablets. ACS Appl. Mater. Interfaces 2019, 11, 23938–23947.
- Infanger, S.; Haemmerli, A.; Iliev, S.; Baier, A.; Stoyanov, E.; Quodbach, J. Powder bed 3D-printing of highly loaded drug delivery devices with hydroxypropyl cellulose as solid binder. Int. J. Pharm. 2019, 555, 198–206.
- Tian, P.; Yang, F.; Yu, L.-P.; Lin, M.-M.; Lin, W.; Lin, Q.-F.; Lv, Z.-F.; Huang, S.-Y.; Chen, Y.-Z. Applications of excipients in the field of 3D printed pharmaceuticals. Drug Dev. Ind. Pharm. 2019, 45, 905–913.
- Yu, D.-G.; Branford-White, C.; Ma, Z.-H.; Zhu, L.-M.; Li, X.-Y.; Yang, X.-L. Novel drug delivery devices for providing linear release profiles fabricated by 3DP. Int. J. Pharm. 2009, 370, 160–166.
- Shi, K.; Tan, D.K.; Nokhodchi, A.; Maniruzzaman, M. Drop-on-powder 3D printing of tablets with an anti-cancer drug, 5-fluorouracil. Pharmaceutics 2019, 11, 150.
- Sen, K.; Manchanda, A.; Mehta, T.; Ma, A.W.; Chaudhuri, B. Formulation design for inkjet-based 3D printed tablets. Int. J. Pharm. 2020, 584, 119430.
- Charoo, N.A.; Barakh Ali, S.F.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective laser sintering 3D printing–an overview of the technology and pharmaceutical applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877.
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594.
- Kafle, A.; Luis, E.; Silwal, R.; Pan, H.M.; Shrestha, P.L.; Bastola, A.K. 3D/4D Printing of polymers: Fused deposition modelling (FDM), selective laser sintering (SLS), and stereolithography (SLA). Polymers 2021, 13, 3101.
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107.
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293.
- Low, K.; Leong, K.; Chua, C.; Du, Z.; Cheah, C. Characterization of SLS parts for drug delivery devices. Rapid Prototyp. J. 2001, 7, 262–267.
- Cheah, C.; Leong, K.; Chua, C.; Low, K.; Quek, H. Characterization of microfeatures in selective laser sintered drug delivery devices. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2002, 216, 369–383.
- Trenfield, S.J.; Goyanes, A.; Telford, R.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. 3D printed drug products: Non-destructive dose verification using a rapid point-and-shoot approach. Int. J. Pharm. 2018, 549, 283–292.
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52.
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective laser sintering 3D printing of orally disintegrating printlets containing ondansetron. Pharmaceutics 2020, 12, 110.
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. An overview of 3D printing technologies for soft materials and potential opportunities for lipid-based drug delivery systems. Pharm. Res. 2019, 36, 1–20.
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14.
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115.
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of geometry on the drug release profiles of stereolithographic (SLA) 3D-printed tablets. AAPS PharmSciTech 2018, 19, 3355–3361.
- Karakurt, I.; Aydoğdu, A.; Çıkrıkcı, S.; Orozco, J.; Lin, L. Stereolithography (SLA) 3D printing of ascorbic acid loaded hydrogels: A controlled release study. Int. J. Pharm. 2020, 584, 119428.
- Konasch, J.; Riess, A.; Mau, R.; Teske, M.; Rekowska, N.; Eickner, T.; Grabow, N.; Seitz, H. A novel hybrid additive manufacturing process for drug delivery systems with locally incorporated drug depots. Pharmaceutics 2019, 11, 661.
- Forouzandeh, F.; Ahamed, N.N.; Hsu, M.-C.; Walton, J.P.; Frisina, R.D.; Borkholder, D.A. A 3D-printed modular microreservoir for drug delivery. Micromachines 2020, 11, 648.
- Park, B.J.; Choi, H.J.; Moon, S.J.; Kim, S.J.; Bajracharya, R.; Min, J.Y.; Han, H.-K. Pharmaceutical applications of 3D printing technology: Current understanding and future perspectives. J. Pharm. Investig. 2019, 49, 575–585.
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D printed medicines: A new branch of digital healthcare. Int. J. Pharm. 2018, 548, 586–596.
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125.
- Tan, L.J.; Zhu, W.; Zhou, K. Recent Progress on Polymer Materials for Additive Manufacturing. Adv. Funct. Mater. 2020, 30, 43.
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.; Ong, J.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Harnessing Artificial Intelligence for the Next Generation of 3D Printed Medicines. Adv. Drug Deliv. Rev. 2021, 175, 113805.
- Basit, A.W.; Gaisford, S. 3D Printing of Pharmaceuticals; Springer: Berlin/Heidelberg, Germany, 2018.
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm 2015, 494, 643–650.
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314.
