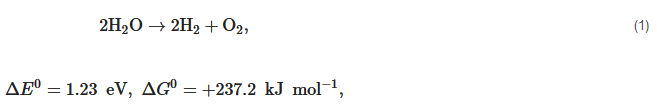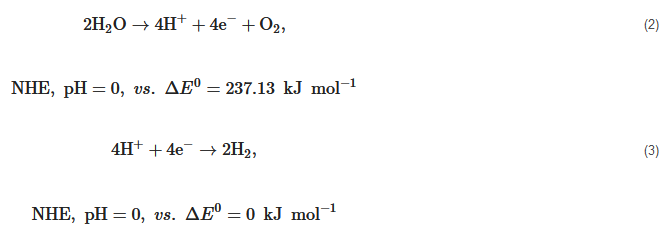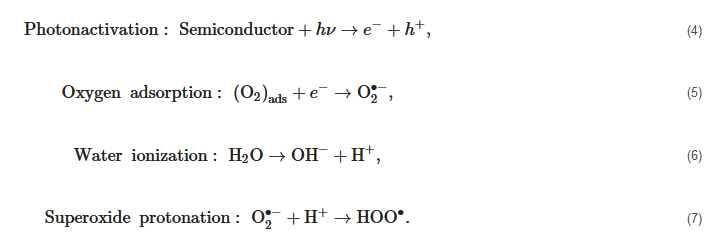A complete view of basic principles and mechanisms with regard to improving the structure stability, physical and chemical properties of the low dimensional semiconductor-based photocatalysts is presented here. Various 2D semiconductor-based photocatalysts show a high electrochemical property and photocatalytic performance due to their ultrathin character, high specific surface area with more activity sites, tunable bandgap to absorb sunlight and versatile options in structural assembly with other nanosheets. At present, most photocatalysts still need rare or expensive noble metals to improve the photocatalytic activity, which inhibits their commercial-scale application extremely. Thus, developing less costly, earth-abundant semiconductor-based photocatalysts with the efficient conversion of sunlight energy remains the primary challenge. A concise overview of different types of 2D semiconductor-mediated photocatalysts is given to figure out the advantages and disadvantages for mentioned semiconductor-based photocatalysis, including the structural property and stability, synthesize method, electrochemical property, and optical properties for H2/O2 production half-reaction along with overall water splitting.
- Ultrathin semiconductor-based photocatalysts
- Hydrogen evolution reaction
- Oxygen evolution reaction
- Overall water splitting
Abstract
Ultrathin two-dimensional (2D) semiconductor-mediated photocatalysts have shown their compelling potential and have arguably received tremendous attention in photocatalysis because of their superior thickness-dependent physical, chemical, mechanical and optical properties. Although numerous comprehensions about 2D semiconductor photocatalysts have been amassed, up to now, low-cost efficiency, degradation, the kinetics of charge transfer along with recycling are still the big challenges to realize a wide application of 2D semiconductor-based photocatalysis. At present, most photocatalysts still need rare or expensive noble metals to improve the photocatalytic activity, which inhibits their commercial-scale application extremely. Thus, developing less costly, earth-abundant semiconductor-based photocatalysts with the efficient conversion of sunlight energy remains the primary challenge. This review begins with a brief description of the general mechanism of overall photocatalytic water splitting. Then a concise overview of different types of 2D semiconductor-mediated photocatalysts is given to figure out the advantages and disadvantages for mentioned semiconductor-based photocatalysis, including the structural property and stability, synthesize method, electrochemical property, and optical properties for \( \rm H_{2}/O_{2} \) production half-reaction along with overall water splitting. Finally, we conclude this review with a perspective, marked on some remaining challenges and new directions of 2D semiconductor-mediated photocatalysts.
1. Introduction
Nowadays, the biggest technological challenges for human beings are the increasing consumption of resources and energy and the related environmental pollution problem all over the world. For example, the worldwide energy consumption will expect to reach up to 815 quadrillion Btu in 2040 due to the growing global production and population [1][2]. However, the huge energy resources in the earth mainly originate from non-renewable coal, oil, natural gas, and nuclear energy, and their reserves are limited. Although the energy is released by combustion on the application of these fossil fuels, a series of critical environmental problems—water contamination, greenhouse gas emissions, global warming, and the emergence of a hole in the ozone layer—occur at the same time. Thus, lots of scientists, politicians, industry groups are encouraged to seek new and sustainable alternative energy resources. Every day the sun radiates an enormous amount of energy for our planet, which has attracted great attention as a promising alternative energy source for cutting greenhouse gas emissions and providing the current and future human energy demand. However, it is not nearly sufficient, therefore, the harvest and conversion of solar energy into other kinds of energy is a priority to resolve [3][4][5]. At present, photovoltaic cells are already a popular sight on rooftops to directly convert solar energy into electricity. However, electricity presents its own challenges in that it would have to be used immediately or be stored in a secondary device at a high energy density, such as capacitors or batteries. In comparison with converting solar energy into electricity, synthesizing storable, transportable chemical fuels via photocatalytic processes would be a more attractive approach, in which the harnessing of energy in sunlight would be stored in the form of molecular bonds through a thermodynamic "uphill reaction" (see Equation (1)). Up to now, a photocatalysis technique has been used in different application fields, such as water splitting to produce hydrogen [6][7], sterilization [8], wastewater treatment and self-cleaning materials [9], decomposition of crude spills [10][11], dye-sensitized solar cell and inactivation of cancer cells [12][13].
Hydrogen (H2) is a clean and carbon-free fuel with high specific enthalpy. Whereas, until now, around 95% of the world’s hydrogen fuel is mainly sourced from natural gas, by reacting methane with steam using fossil fuel to produce hydrogen and carbon dioxide (CO2). Thus the production of hydrogen from methane is a kind of fossil fuel product rather than renewable energy. The critical method to utilize hydrogen production is developing an effective H2-preparation approach without ever seeing fossil fuels. The method using photocatalytic materials to produce hydrogen has been demonstrated since 1972 according to the following reaction equation,
This photocatalytic process is driven by a thermodynamic “uphill reaction” of solar energy with a large change of ΔG
Therefore, the surface of the photocatalytic materials should be struck using suitable energy with the corresponding photons. The estimated solar photon flux density (Q) is 2000 μmols−1m−2 [15]. While the previous reports found the holes took less than 2 μs to react with the water vapor while the oxygen took 10–100 μs to capture the electrons in P25 TiO





There are three major steps to overall photocatalytic split water for a semiconductor photocatalyst: (1) the absorption of photons on the surface of a semiconductor, with appropriate energy, which can stimulate electron transfer to the conduction band position from the valence band position and generate electron-hole pairs (e−−h+), (2) the separation and migration of charge carriers on the surface of the material at a short span of time; (3) the impingement between the corresponding free electrons and holes on the surface of the system to complete the water-splitting reaction to generate H









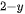

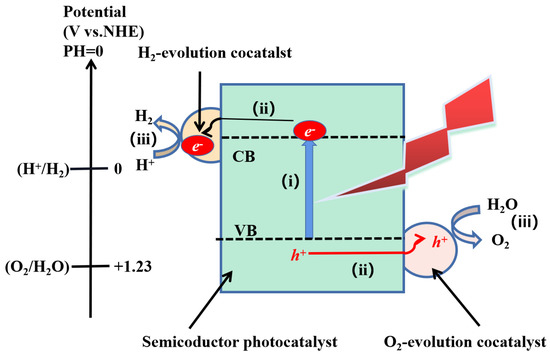
Figure 1. Schematic diagram of the three major steps of overall photocatalytic water splitting on a semiconductor photocatalyst [18].
On the other hand, nanoparticles, porous nanospheres, and nanowires have recently attracted a large amount of attention in the process of water splitting in that they possess unconventional and fancy physical and chemical properties, which may be easier to meet the requirements for overall photocatalytic splitting water compared to their bulk counterparts [30][72][73][74][75][76]. First, these different nanostructures could decrease the rate of charge carriers recombination and let them travel a very small distance, which largely enriches the selectivity of photocatalytic materials and paves a new path to design an effective photocatalysis. Second, 2D nanostructured photocatalysts could have more active sites for electrochemical reactions due to their larger surface areas. Third, quantum confinement effects in 2D metallic and semiconductor nanostructures dependent on the size and morphology of photocatalysts could open a new approach to engineer the processes of sunlight harvesting. It is because the quantum size effects can restrict electrons in order to accommodate the additional atom, platelike nanoparticles, disk, or cluster, resulting in an increase of energy levels and HOMO-LUMO bandgap. For 1D quantum confined materials, it is found that the modification of the aspect ratio of nanorods could largely tune the optical response properties [77]. Last but not least, various 2D nanomaterials provide more sources to integrate heterogeneous structures with multiple functional properties. On the other hand, for the majority of 3D semiconductors, it is commonly ignored to distinguish the electronic and optical bandgaps due to the small exciton binding energy. While the distinction between electronic and optical bandgaps in 2D nanomaterials is significant and obvious. The Bethe–Salpeter equation is frequently used to predict the exciton binding energy and optical bandgap. Furthermore, most of the 2D materials exhibit more soft elastic constants, which could have extra application compared to bulk 3D counterparts. Particularly, the band edge positions and bandgaps are very sensitive to small strains with a significant extent, thus it could facilitate tuning the photocatalytic performance of 2D nanomaterials as photocatalysts. Herein, the current review work covers the fundamental mechanism of overall photocatalytic water splitting and recent progress in the use of two dimensional (2D) semiconductor-based nanomaterials and their nanoheterostructure composites with a focus on the relationship between the structural property of the nanomaterials and their photocatalytic activity. It begins by introducing the various mechanisms of photoexcited carriers on different 2D semiconductor-based nanomaterials during photocatalytic overall water splitting to provide a whole view for the principle of work on the basis of thermodynamics half-reactions. Then, we compare the general structural property, advantages in electronic and surface properties, and electrochemical performances to pursue high efficiency of water splitting on the different 2D semiconductors, including carbon nitride (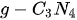
2. Mechanisms of Overall Photocatalytic Water Splitting
In previous researches, the mechanisms of 2D and 3D materials as photocatalysts to split water have been extensively reported. With regard to semiconductors, the absorbed photon should possess higher energy (hν) than the corresponding bandgap of the semiconductor in order to excite the electrons to the empty conduction band (CB) position from the filled valence band (VB) position of the semiconductor photocatalyst. Then the excited electrons and holes pair (e−−h+) is formed according to the following expression,
The bandgap of a single semiconductor photocatalyst should meet the requirements of reduction reaction potential with 0 eV for catalyzing H






In a system with a semiconductor electrode immersing in an electrolyte solution, the electrons transfer take place spontaneously in order to keep the balance between the Fermi level (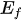
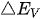


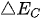
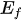
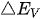
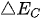
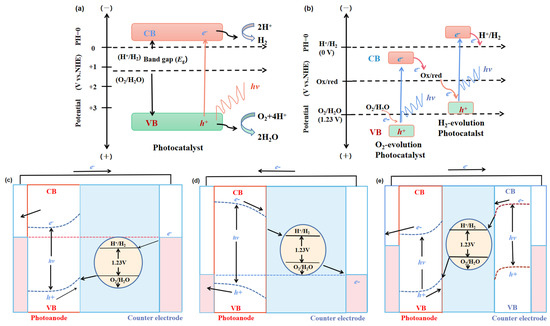
Figure 2. Energy schematic diagram based on (a) one-step water splitting and (b) Z-scheme mechanism; and photoelectrochemical water splitting using (c) a photoanode, (d) photocathode, and (e) photoanode and count electrode in tandem systems for photocatalytic water splitting, respectively [81].
3. Classification of 2D Semiconductors for Photocatalysts
To date, a great amount of 2D nanosheets have been synthesized by various chemical and/or physical methods, which are mainly divided into two types, layered and non-layer structural materials. With regard to layered materials, the in-plane layer is formed by connecting the in-plane atoms by strong chemical bonding. However, the weak van der Waals interaction plays an important role in stacking these monolayers to form bulk crystals [82]. The representative layered material is graphite crystal, which is stacked by many graphene layers via weak van der Waals force. In addition, nitrides (such as g-C
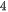
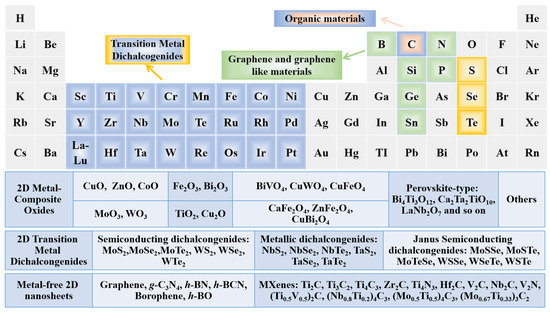
Figure 3. Periodic table with highlighted elements for typical ultrathin 2D materials.
4. Synthesis Methods for 2D Materials
Various approaches have been explored to synthesize 2D monolayers or few-layer nanosheets and tailor the structure with corresponding physical and chemical properties in order to suit different kinds of applications. Generally, the synthesis methods can be classified into two types based on the composition, crystal phase, and surface property of materials: (1) the top-down method, which mainly depends on the exfoliation of 2D monolayers from their parental layered bulk material by overcoming the weak interlayer binding force; (2) the bottom-up method, which is more versatile in principle than most 2D nanosheets, might be gained by this method. In this section, some representative methods to prepare 2D materials are discussed and summarized.
4.1 Micromechanical cleavage using scotch tape
Micromechanical cleavage using scotch tape is a simple and conventional method to fabricate thin flake by peeling and rubbing the surface of inorganic layered bulk materials. The weak van der Waals interaction between the layers of the materials is the key factor so that the applied mechanical force can peel of single or few layers of 2D materials without breaking the chemical bonding of the in-plane layer. The micromechanical cleavage technique is first used by Grim et al. to obtain graphene monolayer from highly oriented graphite [92]. Besides, a more thin flake can be obtained by repeated several times of this process. Then these thin flakes are washed with acetone and transferred on a clean, flat target surface of SiO


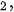






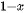

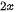
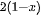



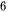
This micromechanical cleavage technique is simple, fast, and low cost, and the size of exfoliated 2D materials can be up to tens of micrometers. Besides, no chemicals are utilized but the shear force is applied during the fabrication process. Thus, the fabricated nanosheets keep the pristine structural integrity with minimum defects from the parental bulk materials, giving rise to many advantages. As a whole, the large lateral size, clean surface, "perfect" crystal quality, high crystallinity make mechanically cleaved 2D materials as remarkable candidates to investigate the corresponding mechanical, electronic, and optical properties and application in electronic and optoelectronic devices. While there are some certain disadvantages that hinder their realistic applications. First, the production yield of this process is extremely low and the nanosheets always coexist with thick flakes on the substrate. Thus, it is suitable for laboratory scale studies but difficult to utilize in various high-end technological applications, high yield, and large-scale production. Second, the fundamental/intrinsic properties, such as size, thickness, and shape of the ultrathin 2D nanosheets are unstable and uncontrollable since the mechanical exfoliation technique by hand is a shortage of precision and repeatability. Third, the utilized substrate is significantly important in the fabrication process. Recently, one advanced micromechanical cleavage method is reported by Sutter et al. to improve the production yield and increase the surface area of graphene and Bi




4.2 Liquid exfoliation
Liquid exfoliation actually also relies on the mechanical forces since the original idea of this technique is to apply the proper mechanical force on layered bulk crystals dispersed in a liquid to exfoliate them into single- or few-layer 2D nanosheets. Thus this technique is mechanical force-assisted liquid exfoliation which can categorize into sonication-assisted liquid exfoliation and shear force-assisted liquid exfoliation. Compare to mechanical exfoliation of 2D nanosheets, liquid exfoliation is a reliable method with high quality and large quantity to produce mono and few-layer 2D sheets at bulk scale. Up to now, the exfoliation method in liquid media has been largely reported to gain ultrathin 2D materials from for their parental layered bulk crystals, such as MoS


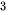



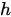


Sonication-assisted liquid exfoliation is an established technique using the simple and common mechanical force to peel the layered bulk materials into 2D sheets in liquid media, in which the bulk crystals are dispersed into a polar solvent and washed using bubbles induced by sonication in the solution. The cavitation bubbles generated by high energy ultrasonic waves could create high energy during the sudden burst with the release of pressure, hence the resultant dispersion is centrifuged to exfoliate of layers in the solution. It is to be noted that the key parameter is the surface energies between the layered bulk materials and the liquid system should be matched. It is because the solvents have the surface tension, using the surface tension of solvent similar to the surface energy of layer bulk crystals could reduce the energetic cost of exfoliation and prohibited the restacking and aggregating between the 2D layers [82][111][114]. For example, it is reported that pure water or pure ethanol is not an efficient way to synthesize TMDs nanosheets due to the large surface energy of the solvent, but the mixing water with ethanol could effectively exfoliate and disperse TMDs. Thus, choosing the optimum solvent could largely utilize the potential of this technique. Besides, the sonication time and centrifugation rate are also significant factors that are related to the quality and quantity of 2D materials. This method is first used to exfoliate graphite into graphene in 2008 [115]. They reported no complicated equipment or expensive chemicals is used during their experiment. Subsequently, more than 25 solvents were studied to naturally exfoliate of 2D nanosheets based on crystallinity, thickness, size, and yield of the resultant layers [111]. It is summarized that dimethylformamide, cyclohexyl-pyrrolidinone, N-methyl-pyrrolidone, Ndodecyl-pyrrolidone, MDSO, NVP, ortho-dichlorobenzene, and IPA are mostly used solvents to ultrasonic exfoliate of MoS


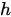

Although the production of 2D nanosheets is increased with concentrations of ~1 mg mL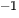
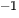
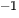
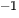
4.3 Chemical vapor deposition
The chemical vapor deposition (CVD) technique is a common and traditional method to synthesize a high-purity 2D layer of metal with large area and uniform thickness, such as Ti, W, Zr, and Si on substrates, for large-scale electronic and flexible optoelectronic devices [122]. The CVD technique is first commercially applied in 1897 to reduce WO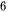


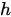












4.4 Van der Waal epitaxial growth on the substrate
There is one important synthesized form for the CVD method, it is van der Waal epitaxial growth on the substrate in which the substrate surface usually plays as a seed crystal. While it is known the substrate often acts as a catalyst. The most predominant of van der Waal epitaxial growth on the substrate is to grow large aspect ratio 2D nanosheets. The epitaxial growth of atom thick, in-plane heterostructure of graphene, and 
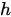
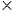
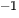

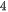







4.5 Hydrothermal synthesis
Hydrothermal synthesis is another popular bottom-up method to crystallize substance at high pressure and high temperature. Particularly, it is suitable to synthesized the materials which possess high vapor pressure at the melting point. Besides, layered 2D nanosheets could be synthesized using the hydrothermal method. Taking MoS















The hydrothermal method is recently widely used in industrial incubation to large-scale synthesize nanosheets. For example, 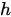








5. Conclusions, Perspectives, and Challenges
In summary, we have attempted to provide a panorama of basic principles and mechanisms with regard to improving the structure stability, physical and chemical properties of low dimensional semiconductor-based photocatalysts. After reporting on a large amount of literature, various 2D semiconductor-based photocatalysts show a high electrochemical property and photocatalytic performance due to their ultrathin character, high specific surface area with more activity sites, tunable bandgap to absorb sunlight, and versatile options in structural assembly with other nanosheets. Particularly, special focus has been placed on various strategies implemented to tackle problems associated with synthetic methods, structural stability, and property, durability, and photodecomposition of 2D semiconductors. From the above, one can know extensive efforts have been devoted in the last few decades to construct various photocatalytic 2D semiconductors. Yet, co-photocatalysts made up of earth-abundant elements with low-cost are still required to further investigate and develop a mechanistic understanding and controllable synthetic strategy in order to achieve high-efficiency, stable, and less costly 2D semiconductor-based photocatalysts. Many challenges, such as the low yield efficiency, stability, durability against etchant solution, kinetics of charge carriers absorption and migration, charge trap, and recycling demand, need to be settled urgently in the near future.
Concurrently, advanced methods for tailoring the electronic and chemical structures of these 2D semiconductor materials have been developed, pushing its rapid development in various photocatalytic applications. For example, homogeneous doping has become a reality [66][159], but how to identify the dopant-induced electronic structures and what is the effect of doping elements on the stability of the system under reaction conditions are rarely being investigated. Until now, only a few examples of improved photocatalyst stability of photocatalysts have been reported in order to deal with the complicated synthetic processes during splitting water. Thus, the present studies on 2D semiconductor photocatalysts are still in the initial stage and further systematic investigations are required. On the other hand, in order to precisely control the dopant distribution, uniformity, composition and the surface states property of photocatalysts, seeking new doping approaches should be addressed for efficient photocatalyst design. In addition, the influence of intrinsic defects, structural shape, the number of layers, and lateral interface size is barely evaluated on the photocatalytic performance, especially in composite photocatalysts. Importantly, an overall apparent efficiency of the entire photocatalytic system is the main topic in the current studies. While the charge transfer process, such as transfer minimum energy pathways, migration diffusivity, charge carrier lifetime recombination reason, etc., in photocatalysts should be addressed and detailedly investigated to further advance the field. With regard to the recycling problem, designing suitable devices or tuning the magnetic properties might be a good choice.
In the theoretical aspect, first-principles calculations based on density functional theory (DFT) are an efficient way to screen the photocatalytic potential spaces, however, a number of key bottlenecks are required to breakthrough. Firstly, the solvation effects are not considered in most of the present theoretical studies. Whereas it is well known that most photocatalytic reactions are related to solid-liquid reaction systems. Hence, it is significantly important and necessary to develop an accurate solvation model as realistically as possible. For example, how the various molecules adsorb on the photocatalyst and influence the calculation of reaction energy and energy barrier heights should be considered in the photocatalyst system in order to realistically simulate the experimental testing conditions. Secondly, various previous theoretical data use the traditional DFT method, whereas the enlightenment of photocatalytic kinetic studies is still rarely studied due to the fact that it is technically challenging. The photoexcitation of a semiconductor photocatalyst, including 1D, 2D, and 3D materials, produces photogenerated electrons and holes, leading to an excited state of the photocatalyst. However, the feature of the excited state and the corresponding reason for the energy barrier reduction under light irradiation are still secrets. Therefore, understanding the dynamics of excited electrons plays a significant role in understanding the whole photocatalytic processes [160][161]. Thirdly, the thermodynamic photocatalytic processes, such as energetic requirement, band edge alignment relative to the hydrogen revolution potential of H2/H+, and oxygen revolution potential of H2O/O2 needs to be deeply understood. For example, the migration behaviors of electrons and holes from substrates are not given much attention, which exhibits significant impacts on photocatalytic efficiency since 2D atomic thin photocatalysts are usually stabilized on various substrates. Last but not least, novel simulation methods or performing dynamic simulation algorithms rather than static crystal models are strongly required since the surfaces of photocatalysts are dynamically and frequently undergoing changes during the reaction proceeds. With rapid advances and rich knowledge about 2D materials in the many joint efforts of research and development sectors in the last few decades, it is the best time for us to do research on photocatalysis. At the same time, one can envision that utilization of the absorbed sunlight energy could largely reduce the fossil energy demand for human development, and further decrease the environmental pollution. Thus one can see photocatalysts play an increasingly important role in that we not only need to improve the performance of the existing photocatalysts but also invent new engineering approaches to better exploit new novel photocatalysts. Thus, joint efforts from various disciplines are needed. We wish that the present mini-review serves as a catalyst to facilitate the discussions by various specialists from physics, chemistry, materials engineering, computer science, and so on.
References
- Conti, J.; Holtberg, P.; Diefenderfer, J.; LaRose, A.; Turnure, J.T.; Westfall, L. International Energy Outlook 2016 with Projections to 2040; Technical Report; USDOE Energy Information Administration (EIA): Washington, DC, USA, 2016; Office of Energy Analysis.
- Yang, X.; Yang, Y.; Lu, Y.; Sun, Z.; Hussain, S.; Zhang, P. First-principles GGA+U calculation investigating the hydriding and diffusion properties of hydrogen in PuH2+x, 0 ≤ x ≤ 1. Int. J. Hydrogen Energy 2018, 43, 13632–13638.
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580.
- Colmenares, J.C.; Luque, R.; Campelo, J.M.; Colmenares, F.; Karpiński, Z.; Romero, A.A. Nanostructured photocatalysts and their applications in the photocatalytic transformation of lignocellulosic biomass: An overview. Materials 2009, 2, 2228–2258.
- Yang, X.; Lu, Y.; Zhang, P. First-principles study of native point defects and diffusion behaviors of helium in zirconium carbide. J. Nucl. Mater. 2015, 465, 161–166.
- Graetzel, M. Artificial photosynthesis: Water cleavage into hydrogen and oxygen by visible light. Accounts Chem. Res. 1981, 14, 376–384.
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473.
- Armelao, L.; Barreca, D.; Bottaro, G.; Gasparotto, A.; Maccato, C.; Maragno, C.; Tondello, E.; Štangar, U.L.; Bergant, M.; Mahne, D. Photocatalytic and antibacterial activity of TiO2 and Au/TiO2 nanosystems. Nanotechnology 2007, 18, 375709.
- Folli, A.; Pade, C.; Hansen, T.B.; De Marco, T.; Macphee, D.E. TiO2 photocatalysis in cementitious systems: Insights into self-cleaning and depollution chemistry. Cement Concerte Res. 2012, 42, 539–548.
- Yue, X.Q. Effect of ZnO-loading method on adsorption and decomposition capacities of expanded graphite/ZnO composites for crude oil. Adv. Mater. Res. Trans. Tech. Publ. 2011, 284, 173–176.
- Kwon, S.; Fan, M.; Cooper, A.T.; Yang, H. Photocatalytic applications of micro-and nano-TiO2 in environmental engineering. Crit. Rev. Environ. Sci. Technol. 2008, 38, 197–226.
- Cai, R.; Hashimoto, K.; Kubota, Y.; Fujishima, A. Increment of photocatalytic killing of cancer cells using TiO2 with the aid of superoxide dismutase. Chem. Lett. 1992, 21, 427–430.
- Tian, C.Y.; Xu, J.J.; Chen, H.Y. A novel aptasensor for the detection of adenosine in cancer cells by electrochemiluminescence of nitrogen doped TiO2 nanotubes. Chem. Commun. 2012, 48, 8234–8236.
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278.
- Ganguly, P.; Byrne, C.; Breen, A.; Pillai, S.C. Antimicrobial activity of photocatalysts: Fundamentals, mechanisms, kinetics and recent advances. Appl. Catal. B Environ. 2018, 225, 51–75.
- Yalavarthi, R.; Naldoni, A.; Kment, Š.; Mascaretti, L.; Kmentová, H.; Tomanec, O.; Schmuki, P.; Zbořil, R. Radiative and non-radiative recombination pathways in mixed-phase TiO2 nanotubes for PEC water-splitting. Catalysts 2019, 9, 204.
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38.
- Yuan, Y.J.; Chen, D.; Yu, Z.T.; Zou, Z.G. Cadmium sulfide-based nanomaterials for photocatalytic hydrogen production. J. Mater. Chem. A 2018, 6, 11606–11630.
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Nat. Rev. Mater. 2012, 24, 229–251.
- Zhang, N.; Wang, L.; Wang, H.; Cao, R.; Wang, J.; Bai, F.; Fan, H. Self-assembled one-dimensional porphyrin nanostructures with enhanced photocatalytic hydrogen generation. Nano Lett. 2018, 18, 560–566.
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043.
- Che, W.; Cheng, W.; Yao, T.; Tang, F.; Liu, W.; Su, H.; Huang, Y.; Liu, Q.; Liu, J.; Hu, F.; et al. Fast photoelectron transfer in (Cring)-C3N4 plane heterostructural nanosheets for overall water splitting. J. Am. Chem. Soc. 2017, 139, 3021–3026.
- Cai, X.; Zhu, M.; Elbanna, O.A.; Fujitsuka, M.; Kim, S.; Mao, L.; Zhang, J.; Majima, T. Au nanorod photosensitized La2Ti2O7 nanosteps: Successive surface heterojunctions boosting visible to near-infrared photocatalytic H2 evolution. ACS Catal. 2018, 8, 122–131.
- Singh, D.; Panda, P.K.; Khossossi, N.; Mishra, Y.K.; Ainane, A.; Ahuja, R. Impact of edge structures on interfacial interactions and efficient visible-light photocatalytic activity of metal–semiconductor hybrid 2D materials. Catal. Sci. Technol. 2020, 10, 3279–3289.
- Singh, D.; Chakraborty, S.; Ahuja, R. Emergence of Si2BN Monolayer as Efficient HER Catalyst under Co-functionalization Influence. ACS Appl. Energy Mater. 2019, 2, 8441–8448.
- Hartley, C.L.; DiRisio, R.J.; Screen, M.E.; Mayer, K.J.; McNamara, W.R. Iron polypyridyl complexes for photocatalytic hydrogen generation. Inorg. Chem. 2016, 55, 8865–8870.
- Zhang, L.Y.; Yin, S.Y.; Pan, M.; Liao, W.M.; Zhang, J.H.; Wang, H.P.; Su, C.Y. Binuclear Ru–Ru and Ir–Ru complexes for deep red emission and photocatalytic water reduction. J. Mater. Chem. A 2017, 5, 9807–9814.
- Greene, B.L.; Schut, G.J.; Adams, M.W.; Dyer, R.B. Pre-Steady-State Kinetics of Catalytic Intermediates of an [FeFe]-Hydrogenase. ACS Catal. 2017, 7, 2145–2150.
- Singh, D.; Gupta, S.K.; Sonvane, Y.; Kumar, A.; Ahuja, R. 2D-HfS2 as an efficient photocatalyst for water splitting. Catal. Sci. Technol. 2016, 6, 6605–6614.
- Zhao, X.; Yang, X.; Singh, D.; Panda, P.K.; Luo, W.; Li, Y.; Ahuja, R. Strain-Engineered Metal-Free h-B2O Monolayer as a Mechanocatalyst for Photocatalysis and Improved Hydrogen Evolution Reaction. J. Phys. Chem. C 2020, 124, 7884–7892.
- Hutton, G.A.; Reuillard, B.; Martindale, B.C.; Caputo, C.A.; Lockwood, C.W.; Butt, J.N.; Reisner, E. Carbon dots as versatile photosensitizers for solar-driven catalysis with redox enzymes. J. Am. Chem. Soc. 2016, 138, 16722–16730.
- Lv, H.; Ruberu, T.P.A.; Fleischauer, V.E.; Brennessel, W.W.; Neidig, M.L.; Eisenberg, R. Catalytic light-driven generation of hydrogen from water by iron dithiolene complexes. J. Am. Chem. Soc. 2016, 138, 11654–11663.
- Yuan, Y.J.; Chen, D.Q.; Xiong, M.; Zhong, J.S.; Wan, Z.Y.; Zhou, Y.; Liu, S.; Yu, Z.T.; Yang, L.X.; Zou, Z.G. Bandgap engineering of (AgIn)xZn2(1−x)S2 quantum dot photosensitizers for photocatalytic H2 generation. Appl. Catal. B Environ. 2017, 204, 58–66.
- Sakai, T.; Mersch, D.; Reisner, E. Photocatalytic hydrogen evolution with a hydrogenase in a mediator-free system under high levels of oxygen. Angew. Chem. 2013, 52, 12313–12316.
- Han, Z.; Shen, L.; Brennessel, W.W.; Holland, P.L.; Eisenberg, R. Nickel pyridinethiolate complexes as catalysts for the light-driven production of hydrogen from aqueous solutions in noble-metal-free systems. J. Am. Chem. Soc. 2013, 135, 14659–14669.
- Yuan, Y.J.; Lu, H.W.; Tu, J.R.; Fang, Y.; Yu, Z.T.; Fan, X.X.; Zou, Z.G. A Noble-Metal-Free Nickel (II) Polypyridyl Catalyst for Visible-Light-Driven Hydrogen Production from Water. ChemPhysChem 2015, 16, 2925–2930.
- Kagalwala, H.N.; Chirdon, D.N.; Mills, I.N.; Budwal, N.; Bernhard, S. Light-driven hydrogen generation from microemulsions using metallosurfactant catalysts and oxalic acid. Inorg. Chem. 2017, 56, 10162–10171.
- Yuan, Y.J.; Ye, Z.J.; Lu, H.W.; Hu, B.; Li, Y.H.; Chen, D.Q.; Zhong, J.S.; Yu, Z.T.; Zou, Z.G. Constructing anatase TiO2 nanosheets with exposed (001) facets/layered MoS2 two-dimensional nanojunctions for enhanced solar hydrogen generation. ACS Catal. 2016, 6, 532–541.
- Lin, Z.; Xiao, J.; Li, L.; Liu, P.; Wang, C.; Yang, G. Nanodiamond-Embedded p-Type Copper (I) Oxide Nanocrystals for Broad-Spectrum Photocatalytic Hydrogen Evolution. Adv. Energy Mater. 2016, 6, 1501865.
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615.
- Liu, L.; Peter, Y.Y.; Chen, X.; Mao, S.S.; Shen, D. Hydrogenation and disorder in engineered black TiO2. Phys. Rev. Lett. 2013, 111, 065505.
- Xu, M.; Gao, Y.; Moreno, E.M.; Kunst, M.; Muhler, M.; Wang, Y.; Idriss, H.; Wöll, C. Photocatalytic activity of bulk TiO2 anatase and rutile single crystals using infrared absorption spectroscopy. Phys. Rev. Lett. 2011, 106, 138302.
- Li, X.; Li, Z.; Yang, J. Proposed photosynthesis method for producing hydrogen from dissociated water molecules using incident near-infrared light. Phys. Rev. Lett. 2014, 112, 018301.
- Ahmed, M.; Guo, X. A review of metal oxynitrides for photocatalysis. Inorg. Chem. Front. 2016, 3, 578–590.
- Yang, X.; Banerjee, A.; Ahuja, R. Probing the active sites of newly predicted stable Janus scandium dichalcogenides for photocatalytic water-splitting. Catal. Sci. Technol. 2019, 9, 4981–4989.
- Serpone, N.; Emeline, A.; Ryabchuk, V.; Kuznetsov, V. Why do hydrogen and oxygen yields from semiconductor-based photocatalyzed water splitting remain disappointingly low? Intrinsic and extrinsic factors impacting surface redox reactions. ACS Energy Lett. 2016, 1, 931–948.
- Fukuzumi, S.; Hong, D.; Yamada, Y. Bioinspired photocatalytic water reduction and oxidation with earth-abundant metal catalysts. J. Phys. Chem. Lett. 2013, 4, 3458–3467.
- Guzman, F.; Chuang, S.S.; Yang, C. Role of methanol sacrificing reagent in the photocatalytic evolution of hydrogen. Ind. Eng. Chem. Res 2013, 52, 61–65.
- Salzl, S.; Ertl, M.; Knör, G. Evidence for photosensitised hydrogen production from water in the absence of precious metals, redox-mediators and co-catalysts. Phys. Chem. Chem. Phys. 2017, 19, 8141–8147.
- Simon, T.; Bouchonville, N.; Berr, M.J.; Vaneski, A.; Adrović, A.; Volbers, D.; Wyrwich, R.; Döblinger, M.; Susha, A.S.; Rogach, A.L.; et al. Redox shuttle mechanism enhances photocatalytic H2 generation on Ni-decorated CdS nanorods. Nat. Mater. 2014, 13, 1013–1018.
- Wang, J.; Chen, Y.; Zhou, W.; Tian, G.; Xiao, Y.; Fu, H.; Fu, H. Cubic quantum dot/hexagonal microsphere ZnIn2 S4 heterophase junctions for exceptional visible-light-driven photocatalytic H2 evolution. J. Mater. Chem. A 2017, 5, 8451–8460.
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80.
- Han, Z.; Qiu, F.; Eisenberg, R.; Holland, P.L.; Krauss, T.D. Robust photogeneration of H2 in water using semiconductor nanocrystals and a nickel catalyst. Science 2012, 338, 1321–1324.
- Xu, J.; Cao, X. Characterization and mechanism of MoS2/CdS composite photocatalyst used for hydrogen production from water splitting under visible light. Chem. Eng. J. 2015, 260, 642–648.
- Kim, W.; Tachikawa, T.; Majima, T.; Li, C.; Kim, H.J.; Choi, W. Tin-porphyrin sensitized TiO2 for the production of H2 under visible light. Energy Environ. Sci. 2010, 3, 1789–1795.
- Ye, C.; Li, J.X.; Li, Z.J.; Li, X.B.; Fan, X.B.; Zhang, L.P.; Chen, B.; Tung, C.H.; Wu, L.Z. Enhanced driving force and charge separation efficiency of protonated g-C3N4 for photocatalytic O2 evolution. ACS Catal. 2015, 5, 6973–6979.
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271.
- Yang, X.; Banerjee, A.; Xu, Z.; Wang, Z.; Ahuja, R. Interfacial aspect of ZnTe/In2Te3 heterostructures as an efficient catalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 27441–27449.
- Wen, C.Z.; Hu, Q.H.; Guo, Y.N.; Gong, X.Q.; Qiao, S.Z.; Yang, H.G. From titanium oxydifluoride (TiOF2) to titania (TiO2): Phase transition and non-metal doping with enhanced photocatalytic hydrogen (H2) evolution properties. Chem. Commun. 2011, 47, 6138–6140.
- Yu, J.; Zhang, J.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2-production activity of CdS quantum dots-sensitized Zn1−xCdxS solid solution. Green Chem. 2010, 12, 1611–1614.
- Ning, Z.; Tian, H.; Yuan, C.; Fu, Y.; Qin, H.; Sun, L.; Ågren, H. Solar cells sensitized with type-II ZnSe–CdS core/shell colloidal quantum dots. Chem. Commun. 2011, 47, 1536–1538.
- Chen, X.; Liu, L.; Peter, Y.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750.
- Thimsen, E.; Le Formal, F.; Gratzel, M.; Warren, S.C. Influence of plasmonic Au nanoparticles on the photoactivity of Fe2O3 electrodes for water splitting. Nano Lett. 2011, 11, 35–43.
- Wu, H.B.; Hng, H.H.; Lou, X.W. Direct synthesis of anatase TiO2 nanowires with enhanced photocatalytic activity. Adv. Mater. 2012, 24, 2567–2571.
- Chen, J.S.; Chen, C.; Liu, J.; Xu, R.; Qiao, S.Z.; Lou, X.W. Ellipsoidal hollow nanostructures assembled from anatase TiO2 nanosheets as a magnetically separable photocatalyst. Chem. Commun. 2011, 47, 2631–2633.
- Meng, F.; Li, J.; Cushing, S.K.; Zhi, M.; Wu, N. Solar hydrogen generation by nanoscale p-n junction of p-type molybdenum disulfide/n-type nitrogen-doped reduced graphene oxide. J. Am. Chem. Soc. 2013, 135, 10286–10289.
- Wang, X.; Xu, Q.; Li, M.; Shen, S.; Wang, X.; Wang, Y.; Feng, Z.; Shi, J.; Han, H.; Li, C. Photocatalytic overall water splitting promoted by an α–β phase junction on Ga2O3. Angew. Chem. 2012, 124, 13266–13269.
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic Activities of Noble Metal Ion Doped SrTiO3 under Visible Light Irradiation. J. Phys. Chem. B 2004, 108, 8992–8995.
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295.
- Maeda, K.; Teramura, K.; Lu, D.; Saito, N.; Inoue, Y.; Domen, K. Noble-metal/Cr2O3 core/shell nanoparticles as a cocatalyst for photocatalytic overall water splitting. Angew. Chem. 2006, 118, 7970–7973.
- Maeda, K.; Sakamoto, N.; Ikeda, T.; Ohtsuka, H.; Xiong, A.; Lu, D.; Kanehara, M.; Teranishi, T.; Domen, K. Preparation of Core–Shell-Structured Nanoparticles (with a Noble-Metal or Metal Oxide Core and a Chromia Shell) and Their Application in Water Splitting by Means of Visible Light. Chem. Eur. J. 2010, 16, 7750–7759.
- Qu, Y.; Liao, L.; Cheng, R.; Wang, Y.; Lin, Y.C.; Huang, Y.; Duan, X. Rational design and synthesis of freestanding photoelectric nanodevices as highly efficient photocatalysts. Nano Lett. 2010, 10, 1941–1949.
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613.
- Zhou, H.; Qu, Y.; Zeid, T.; Duan, X. Towards highly efficient photocatalysts using semiconductor nanoarchitectures. Energy Environ. Sci. 2012, 5, 6732–6743.
- Hochbaum, A.I.; Yang, P. Semiconductor nanowires for energy conversion. Chem. Rev. 2010, 110, 527–546.
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 1–11.
- Tafen, D.N.; Long, R.; Prezhdo, O.V. Dimensionality of nanoscale TiO2 determines the mechanism of photoinduced electron injection from a CdSe nanoparticle. Nano Lett. 2014, 14, 1790–1796.
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Rodrigues, M.T.F.; et al. High efficiency photocatalytic water splitting using 2D α-Fe2O3/g-C3N4 Z-scheme catalysts. Adv. Energy Mater. 2017, 7, 1700025.
- Han, Q.; Wang, B.; Gao, J.; Cheng, Z.; Zhao, Y.; Zhang, Z.; Qu, L. Atomically thin mesoporous nanomesh of graphitic C3N4 for high-efficiency photocatalytic hydrogen evolution. ACS Nano 2016, 10, 2745–2751.
- Sivula, K. Metal oxide photoelectrodes for solar fuel production, surface traps, and catalysis. J. Phys. Chem. Lett. 2013, 4, 1624–1633.
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535.
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419.
- Son, J.S.; Yu, J.H.; Kwon, S.G.; Lee, J.; Joo, J.; Hyeon, T. Colloidal Synthesis of Ultrathin Two-Dimensional Semiconductor Nanocrystals. Adv. Mater. 2011, 23, 3214–3219.
- Oh, S.M.; Patil, S.B.; Jin, X.; Hwang, S.J. Recent applications of 2D inorganic nanosheets for emerging energy storage system. Chem. Eur. J. 2018, 24, 4757–4773.
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469.
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712.
- Fan, Z.; Zhang, H. Crystal phase-controlled synthesis, properties and applications of noble metal nanomaterials. Chem. Soc. Rev. 2016, 45, 63–82.
- Chhowalla, M.; Voiry, D.; Yang, J.; Shin, H.S.; Loh, K.P. Phase-engineered transition-metal dichalcogenides for energy and electronics. MRS Bull. 2015, 40, 585.
- Ambrosi, A.; Sofer, Z.; Pumera, M. 2H → 1T phase transition and hydrogen evolution activity of MoS2, MoSe2, WS2 and WSe2 strongly depends on the MX2 composition. Chem. Commun. 2015, 51, 8450–8453.
- Chang, K.; Hai, X.; Pang, H.; Zhang, H.; Shi, L.; Liu, G.; Liu, H.; Zhao, G.; Li, M.; Ye, J. Targeted synthesis of 2H-and 1T-phase MoS2 monolayers for catalytic hydrogen evolution. Adv. Mater. 2016, 28, 10033–10041.
- Qu, Y.; Medina, H.; Wang, S.W.; Wang, Y.C.; Chen, C.W.; Su, T.Y.; Manikandan, A.; Wang, K.; Shih, Y.C.; Chang, J.W.; et al. Wafer Scale Phase-Engineered 1T-and 2H-MoSe2/Mo Core–Shell 3D-Hierarchical Nanostructures toward Efficient Electrocatalytic Hydrogen Evolution Reaction. Nat. Rev. Mater. 2016, 28, 9831–9838.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669.
- Pacile, D.; Meyer, J.; Girit, Ç.; Zettl, A. The two-dimensional phase of boron nitride: Few-atomic-layer sheets and suspended membranes. Appl. Phys. Lett. 2008, 92, 133107.
- Li, H.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of single-and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 2012, 8, 63–67.
- Late, D.J.; Doneux, T.; Bougouma, M. Single-layer MoSe2 based NH3 gas sensor. Appl. Phys. Lett. 2014, 105, 233103.
- Li, H.; Lu, G.; Wang, Y.; Yin, Z.; Cong, C.; He, Q.; Wang, L.; Ding, F.; Yu, T.; Zhang, H. Mechanical exfoliation and characterization of single-and few-layer nanosheets of WSe2, TaS2, and TaSe2. Small 2013, 9, 1974–1981.
- Pezeshki, A.; Hosseini Shokouh, S.H.; Jeon, P.J.; Shackery, I.; Kim, J.S.; Oh, I.K.; Jun, S.C.; Kim, H.; Im, S. Static and dynamic performance of complementary inverters based on nanosheet α-MoTe2 p-channel and MoS2 n-channel transistors. ACS Nano 2016, 10, 1118–1125.
- Liu, F.; Zheng, S.; He, X.; Chaturvedi, A.; He, J.; Chow, W.L.; Mion, T.R.; Wang, X.; Zhou, J.; Fu, Q.; et al. Highly sensitive detection of polarized light using anisotropic 2D ReS2. Adv. Funct. Mater. 2016, 26, 1169–1177.
- Dumcenco, D.O.; Kobayashi, H.; Liu, Z.; Huang, Y.S.; Suenaga, K. Visualization and quantification of transition metal atomic mixing in Mo1−xWxS2 single layers. Nat. Commun. 2013, 4, 1–5.
- Chen, Y.; Xi, J.; Dumcenco, D.O.; Liu, Z.; Suenaga, K.; Wang, D.; Shuai, Z.; Huang, Y.S.; Xie, L. Tunable band gap photoluminescence from atomically thin transition-metal dichalcogenide alloys. ACS Nano 2013, 7, 4610–4616.
- Chen, Y.; Dumcenco, D.O.; Zhu, Y.; Zhang, X.; Mao, N.; Feng, Q.; Zhang, M.; Zhang, J.; Tan, P.H.; Huang, Y.S.; et al. Composition-dependent Raman modes of Mo1−xWxS2 monolayer alloys. Nanoscale 2014, 6, 2833–2839.
- Liu, F.; Zheng, S.; Chaturvedi, A.; Zólyomi, V.; Zhou, J.; Fu, Q.; Zhu, C.; Yu, P.; Zeng, Q.; Drummond, N.D.; et al. Optoelectronic properties of atomically thin ReSSe with weak interlayer coupling. Nanoscale 2016, 8, 5826–5834.
- Goyal, V.; Teweldebrhan, D.; Balandin, A.A. Mechanically-exfoliated stacks of thin films of Bi2Te3 topological insulators with enhanced thermoelectric performance. Appl. Phys. Lett. 2010, 97, 133117.
- Shahil, K.; Hossain, M.; Goyal, V.; Balandin, A. Micro-Raman spectroscopy of mechanically exfoliated few-quintuple layers of Bi2Te3, Bi2Se3, and Sb2Te3 materials. J. Appl. Phys. 2012, 111, 054305.
- Du, K.z.; Wang, X.z.; Liu, Y.; Hu, P.; Utama, M.I.B.; Gan, C.K.; Xiong, Q.; Kloc, C. Weak van der Waals stacking, wide-range band gap, and Raman study on ultrathin layers of metal phosphorus trichalcogenides. ACS Nano 2016, 10, 1738–1743.
- Castellanos-Gomez, A.; Vicarelli, L.; Prada, E.; Island, J.O.; Narasimha-Acharya, K.; Blanter, S.I.; Groenendijk, D.J.; Buscema, M.; Steele, G.A.; Alvarez, J.V.; et al. Isolation and characterization of few-layer black phosphorus. 2D Mater. 2014, 1, 025001.
- Buscema, M.; Groenendijk, D.J.; Blanter, S.I.; Steele, G.A.; Van Der Zant, H.S.; Castellanos-Gomez, A. Fast and broadband photoresponse of few-layer black phosphorus field-effect transistors. Nano Lett. 2014, 14, 3347–3352.
- Liu, F.; You, L.; Seyler, K.L.; Li, X.; Yu, P.; Lin, J.; Wang, X.; Zhou, J.; Wang, H.; He, H.; et al. Room-temperature ferroelectricity in CuInP2S6 ultrathin flakes. Nat. Commun. 2016, 7, 1–6.
- Huang, Y.; Sutter, E.; Shi, N.N.; Zheng, J.; Yang, T.; Englund, D.; Gao, H.J.; Sutter, P. Reliable exfoliation of large-area high-quality flakes of graphene and other two-dimensional materials. ACS Nano 2015, 9, 10612–10620.
- Desai, S.B.; Madhvapathy, S.R.; Amani, M.; Kiriya, D.; Hettick, M.; Tosun, M.; Zhou, Y.; Dubey, M.; Ager, J.W., III; Chrzan, D.; et al. Gold-mediated exfoliation of ultralarge optoelectronically-perfect monolayers. Adv. Mater. 2016, 28, 4053–4058.
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571.
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712.
- Smith, R.J.; King, P.J.; Lotya, M.; Wirtz, C.; Khan, U.; De, S.; O’Neill, A.; Duesberg, G.S.; Grunlan, J.C.; Moriarty, G.; et al. Large-scale exfoliation of inorganic layered compounds in aqueous surfactant solutions. Adv. Mater. 2011, 23, 3944–3948.
- Coleman, J.N. Liquid exfoliation of defect-free graphene. Accounts Chem. Res. 2013, 46, 14–22.
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568.
- O’Neill, A.; Khan, U.; Coleman, J.N. Preparation of high concentration dispersions of exfoliated MoS2 with increased flake size. Chem. Mater. 2012, 24, 2414–2421.
- Feng, J.; Peng, L.; Wu, C.; Sun, X.; Hu, S.; Lin, C.; Dai, J.; Yang, J.; Xie, Y. Giant moisture responsiveness of VS2 ultrathin nanosheets for novel touchless positioning interface. Adv. Mater. 2012, 24, 1969–1974.
- Zhou, K.G.; Mao, N.N.; Wang, H.X.; Peng, Y.; Zhang, H.L. A mixed-solvent strategy for efficient exfoliation of inorganic graphene analogues. Angew. Chem. 2011, 123, 11031–11034.
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715.
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630.
- Liu, L.; Shen, Z.; Yi, M.; Zhang, X.; Ma, S. A green, rapid and size-controlled production of high-quality graphene sheets by hydrodynamic forces. Rsc Adv. 2014, 4, 36464–36470.
- Yu, J.; Li, J.; Zhang, W.; Chang, H. Synthesis of high quality two-dimensional materials via chemical vapor deposition. Chem. Sci. 2015, 6, 6705–6716.
- Jones, A.C.; Hitchman, M.L. Overview of chemical vapour deposition. Chem. Vapor. Depos. 2009, 1, 1–36.
- Somani, P.R.; Somani, S.P.; Umeno, M. Planer nano-graphenes from camphor by CVD. Chem. Phys. Lett. 2006, 430, 56–59.
- Pollard, A.; Nair, R.; Sabki, S.; Staddon, C.; Perdigao, L.; Hsu, C.; Garfitt, J.; Gangopadhyay, S.; Gleeson, H.; Geim, A.; et al. Formation of monolayer graphene by annealing sacrificial nickel thin films. J. Phys. Chem. C 2009, 113, 16565–16567.
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35.
- Kim, G.; Jang, A.R.; Jeong, H.Y.; Lee, Z.; Kang, D.J.; Shin, H.S. Growth of high-crystalline, single-layer hexagonal boron nitride on recyclable platinum foil. Nano Lett. 2013, 13, 1834–1839.
- Zhao, Y.; Luo, X.; Zhang, J.; Wu, J.; Bai, X.; Wang, M.; Jia, J.; Peng, H.; Liu, Z.; Quek, S.Y.; et al. Interlayer vibrational modes in few-quintuple-layer Bi2Te3 and Bi2Se3 two-dimensional crystals: Raman spectroscopy and first-principles studies. Phys. Rev. B 2014, 90, 245428.
- Zheng, W.; Xie, T.; Zhou, Y.; Chen, Y.; Jiang, W.; Zhao, S.; Wu, J.; Jing, Y.; Wu, Y.; Chen, G.; et al. Patterning two-dimensional chalcogenide crystals of Bi2 Se3 and In2Se3 and efficient photodetectors. Nat. Commun. 2015, 6, 1–8.
- Mannix, A.J.; Zhou, X.F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science 2015, 350, 1513–1516.
- Gogotsi, Y. Chemical vapour deposition: Transition metal carbides go 2D. Nat. Mater. 2015, 14, 1079–1080.
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.L.; Cheng, H.M.; Ren, W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141.
- Fleurence, A.; Friedlein, R.; Ozaki, T.; Kawai, H.; Wang, Y.; Yamada-Takamura, Y. Experimental evidence for epitaxial silicene on diboride thin films. Phy. Rev. Lett. 2012, 108, 245501.
- Zhan, Y.; Liu, Z.; Najmaei, S.; Ajayan, P.M.; Lou, J. Large-area vapor-phase growth and characterization of MoS2 atomic layers on a SiO2 substrate. Small 2012, 8, 966–971.
- Zhang, Y.; Zhang, Y.; Ji, Q.; Ju, J.; Yuan, H.; Shi, J.; Gao, T.; Ma, D.; Liu, M.; Chen, Y.; et al. Controlled growth of high-quality monolayer WS2 layers on sapphire and imaging its grain boundary. ACS Nano 2013, 7, 8963–8971.
- Wang, X.; Gong, Y.; Shi, G.; Chow, W.L.; Keyshar, K.; Ye, G.; Vajtai, R.; Lou, J.; Liu, Z.; Ringe, E.; et al. Chemical vapor deposition growth of crystalline monolayer MoSe2. ACS Nano 2014, 8, 5125–5131.
- Docherty, C.J.; Parkinson, P.; Joyce, H.J.; Chiu, M.H.; Chen, C.H.; Lee, M.Y.; Li, L.J.; Herz, L.M.; Johnston, M.B. Ultrafast transient terahertz conductivity of monolayer MoS2 and WSe2 grown by chemical vapor deposition. ACS Nano 2014, 8, 11147–11153.
- Zhang, M.; Zhu, Y.; Wang, X.; Feng, Q.; Qiao, S.; Wen, W.; Chen, Y.; Cui, M.; Zhang, J.; Cai, C.; et al. Controlled synthesis of ZrS2 monolayer and few layers on hexagonal boron nitride. J. Am. Chem. Soc. 2015, 137, 7051–7054.
- Keyshar, K.; Gong, Y.; Ye, G.; Brunetto, G.; Zhou, W.; Cole, D.P.; Hackenberg, K.; He, Y.; Machado, L.; Kabbani, M.; et al. Chemical vapor deposition of monolayer rhenium disulfide (ReS2). Adv. Mater. 2015, 27, 4640–4648.
- Liu, L.; Park, J.; Siegel, D.A.; McCarty, K.F.; Clark, K.W.; Deng, W.; Basile, L.; Idrobo, J.C.; Li, A.P.; Gu, G. Heteroepitaxial growth of two-dimensional hexagonal boron nitride templated by graphene edges. Science 2014, 343, 163–167.
- De Parga, A.V.; Calleja, F.; Borca, B.; Passeggi Jr, M.; Hinarejos, J.; Guinea, F.; Miranda, R. Periodically rippled graphene: Growth and spatially resolved electronic structure. Phy. Rev. Lett. 2008, 100, 056807.
- Li, M.Y.; Shi, Y.; Cheng, C.C.; Lu, L.S.; Lin, Y.C.; Tang, H.L.; Tsai, M.L.; Chu, C.W.; Wei, K.H.; He, J.H.; et al. Epitaxial growth of a monolayer WSe2-MoS2 lateral p-n junction with an atomically sharp interface. Science 2015, 349, 524–528.
- Yu, Y.; Hu, S.; Su, L.; Huang, L.; Liu, Y.; Jin, Z.; Purezky, A.A.; Geohegan, D.B.; Kim, K.W.; Zhang, Y.; et al. Equally efficient interlayer exciton relaxation and improved absorption in epitaxial and nonepitaxial MoS2/WS2 heterostructures. Nano Lett. 2015, 15, 486–491.
- Heo, H.; Sung, J.H.; Jin, G.; Ahn, J.H.; Kim, K.; Lee, M.J.; Cha, S.; Choi, H.; Jo, M.H. Rotation-Misfit-Free Heteroepitaxial Stacking and Stitching Growth of Hexagonal Transition-Metal Dichalcogenide Monolayers by Nucleation Kinetics Controls. Adv. Mater. 2015, 27, 3803–3810.
- Duesberg, G.S. Heterojunctions in 2D semiconductors: A perfect match. Nat. Mater. 2014, 13, 1075–1076.
- Li, M.Y.; Chen, C.H.; Shi, Y.; Li, L.J. Heterostructures based on two-dimensional layered materials and their potential applications. Mater. Today 2016, 19, 322–335.
- Shi, Z.T.; Kang, W.; Xu, J.; Sun, Y.W.; Jiang, M.; Ng, T.W.; Xue, H.T.; Denis, Y.; Zhang, W.; Lee, C.S. Hierarchical nanotubes assembled from MoS2-carbon monolayer sandwiched superstructure nanosheets for high-performance sodium ion batteries. Nano Energy 2016, 22, 27–37.
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813.
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888.
- Cai, L.; He, J.; Liu, Q.; Yao, T.; Chen, L.; Yan, W.; Hu, F.; Jiang, Y.; Zhao, Y.; Hu, T.; et al. Vacancy-induced ferromagnetism of MoS2 nanosheets. J. Am. Chem. Soc. 2015, 137, 2622–2627.
- Song, H.J.; You, S.; Jia, X.H.; Yang, J. MoS2 nanosheets decorated with magnetic Fe3O4 nanoparticles and their ultrafast adsorption for wastewater treatment. Ceram. Int. 2015, 41, 13896–13902.
- Jeong, S.; Yoo, D.; Jang, J.t.; Kim, M.; Cheon, J. Well-defined colloidal 2-D layered transition-metal chalcogenide nanocrystals via generalized synthetic protocols. J. Am. Chem. Soc. 2012, 134, 18233–18236.
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater. Sci. 2015, 73, 44–126.
- Wang, L.; Sun, C.; Xu, L.; Qian, Y. Convenient synthesis and applications of gram scale boron nitride nanosheets. Catal. Sci. Technol. 2011, 1, 1119–1123.
- Zhang, N.; Liu, X.; Yi, R.; Shi, R.; Gao, G.; Qiu, G. Selective and controlled synthesis of single-crystalline yttrium hydroxide/oxide nanosheets and nanotubes. J. Phys. Chem. C 2008, 112, 17788–17795.
- Yang, S.; Gong, Y.; Liu, Z.; Zhan, L.; Hashim, D.P.; Ma, L.; Vajtai, R.; Ajayan, P.M. Bottom-up approach toward single-crystalline VO2-graphene ribbons as cathodes for ultrafast lithium storage. Nano Lett. 2013, 13, 1596–1601.
- Shen, J.; Dong, P.; Baines, R.; Xu, X.; Zhang, Z.; Ajayan, P.M.; Ye, M. Controlled synthesis and comparison of NiCo2S4/graphene/2D TMD ternary nanocomposites for high-performance supercapacitors. Chem. Commun. 2016, 52, 9251–9254.
- Shen, J.; Ji, J.; Dong, P.; Baines, R.; Zhang, Z.; Ajayan, P.M.; Ye, M. Novel FeNi2S4/TMD-based ternary composites for supercapacitor applications. J. Mater. Chem. A 2016, 4, 8844–8850.
- Meng, F.; Hong, Z.; Arndt, J.; Li, M.; Zhi, M.; Yang, F.; Wu, N. Visible light photocatalytic activity of nitrogen-doped La2Ti2O7 nanosheets originating from band gap narrowing. Nano Res. 2012, 5, 213–221.
- Ghuman, K.K.; Hoch, L.B.; Szymanski, P.; Loh, J.Y.; Kherani, N.P.; El-Sayed, M.A.; Ozin, G.A.; Singh, C.V. Photoexcited surface frustrated Lewis pairs for heterogeneous photocatalytic CO2 reduction. J. Am. Chem. Soc. 2016, 138, 1206–1214.
- Hoch, L.B.; Szymanski, P.; Ghuman, K.K.; He, L.; Liao, K.; Qiao, Q.; Reyes, L.M.; Zhu, Y.; El-Sayed, M.A.; Singh, C.V.; et al. Carrier dynamics and the role of surface defects: Designing a photocatalyst for gas-phase CO2 reduction. Proc. Natl. Acad. Sci. USA 2016, 113, E8011–E8020.