Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Amina Yu and Version 2 by Amina Yu.
Drug reaction with eosinophilia and systemic symptoms (DReSS), also known as drug-induced hypersensitivity syndrome (DiHS), is a severe, systemic, T cell mediated drug reaction with combinations of cutaneous, hematologic, and internal organ involvement. Pathogenesis of DReSS is multi-factorial, involving drug-exposure, genetic predisposition through specific human leukocyte antigen (HLA) alleles and metabolism defects, viral reactivation, and immune dysregulation. Clinical features of this condition are delayed, stepwise, and heterogenous, making this syndrome challenging to recognize and diagnose.
- drug reaction
- drug-induced hypersensitivity syndrome
- drug reaction with eosinophilia and systemic symptoms
1. Pathogenesis
Significant advances in the understanding of drug reaction with eosinophilia and systemic symptoms (DReSS) pathogenesis have been made recently. DReSS is a severe, idiosyncratic, T cell mediated drug reaction, classified as a delayed type IVb, and sometimes IVc, hypersensitivity reaction [1]. It is assumed that DReSS is the result of a complex interaction between drug (or vaccine or biologic) exposure, genetic predisposition, and viral reactivation [1]. Why some develop this condition while others do not, despite the same exposure, is thought to be a result of the cumulative effect of aligned risks that can be likened to a “Swiss cheese” model as illustrated in Figure 1. There are likely other risk factors and predisposing conditions leading to this disease still unknown that need to be accounted for in this model.
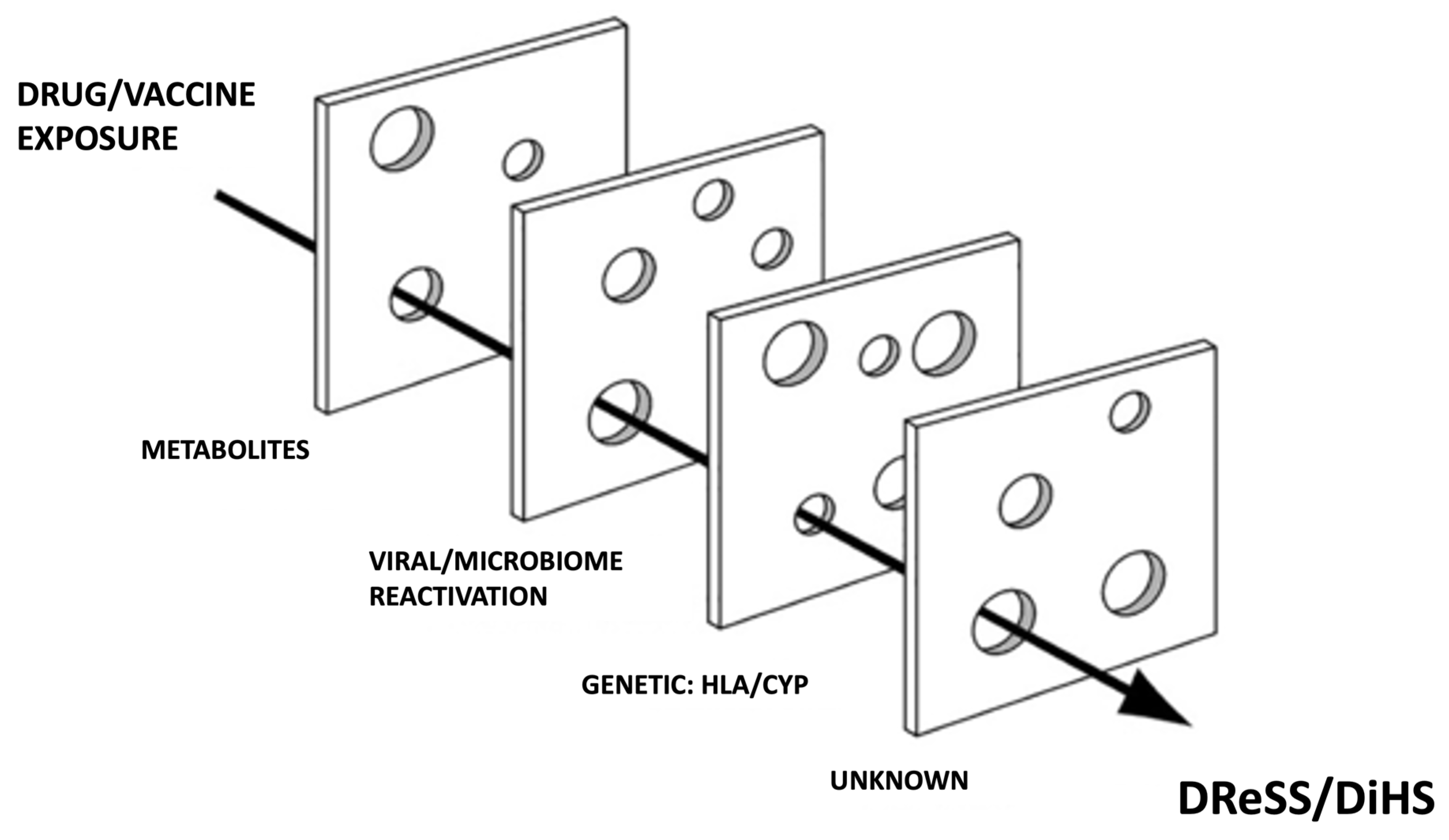
Figure 1. “Swiss cheese” risk model of DReSS/DiHS. CYP cytochrome P450, HLA human leukocyte antigen, DReSS drug reaction with eosinophilia and systemic symptoms, DiHS drug induced hypersensitivity syndrome.
1.1. Viral Reactivation
The relationship between viral reactivation and DReSS has been learned extensively [2]. Despite this, there is still much controversy surrounding this topic. Even with the abundance of research on this matter, questions regarding its clinical relevance, role as a causative factor vs. a complication, and validity of viral testing techniques, remain mostly unanswered.
Historically, human herpes virus-6 (HHV-6) has been most associated with DReSS, although other human herpes viruses have been reported including HHV-7, cytomegalovirus (CMV), Epstein-Barr virus (EBV), and herpes simplex virus (HSV) [3][4]. There are multiple ones demonstrating a connection between DReSS and viral reactivation, so much so that the diagnosis of DiHS by the Japanese consensus group requires evidence of HHV-6 infection [5][6]. However, viral reactivation is less reported in cases outside of Japan, and some have suggested the incidence may be over-estimated owing to testing techniques [7][8].
The incidence rates of HHV-6 reactivation are highly variable across studies, ranging from 36% in the multi-national RegiSCAR one, to 62% in a Japanese-specific population [7][9]. A 2013 one was found that less than 50% tested for viral reactivation, but when performed, the majority showed evidence of reactivation with an HHV-6 reactivation rate of 80% [10]. A 2010 prospective one by Picard et al. [11] found 76% of DReSS patients had evidence of viral reactivation with some combination of EBV, HHV-6 and/or HHV-7. Rates of viral reactivation in the healthy control group, comparatively, were 0%.
Viral reactivation typically occurs 2–4 weeks after symptom onset and has been associated with longer disease duration, flares, and more severe outcomes [3][5][6][9][12][13][14][15]. Specifically, patients with viral reactivation were found to have a longer disease duration, an increased number of relapses, and increased rates of lymphadenopathy, hepatitis, renal failure, encephalitis, myocarditis, severe lymphopenia, and death, compared to DReSS patients with no evidence of viral reactivation [9][14][15]. diKano et al. [16] found patients can experience sequential reactivation of multiple viruses that may explain the symptom relapses seen in DReSS despite drug withdrawal. This finding has been compared to the sequential viral reactivation seen in acute graft versus host disease (GVHD) [16].
Multiple mechanisms have been proposed to explain why viral reactivation occurs in DReSS. In the acute phase, a relatively immunocompromised profile is seen with the rapid expansion of immune suppressive T regulatory cells (specifically CD4+CD25+FoxP3+) and concomitant decreases in immunoglobulins and peripheral B-cells [16][17] This clonal expansion may reduce the function of anti-viral T lymphocytes leading to viral reactivation. Others have suggested that viral reactivation may be due to immunosuppression from corticosteroid treatment [18]. However, Tohyama et al. [9] showed HHV-6 reactivation occurred more frequently in patients not treated with steroids compared to high-dose steroid groups [12]. While high-dose pulsed steroids have been associated with CMV reactivation in other conditions, Mizukawa et al. [19] suggest that the rapid dose reduction in steroids may induce a rapid recovery of immune response that could contribute to the development of CMV reactivation and symptom worsening. Indeed, several have shown the steroid tapering phase to be a high-risk time for symptom recurrence [6][14][18]. Flares during the steroid tapering are theorized to either be secondary to the underlying inflammation of DReSS recurring, or possibly, reactivation of pre-existent but clinically undetectable viruses [20]. Shiohara et al. liken the latter phenomenon to the immune reconstitution syndrome seen in HIV [20]. It has been suggested that if an immune reconstitution-like syndrome is occurring in DReSS, it may indicate that viral infection is playing a more causative role in DReSS despite being undetectable in patients at initial presentation owed to robust host immune responses [19][20][21].
Recently, there has been interest in the idea that drug-reactive DReSS-inducing T cells are virus-specific memory T cells [22]. In other words, T cells previously generated in response to a viral infection are stimulated upon presentation with HLA-drug complexes and mistakenly reactivate [22] This is supported by evidence from Picard et al. [11] showing the expansion of EBV-specific CD8+ T lymphocytes in blood, skin, liver, and lungs of DReSS patients. Furthermore, in vitro ones showed HIV-specific T cells cross-reacted with HLA-B*57:01-positive cells in the presence of abacavir [23]. Kim et al. [24], single cell RNA sequencing was used to examine the aberrant immune response in a patient with persistent DReSS from Trimethoprim-Sulfamethoxazole (TMP-SMX). Not only did they find the activation of the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway, leading to successful treatment with the JAK-inhibitor tofacitinib, but they also found HHV-6b DNA was enriched in central memory CD4+ T cells. Impressively, in vitro ones demonstrated anti-viral medications targeting HHV6b were able to suppress TMP/SMX-induced CD4+ T cell proliferation in dose-dependent manners [24].
In summary, there is no global consensus as to if, or how, viral reactivation is associated with DReSS. As to whether it is one of the many contributing risk factors in the development of DReSS, a consequence of the drug-induced immune dysregulation, or a side-effect of the immunosuppression used in treating DReSS, this is still unknown [1][8][22][25].
1.2. Drugs
The most common DReSS-inducing drugs are anticonvulsants, allopurinol, sulfonamides, and antibiotics [7][26][27]. In the RegiSCAR one, aromatic anti-epileptics made up 35% of all drug-triggers, with carbamazepine alone making up 27% in one [7][10]. Carbamazepine is the most common cause of DReSS both overall and within the anticonvulsant grouping, with lamotrigine, phenytoin and phenobarbital also frequently reported [7][10][19][28][29]. Allopurinol has been the attributable drug in 11–18% of DReSS [7][10]. Sulfonamides are the probable causative drugs in around 12%, with sulfasalazine often making up more than half of that grouping, although TMP-SMX and dapsone are also reported [7][10][27]. Non-antibiotic sulfa-drugs like furosemide are less associated with DReSS, but related information do exist [27][30]. Sharifzadeh et al. [27] found anti-tuberculosis medications made up 42% of all antibiotic-related DReSS, with rifampin being the most common offending agent. Vancomycin was the second most common antibiotic comprising 18% of antibiotic related ones [27].
1.3. Immune Changes
DReSS is characterized by a variety of hematological abnormalities including leukocytosis, atypical lymphocytosis, and eosinophilia [31]. Furthermore, a heterogenous profile of cytokines and chemokines have been found in DReSS [32]. While eosinophilia is not universally present, a Th2-type response can be seen with eosinophil-associated cytokines such as IL-4, Il-5 and IL-13 [32][33][34]. Serum thymus and activation-regulated chemokine (TARC/CCL17), commonly associated with the Th2 response, may also be elevated and possibly correlate with disease activity [35][36]. Specifically, TARC/CCL17 recruits Th2-polarized T lymphocytes into local inflammation sites. Other cytokines reported to be elevated in DReSS include IFN-γ, TNF-α, IL-2, IL-6, and granulysin [11][32][37][38][39].
By Nishio et al. [37], CD4+ cells increased early on in DReSS and decreased thereafter. They also found CD8+ T cells and Th1-cells increased later in disease, often concomitantly with disease flare, worsening hepatitis, and rises in anti-HHV-6 antibodies. TheseIt authorwas proposed that CD4+ and CD8+ T cells might respond to causative drug and virus-infected cells, respectively. This trend in CD4+ and CD8+ T cell fluctuation has been replicated by Shiohara et al. [20].
Several have been shown a dramatic expansion of the immune-suppressing CD4+CD25+FoxP3+ Treg cells in the early stages of DReSS, both in peripheral blood samples and in cutaneous lesions [17][40][41]. Skin homing Tregs are postulated to limit the severity of skin disease in DReSS through their suppressive effect on cytotoxic T cells [15]. While skin lesion severity may be limited through this mechanism, this expanded population might serve to prevent the activation and expansion of antiviral T cells, allowing latent herpesviruses to reactivate in an uncontrolled fashion [17]. At the resolution stage of DReSS, Tregs are found to be functionally impaired despite normal cell frequencies [17]. Some have been shown an association between autoimmune conditions and the decreased suppressive function of Treg cells, which could explain a pathogenic mechanism for the development of autoimmune conditions seen in some DReSS patients [18][41][42][43].
1.4. Genetic Predisposition
HLA alleles are one of the most important risk factors in the development of DReSS. Importantly, certain high-risk alleles are present in some ethnicities more than others, making ethnic background an important predisposing factor to DReSS [44]. Mechanistically, it is thought that the culprit drug interacts with a particular HLA to form a complex-hapten, which is then presented to naive T cells via the T cell receptor to stimulate an immune response [45].
One of the first examples of HLA association with drug hypersensitivity was in 2002 when the association between abacavir-induced hypersensitivity syndrome in HIV patients and HLA-B*57:01 was found [46][47]. With a negative predictive value of 100% in the PREDICT-1 trial and complete elimination of abacavir-hypersensitivity, screening for HLA-B*57:01 is now recommended by drug regulatory agencies in every patient prior to initiating treatment [46]. A 2005 one examining Han-Chinese patients found a strong association with HLA-B*58:01 and allopurinol-induced DReSS (among other SCARs), present in 100% examined [48]. The 2012 American College of Rheumatology Guidelines recommend testing for the HLA-B*58:01 allele in selected subpopulations (individuals of Korean descent with stage 3 or worse chronic kidney disease and those of Han-Chinese or Thai descent) prior to the initiation of allopurinol.
Similarly, multiple ones have shown an association between HLA-A*31:01 and carbamazepine-induced DReSS, with positivity rates ranging from 37–67% among multiple ethnicities [49][50][51][52][53]. Per the Canadian Pharmacogenomics Network for Drug Safety [54], genetic testing for HLA-A*31:01 is recommended for all carbamazepine-naive patients before the initiation of therapy. While the link between A*31:01 and carbamazepine-induced DReSS may not be as strong as with other alleles, it is one of the most common alleles in most populations, making it an important screening tool to prevent DReSS [54].
Importantly, drug-HLA associations often have a high negative predictive value (NPV) and moderate-to-low positive predictive values (PPV). To date, the highest PPV known is around 50% in abacavir-induced DReSS [55]. In other words, the low PPV of HLA allele associations suggests that additional factors contribute to the onset of disease. Furthermore, allelic frequencies vary greatly between different ethnicities, making it important that recommendations not only be made based on the drug, but on ethnic origin as well. With the explosion of research on this topic, the list of DReSS related drug-HLA associations is expected to grow in the coming years, further contributing in the movement towards personalized medicine.
Mutations in several drug detoxification enzymes have also been linked to DReSS. Aromatic anticonvulsants are metabolized by the cytochrome P450 (CYP-450) system to arene oxide metabolites, normally detoxified by epoxide hydrolase or glutathione transferase to inactive metabolites [56]. Through in vitro lymphocyte transformation assays Shear and Spielberg [57] showed evidence of epoxide hydrolase deficiency in anticonvulsant-induced DReSS patients [58]. The arene oxide intermediates may act as haptens to stimulate the immune response or bind to tissue macromolecules and cause cell damage directly.
Shear et al. [57] describe a similar phenomenon in sulfonamide-related DReSS, finding high incidence of slow N-acetylation status in these patients [57]. Because of the relative N-acetyltransferase deficiency, the CYP-450 oxidative pathway is favored, leading to toxic levels of hydroxylamine metabolites capable of causing cell damage and immune activation. Examining patients from Taiwan, Japan and Malaysia, the CYP2C9*3 gene variant (known to significantly reduce phenytoin clearance) was significantly associated with phenytoin-related SCARs including DReSS [59].
It has been shown increased risk of allopurinol-related DReSS in HLA-B*58:01 positive patients with reduced kidney function, it is possible that elevated levels of drug or toxic drug metabolites may interact with specific HLA to further increase the risk of DReSS development [60][61]. Indeed, it does not seem that the presence of drug-specific HLA-alleles or drug metabolism polymorphisms alone are enough to trigger DReSS, but taken together may function synergistically to elevate risk.
2. Clinical Features
DReSS is characterized by stepwise multi-organ involvement that may include skin, hematological systems, and solid organs. Most commonly it begins with a flu-like prodrome of malaise, pharyngitis, fever, and lymphadenopathy [10]. The progression of signs and symptoms can be slow and in varied combinations, but most fever in most patients (between 75–100%) [1][7][11][28][62]. Fever typically precedes the cutaneous eruption by several days.
Compared to other SCARs, the lag time between drug exposure and symptom onset is more delayed, typically between 2–8 weeks (although longer and shorter times have been reported) [7][10]. Upon re-exposure to the culprit drug symptoms can develop in hours to days [7][10]. Lag time may also differ depending on the drug [7]. For example, antiepileptics and allopurinol tend to have longer latency periods compared to antibiotics or radiocontrast media, which have been shown to have lag times less than 14 days from exposure [63][64][65]. Longer latency periods may correlate with more severe disease [62].
The cutaneous manifestations of DReSS are diverse. Typically, more than 50% of total body surface area (BSA) is involved [7][10][66]. The most common morphologies reported are monomorphic maculopapular/morbilliform, urticated papular, and exfoliative erythroderma [7]. Shiohara et al. [67] describe the rash starting as patchy erythematous macules, pustular, target-like or eczema-like lesions, that can become purpuric and confluent over time. An erythema multiforme (EM)-like eruption with atypical targetoid lesions has also been described, which was associated with more severe hepatic involvement in [68]. Distribution is typically symmetric, often starting on the face, upper trunk and upper extremities and then spreading to the lower extremities [67]. Cutaneous manifestations are polymorphic in around 85%, which can include secondary features such as pustules, purpura, vesicles, bullae and cheilitis [7][28][69]. Facial edema is also characteristic of DReSS (reported in up to 76% ), and may be a distinguishing feature from more mild forms of DReSS or maculopapular eruption (MPE) [7][62][67][70]. Mucosal involvement can be seen in up to 56% of patients, however it is typically mild and non-hemorrhagic, distinguishing it from SJS/TEN [7][28][70]. It was reported that in pediatric DReSS, children are more likely to have a morbilliform exanthem, fever, and lymphadenopathy, but less likely to have facial edema [71][72].
There are a range of hematological abnormalities seen in DReSS. Hypereosinophilia is the most common finding, present in 52–92% of patients across multiple ones (although the majority show greater than 80%) [7][10][19][26][28][62][64][70]. Eosinophil counts are often dramatically increased with an average eosinophil count of 3.5 (×109 L−1) [10]. Leukocytosis with early neutrophilia and delayed monocytosis is the next most common, followed by atypical lymphocytosis in 27–67% of patients [7][10][11][28][64][73]. Other less frequent findings include lymphopenia, leukopenia, thrombocytopenia, thrombocytosis, and pancytopenia, which are associated with a more severe prognosis [7][28][64][70].
Liver injury is the most common visceral manifestation in DReSS, seen in up to 97% [7][10][11][74][75]. Elevated liver enzymes (cholestatic, mixed and hepatocellular have all been reported) is the most common finding; however, liver failure with or without subsequent transplant does occur [70]. The hepatitis is typically anicteric and liver enzyme elevation may take months to completely resolve [21].
The next most involved organ is the kidney [1][7][76]. Renal involvement in DReSS ranges from mild acute kidney injury (AKI) to severe interstitial nephritis, sometimes resulting in permanent end-stage renal disease. Elderly patients, allopurinol-associated DReSS, and those with pre-existing kidney disease are at the highest risk of renal impairment [75]. The lung is the third most frequently impaired organ, with interstitial pneumonitis being the most common manifestation [76]. Minocycline-associated DReSS has been associated with a higher incidence of pneumonitis [66]. Cardiac involvement in DReSS is becoming more frequently recognized, typically presenting as myo- or pericarditis [77]. Cardiac disease is often delayed in DReSS, occurring on average 70 days after the initial symptoms. The most common signs and symptoms of cardiac DReSS are dyspnea, cardiogenic shock, chest pain, and tachycardia.
More incidentally there have been reports of the following: pancreatitis, colitis, cholangitis, encephalitis/meningoencephalitis, hemophagocytic syndrome, and thyroiditis [7][10][11][75][78].
3. Diagnosis
3.1. DReSS Minor/DReSS Major
There has been recent interest in further defining the spectrum of DReSS severity. Some have proposed separating into categories of simple MPE (maculopapular eruption), DReSS minor (other terms include mini-DReSS, MPE/DReSS overlap, and systemic MPE), and DReSS or “DReSS major” [62][70][79]. Determining predictive factors of disease severity and the clinical features that may help delineate between these phenotypes has also been investigated. Momen et al. [62] defined DReSS major as a RegiSCAR score of ≥4 (with DReSS minor as 1–3) and found that DReSS major patients experienced significantly more facial edema, higher liver enzyme elevations, and required longer courses of immunosuppression than DReSS minor patients. It was concluded that DReSS minor should therefore be distinguished as a milder form of the disease from DReSS major, and that facial edema can help discriminate between major and minor forms. Skowron et al. [79] classified “systemic MPE” (sME) as MPE with the presence of fever or organ involvement without meeting DReSS criteria. Similar to the results by Momen et al. [62], they found facial edema, hepatic involvement, fever, and eosinophilia were significantly more frequent in DReSS compared to sMPE. Gouveia et al. [70] defined MPE/DReSS overlap as an exanthem plus one or two systemic symptoms or laboratory abnormalities, but a RegiSCAR score < 4. Somewhat contrary to the previous ones, they found that while MPE/DReSS overlap presented with fewer features of DReSS, these patients showed similar disease severity and length of hospitalization. TheIt authorwas therefore concluded that this overlap syndrome should be considered within the same category as DReSS with regards to management and follow-up.
As evidenced by these three, there is still much controversy regarding the milder forms of DReSS and how they should be classified and managed moving forward. What can be agreed upon is that DReSS clearly exists on a spectrum, with mild ones showing fewer systemic signs and symptoms, and patients at the opposite end potentially developing life-threatening organ dysfunction [70]. This recent focus on the variability in DReSS presentation highlights the collective shift towards a broadened, deeper, and more inclusive understanding of DReSS.
3.2. Causality Assessment and Confirmatory Testing
There is no clear consensus on the ideal method of determining the causative drug in DReSS, although several systems have been proposed. The Spanish Guidelines for Diagnosis, Management, Treatment, and Prevention of DRESS Syndrome suggest using the Algorithm of the Spanish Pharmacovigilance System, which considers chronology (suggestive if the drug was initiated less than 6 months previously and stopped less than 14 days before the index day), known drug association, an improvement with drug withdrawal, and the positive re-challenge effect [80]. Kardaun et al. [7] suggest excluding a drug from consideration if the drug was taken for more than 3 months, had been stopped more than 2 weeks before the index day, or had been initiated less than 3 days before the probable index day.
There are two in vitro tests, the lymphocyte transformation test (LTT) and the enzyme-linked immunospot (ELISpot) assay, that can be used to help identify the culprit drug. The LTT measures T cell proliferation, specifically H-thymidine, in response to a drug after incubation [81]. While laboratories have reached a consensus regarding the protocol and cut-offs for positivity, there are no standard values for each drug [82]. Sensitivity and specificity ranges from 58–73% and 82–95%, respectively, with higher values for anticonvulsants, antituberculosis drugs and B-lactams [83][84]. A negative result is not overly helpful owing to its lower sensitivity, but a positive result reflects specific sensitization to the test drug that can help support the diagnosis and determine the culprit agent if the patient took several drugs. The ELISpot determines the number of cells that release relevant cytokines and cytotoxic markers after their activation by the culprit drug [81]. Positive ELISpot assays for IFN-γ production have been reported in DRESS, however no consensus exists on the criteria for positivity, and it is less frequently employed [80]. Neither test should be performed until at least 4–8 weeks after recovery and 4 weeks after stopping steroids.
In-vivo confirmatory test options include patch testing, skin pricks, delayed intradermal and controlled re-exposure. Patch testing was found to be positive between 57–64% of DReSS, with significant variability depending on the drug [85][86]. While significantly less studied, delayed skin prick and intradermal testing has been shown to have positive results in DReSS and is suggested by the Spanish guidelines if initial patch testing is negative [80]. Despite the relatively good safety profile of patch and intradermal testing, both have been known to cause the recurrence of DReSS symptoms in some situations [86]. Controlled re-exposure is absolutely contraindicated unless special circumstances exist, such as DReSS related to anti-tuberculosis medications and beta-lactams [80]. For exclusively anti-tuberculosis drug related DReSS, reintroduction led to a relapse of symptoms in seven out of the 13 patients, with three meeting criteria for recurrent DReSS [87].
At present, the LTT is the best documented assay for the in vitro diagnosis of DReSS and the Spanish guidelines strongly recommend performing the LTT and/or the ELISPOT to confirm the diagnosis [80]. The international consensus of in vitro methods for the diagnosis of drug hypersensitivity reactions by the European Academy of Allergy and Clinical Immunology (EAACI) gave a grade C recommendation to both the LTT and ELISpot [81]. A comparison of skin tests and LTT confirms a higher sensitivity and specificity of LTT in DReSS syndrome, and both the Spanish guidelines and EAACI suggest performing in vitro tests prior to in-vivo tests. If in vitro testing is not available, patch testing may be considered first-line.
Overall, confirmatory testing is reasonable and may be advised in high-risk patients when the potential benefits outweigh the risks. This may include patients where there is diagnostic uncertainty, multiple possible causative agents, and when the suspected culprit drug is necessary for a critical health condition and no reasonable alternatives exist [80][81][87].
References
- Shear, N.H.; Dodiuk-Gad, R. Advances in Diagnosis and Management of Cu-Taneous Adverse Drug Reactions: Current and Future Trends; Shear, N., Dodi-uk-Gad, R., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 87–104. ISBN 9789811314889.
- Miyagawa, F.; Asada, H. Current Perspective Regarding the Immunopathogenesis of Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms (Dihs/Dress). Int. J. Mol. Sci. 2021, 22, 2147.
- Seishima, M.; Yamanaka, S.; Fujisawa, T.; Tohyama, M.; Hashimoto, K. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2006, 155, 344–349.
- Drago, F.; Cogorno, L.; Broccolo, F.; Ciccarese, G.; Parodi, A. A fatal case of DRESS induced by strontium ranelate associated with HHV-7 reactivation. Osteoporos. Int. 2015, 27, 1261–1264.
- Shiohara, T.; Iijima, M.; Ikezawa, Z.; Hashimoto, K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br. J. Dermatol. 2007, 156, 1083–1084.
- Descamps, V.; Brunet-Possenti, F. Human Herpesvirus 6 Reactivation in DRESS with Acute Liver Failure. Transplant. 2017, 101, e224–e225.
- Kardaun, S.H.; Sekula, P.; Valeyrie-Allanore, L.; Liss, Y.; Chu, C.-Y.; Creamer, D.; Sidoroff, A.; Naldi, L.; Mockenhaupt, M.; Roujeau, J.C.; et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br. J. Dermatol. 2013, 169, 1071–1080.
- Roujeau, J.-C.; Dupin, N. Virus Reactivation in Drug Reaction with Eosinophilia and Systemic Symptoms (Dress) Results from a Strong Drug-Specific Immune Response. J. Allergy Clin. Immunol. Pract. 2017, 5, 811–812.
- Tohyama, M.; Hashimoto, K.; Yasukawa, M.; Kimura, H.; Horikawa, T.; Nakajima, K.; Urano, Y.; Matsumoto, K.; Iijima, M.; Shear, N. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2007, 157, 934–940.
- Cacoub, P.; Musette, P.; Descamps, V.; Meyer, O.; Speirs, C.; Finzi, L.; Roujeau, J.C. The DRESS Syndrome: A Literature Review. Am. J. Med. 2011, 124, 588–597.
- Picard, D.; Janela, B.; Descamps, V.; D’Incan, M.; Courville, P.; Jacquot, S.; Rogez, S.; Mardivirin, L.; Moins-Teisserenc, H.; Toubert, A.; et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): A Multiorgan Antiviral T Cell Response. Sci. Transl. Med. 2010, 2, 46ra62.
- Tohyama, M.; Hashimoto, K.; Oda, F.; Namba, C.; Sayama, K. Influence of corticosteroid therapy on viral reactivation in drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. J. Dermatol. 2020, 47, 476–482.
- Shiohara, T.; Kano, Y.; Hirahara, K.; Aoyama, Y. Prediction and management of drug reaction with eosinophilia and systemic symptoms (DRESS). Expert Opin. Drug Metab. Toxicol. 2017, 13, 701–704.
- Ichai, P.; Laurent-Bellue, A.; Saliba, F.; Moreau, D.; Besch, C.; Francoz, C.; Valeyrie-Allanore, L.; Bretagne, S.R.; Boudon, M.; Antonini, T.M.; et al. Acute Liver Failure/Injury Related to Drug Reaction with Eosinophilia and Systemic Symptoms. Transplant. 2017, 101, 1830–1837.
- Descamps, V.; Brunet-Possenti, F. Monitoring of human herpesvirus 6 infection in the management of drug reaction with eosinophilia and systemic symptoms. Clin. Exp. Dermatol. 2021, 46, 351–352.
- Kano, Y.; Hiraharas, K.; Sakuma, K.; Shiohara, T. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br. J. Dermatol. 2006, 155, 301–306.
- Takahashi, R.; Kano, Y.; Yamazaki, Y.; Kimishima, M.; Mizukawa, Y.; Shiohara, T. Defective Regulatory T Cells In Patients with Severe Drug Eruptions: Timing of the Dysfunction Is Associated with the Pathological Phenotype and Outcome. J. Immunol. 2009, 182, 8071–8079.
- Ushigome, Y.; Kano, Y.; Ishida, T.; Hirahara, K.; Shiohara, T. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J. Am. Acad. Dermatol. 2013, 68, 721–728.
- Mizukawa, Y.; Hirahara, K.; Kano, Y.; Shiohara, T. Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms severity score: A useful tool for assessing disease severity and predicting fatal cytomegalovirus disease. J. Am. Acad. Dermatol. 2019, 80, 670–678.e2.
- Shiohara, T.; Kurata, M.; Mizukawa, Y.; Kano, Y. Recognition of Immune Reconstitution Syndrome Necessary for Better Management of Patients with Severe Drug Eruptions and Those under Immunosuppressive Therapy. Allergol. Int. 2010, 59, 333–343.
- Shiohara, T.; Inaoka, M.; Kano, Y. Drug-Induced Hypersensitivity Syndrome (DIHS): A Reaction Induced by a Complex Interplay among Herpesviruses and Antiviral and Antidrug Immune Responses. Allergol. Int. 2006, 55, 1–8.
- Schunkert, E.M.; Divito, S.J. Updates and Insights in the Diagnosis and Management of DRESS Syndrome. Curr. Dermatol. Rep. 2021, 10, 192–204.
- Almeida, C.-A.; van Miert, P.; O’Driscoll, K.; Zoet, Y.M.; Chopra, A.; Witt, C.; John, M.; Claas, F.H.J.; D’Orsogna, L.J. Virus-Specific T-Cell Clonotypes Might Contribute to Drug Hypersensitivity Reactions through Heterologous Immunity. J. Allergy Clin. Immunol. 2019, 144, 608–611.e4.
- Kim, D.Y.; Kobayashi, T.; Voisin, B.; Jo, J.-H.; Sakamoto, K.; Jin, S.-P.; Kelly, M.; Pasieka, H.B.; Naff, J.L.; Meyerle, J.H.; et al. Targeted therapy guided by single-cell transcriptomic analysis in drug-induced hypersensitivity syndrome: A case report. Nat. Med. 2020, 26, 236–243.
- Descamps, V.; Brunet-Possenti, F. Drug Reaction with Eosinophilia and Sys-temic Symptoms or Virus Reactivation with Eosinophilia and Systemic Symptoms. Pediatr. Dermatol. 2016, 33, 562.
- Wolfson, A.R.; Zhou, L.; Li, Y.; Phadke, N.A.; Chow, O.A.; Blumenthal, K.G. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome Identified in the Electronic Health Record Allergy Module. J. Allergy Clin. Immunol. Pract. 2019, 7, 633–640.
- Sharifzadeh, S.; Mohammadpour, A.H.; Tavanaee, A.; Elyasi, S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: A literature review. Eur. J. Clin. Pharmacol. 2021, 77, 275–289.
- Chen, Y.-C.; Chiu, H.-C.; Chu, C.-Y. Drug Reaction with Eosinophilia and Systemic Symptoms. Arch. Dermatol. 2010, 146, 1373–1379.
- Bommersbach, T.J.; Lapid, M.I.; Leung, J.G.; Cunningham, J.L.; Rummans, T.A.; Kung, S. Management of Psychotropic Drug–Induced DRESS Syndrome: A Systematic Review. Mayo Clin. Proc. 2016, 91, 787–801.
- Ben Fadhel, N.; Ben Romdhane, H.; Chaabane, A.; Ali, H.B.; Boughattas, N.; Aouam, K.; Ben Fredj, N. DRESS syndrome following furosemide administration: An unusual association. Néphrologie Thérapeutique 2020, 16, 437–438.
- Walsh, S.A.; Creamer, D. Drug reaction with eosinophilia and systemic symptoms (DRESS): A clinical update and review of current thinking. Clin. Exp. Dermatol. 2010, 36, 6–11.
- Beeler, A.; Engler, O.; Gerber, B.O.; Pichler, W.J. Long-lasting reactivity and high frequency of drug-specific T cells after severe systemic drug hypersensitivity reactions. J. Allergy Clin. Immunol. 2006, 117, 455–462.
- Hansel, K.; Bellini, V.; Bianchi, L.; Brozzi, J.; Stingeni, L. Drug reaction with eosinophilia and systemic symptoms from ceftriaxone confirmed by positive patch test: An immunohistochemical study. J. Allergy Clin. Immunol. Pract. 2017, 5, 808–810.
- Kang, S.-Y.; Kim, J.; Ham, J.; Cho, S.-H.; Kang, H.-R.; Kim, H.Y. Altered T cell and monocyte subsets in prolonged immune reconstitution inflammatory syndrome related with DRESS (drug reaction with eosinophilia and systemic symptoms). Asia Pac. Allergy 2020, 10, e2.
- Ogawa, K.; Morito, H.; Hasegawa, A.; Miyagawa, F.; Kobayashi, N.; Watanabe, H.; Sueki, H.; Tohyama, M.; Hashimoto, K.; Kano, Y.; et al. Elevated serum thymus and activation-regulated chemokine (TARC/CCL17) relates to reactivation of human herpesvirus 6 in drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS). Br. J. Dermatol. 2014, 171, 425–427.
- Ogawa, K.; Morito, H.; Hasegawa, A.; Daikoku, N.; Miyagawa, F.; Okazaki, A.; Fukumoto, T.; Kobayashi, N.; Kasai, T.; Watanabe, H.; et al. Identification of thymus and activation-regulated chemokine (TARC/CCL17) as a potential marker for early indication of disease and prediction of disease activity in drug-induced hypersensitivity syndrome (DIHS)/drug rash with eosinophilia and systemic symptoms (DRESS). J. Dermatol. Sci. 2013, 69, 38–43.
- Nishio, D.; Izu, K.; Kabashima, K.; Tokura, Y. T cell populations propagating in the peripheral blood of patients with drug eruptions. J. Dermatol. Sci. 2007, 48, 25–33.
- Weinborn, M.; Barbaud, A.; Truchetet, F.; Beurey, P.; Germain, L.; Cribier, B. Histopathological study of six types of adverse cutaneous drug reactions using granulysin expression. Int. J. Dermatol. 2016, 55, 1225–1233.
- Yoshikawa, T.; Fujita, A.; Yagami, A.; Suzuki, K.; Matsunaga, K.; Ihira, M.; Asano, Y. Human herpesvirus 6 reactivation and inflammatory cytokine production in patients with drug-induced hypersensitivity syndrome. J. Clin. Virol. 2006, 37, S92–S96.
- Dosch, H.-M.; Jason, J.; Gelfand, E.W. Transient Antibody Deficiency and Abnormal T Suppressor Cells Induced by Phenytoin. N. Engl. J. Med. 1982, 306, 406–409.
- Kumar, P.; Saini, S.; Khan, S.; Lele, S.S.; Prabhakar, B.S. Restoring self-tolerance in autoimmune diseases by enhancing regulatory T-cells. Cell. Immunol. 2019, 339, 41–49.
- Matta, J.M.R.; Flores, S.M.; Cherit, J.D. Drug reaction with eosinophilia and systemic symptoms (DRESS) and its relation with autoimmunity in a reference center in Mexico. An. Bras. Dermatol. 2017, 92, 30–33.
- Deng, M.; Wu, H.; Yu, M.; Tian, Y.; Li, Y.; Xiao, X. Co-Occurrence of Multiple Endocrine Abnormalities Induced by the DIHS/DRESS. Int. J. Endocrinol. 2019, 2019, 7959615.
- Deshpande, P.; Hertzman, R.J.; Palubinsky, A.M.; Giles, J.B.; Karnes, J.H.; Gibson, A.; Phillips, E.J. Immunopharmacogenomics: Mechanisms of HLA-Associated Drug Reactions. Clin. Pharmacol. Ther. 2021, 110, 607–615.
- Husain, Z.; Reddy, B.Y.; Schwartz, R.A. DRESS syndrome. J. Am. Acad. Dermatol. 2013, 68, 693.e1–693.e14.
- Saag, M.; Balu, R.; Phillips, E.; Brachman, P.; Martorell, C.; Burman, W.; Stancil, B.; Mosteller, M.; Brothers, C.; Wannamaker, P.; et al. High Sensitivity of Human Leukocyte Antigen–B*5701 as a Marker for Immunologically Confirmed Abacavir Hypersensitivity in White and Black Patients. Clin. Infect. Dis. 2008, 46, 1111–1118.
- Hetherington, S.; Hughes, A.R.; Mosteller, M.; Shortino, D.; Baker-Neblett, K.; Spreen, W.; Lai, E.; Davies, K.; Handley, A.; Dow, D.J.; et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet 2002, 359, 1121–1122.
- Hung, S.-I.; Chung, W.-H.; Liou, L.-B.; Chu, C.-C.; Lin, M.; Huang, H.-P.; Lin, Y.-L.; Lan, J.-L.; Yang, L.-C.; Hong, H.-S.; et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. USA 2005, 102, 4134–4139.
- Ponzo, M.G.; Miliszewski, M.; Kirchhof, M.G.; Keown, P.A.; Dutz, J.P. HLA-B*58:01 Genotyping to Prevent Cases of DRESS and SJS/TEN in East Asians Treated with Allopurinol—A Canadian Missed Opportunity. J. Cutan. Med. Surg. 2019, 23, 595–601.
- Genin, E.; Chen, D.-P.; Hung, S.-I.; Sekula, P.; Schumacher, M.E.; Chang, P.-Y.; Tsai, S.-H.; Wu, T.-L.; Bellón, T.; Tamouza, R.; et al. HLA-A*31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: An international study and meta-analysis. Pharm. J. 2014, 14, 281–288.
- McCormack, M.; Alfirevic, A.; Bourgeois, S.; Farrell, J.J.; Kasperavičiūtė, D.; Carrington, M.; Sills, G.J.; Marson, T.; Jia, X.; De Bakker, P.I.; et al. HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. N. Engl. J. Med. 2011, 364, 1134–1143.
- Ozeki, T.; Mushiroda, T.; Yowang, A.; Takahashi, A.; Kubo, M.; Shirakata, Y.; Ikezawa, Z.; Iijima, M.; Shiohara, T.; Hashimoto, K.; et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum. Mol. Genet. 2011, 20, 1034–1041.
- Kim, S.-H.; Lee, K.W.; Song, W.-J.; Kim, S.-H.; Jee, Y.-K.; Lee, S.-M.; Kang, H.-R.; Park, H.-W.; Cho, S.-H.; Park, S.-H.; et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011, 97, 190–197.
- Amstutz, U.; Shear, N.H.; Rieder, M.J.; Hwang, S.; Fung, V.; Nakamura, H.; Connolly, M.; Ito, S.; Carleton, B.C.; The CPNDS Clinical Recommendation Group. Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia 2014, 55, 496–506.
- Mallal, S.; Nolan, D.; Witt, C.; Masel, G.; Martin, A.; Moore, C.; Sayer, D.; Castley, A.; Mamotte, C.; Maxwell, D.; et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 2002, 359, 727–732.
- Spielberg, S.P.; Gordon, G.B.; Blake, D.A.; Mellits, E.D.; Bross, D.S. Anticonvulsant Toxicity in Vitro: Possible Role of Arene Oxides. J. Pharmacol. Exp. Ther. 1981, 217, 386–389.
- Shear, N.H.; Spielberg, S.P.; Grant, D.M.; Tang, B.K.; Kalow, W. Differences in Metabolism of Sulfonamides Predisposing to Idiosyncratic Toxicity. Ann. Intern. Med. 1986, 105, 179–184.
- Shear, N.H.; Spielberg, S.P. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J. Clin. Investig. 1988, 82, 1826–1832.
- Chung, W.-H.; Chang, W.-C.; Lee, Y.-S.; Wu, Y.-Y.; Yang, C.-H.; Ho, H.-C.; Chen, M.-J.; Lin, J.-Y.; Hui, R.C.-Y.; Ho, J.-C.; et al. Genetic Variants Associated with Phenytoin-Related Severe Cutaneous Adverse Reactions. JAMA J. Am. Med. Assoc. 2014, 312, 525–534.
- Ng, C.Y.; Yeh, Y.-T.; Wang, C.-W.; Hung, S.-I.; Yang, C.-H.; Chang, Y.-C.; Chang, W.-C.; Lin, Y.-J.; Chang, C.-J.; Su, S.-C.; et al. Impact of the HLA-B58:01 Allele and Renal Impairment on Allopurinol-Induced Cutaneous Adverse Reactions. J. Investig. Dermatol. 2016, 136, 1373–1381.
- Chung, W.-H.; Chang, W.-C.; Stocker, S.L.; Juo, C.-G.; Graham, G.G.; Lee, M.-H.H.; Williams, K.M.; Tian, Y.-C.; Juan, K.-C.; Wu, Y.-J.J.; et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: The impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann. Rheum. Dis. 2014, 74, 2157–2164.
- Momen, S.E.; Diaz-Cano, S.; Walsh, S.; Creamer, D. Discriminating minor and major forms of drug reaction with eosinophilia and systemic symptoms: Facial edema aligns to the severe phenotype. J. Am. Acad. Dermatol. 2021, 85, 645–652.
- Soria, A.; Bernier, C.; Veyrac, G.; Barbaud, A.; Puymirat, E.; Milpied, B. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: A retrospective study. J. Am. Acad. Dermatol. 2020, 82, 606–611.
- Um, S.J.; Lee, S.K.; Kim, Y.H.; Kim, K.H.; Son, C.H.; Roh, M.S.; Lee, M.K. Clinical features of drug-induced hypersensitivity syndrome in 38 patients. J. Investig. Allergy Clin. Immunol. 2010, 20, 556–562.
- Soria, A.; Amsler, E.; Bernier, C.; Milpied, B.; Tétart, F.; Morice, C.; Dezoteux, F.; Bouedec, M.-C.F.-L.; Barbaud, A.; Staumont-Sallé, D.; et al. DRESS and AGEP Reactions to Iodinated Contrast Media: A French Case Series. J. Allergy Clin. Immunol. Pract. 2021, 9, 3041–3050.
- Eshki, M.; Allanore, L.; Musette, P.; Milpied, B.; Grange, A.; Guillaume, J.-C.; Chosidow, O.; Guillot, I.; Paradis, V.; Joly, P.; et al. Twelve-Year Analysis of Severe Cases of Drug Reaction with Eosinophilia and Systemic Symptoms. Arch. Dermatol. 2009, 145, 67–72.
- Shiohara, T.; Mizukawa, Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol. Int. 2019, 68, 301–308.
- Walsh, S.; Diaz-Cano, S.; Higgins, E.; Morris-Jones, R.; Bashir, S.; Bernal, W.; Creamer, D. Drug reaction with eosinophilia and systemic symptoms: Is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of 27 cases. Br. J. Dermatol. 2013, 168, 391–401.
- Bocquet, H.; Bagot, M.; Roujeau, J.C. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin. Cutan. Med. Surg. 1996, 15, 250–257.
- Gouveia, M.P.; Gameiro, A.; Coutinho, I.; Pereira, N.; Cardoso, J.; Gonçalo, M. Overlap between maculopapular exanthema and drug reaction with eosinophilia and systemic symptoms among cutaneous adverse drug reactions in a dermatology ward. Br. J. Dermatol. 2016, 175, 1274–1283.
- Metterle, L.; Hatch, L.; Seminario-Vidal, L. Pediatric drug reaction with eosinophilia and systemic symptoms: A systematic review of the literature. Pediatr. Dermatol. 2019, 37, 124–129.
- Bedouelle, E.; Ben Said, B.; Tetart, F.; Milpied, B.; Welfringer-Morin, A.; Maruani, A.; Catteau, B.; Dezoteux, F.; Staumont-Sallé, D.; Mazereeuw-Hautier, J.; et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Series of 49 French Pediatric Cases. J. Allergy Clin. Immunol. Pract. 2021, 10, 267–274.e5.
- Martínez-Cabriales, S.A.; Rodríguez-Bolaños, F.; Shear, N.H. Drug Reaction with Eosinophilia and Systemic Symptoms (DReSS): How Far Have We Come? Am. J. Clin. Dermatol. 2019, 20, 217–236.
- Lin, I.-C.; Yang, H.-C.; Strong, C.; Yang, C.-W.; Cho, Y.-T.; Chen, K.-L.; Chu, C.-Y. Liver injury in patients with DRESS: A clinical study of 72 cases. J. Am. Acad. Dermatol. 2015, 72, 984–991.
- Kano, Y.; Ishida, T.; Hirahara, K.; Shiohara, T. Visceral Involvements and Long-term Sequelae in Drug-induced Hypersensitivity Syndrome. Med. Clin. N. Am. 2010, 94, 743–759.
- Taweesedt, P.T.; Nordstrom, C.W.; Stoeckel, J.; Dumic, I. Pulmonary Manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Systematic Review. BioMed Res. Int. 2019, 2019, 7863815.
- Radovanovic, M.; Jevtic, D.; Calvin, A.D.; Petrovic, M.; Paulson, M.; Prada, L.R.; Sprecher, L.; Savic, I.; Dumic, I. “Heart in DRESS&rdquo: Cardiac Manifestations, Treatment and Outcome of Patients with Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome: A Systematic Review. J. Clin. Med. 2022, 11, 704.
- Thongsri, T.; Chularojanamontri, L.; Pichler, W.J. Cardiac involvement in DRESS syndrome. Asian Pac. J. Allergy Immunol. 2016, 35, 3–10.
- Skowron, F.; Bensaid, B.; Balme, B.; Depaepe, L.; Kanitakis, J.; Nosbaum, A.; Maucort-Boulch, D.; Berard, F.; D’Incan, M.; Kardaun, S.H.; et al. Comparative histological analysis of drug-induced maculopapular exanthema and DRESS. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2085–2090.
- Cabañas, R.; Ramírez, E.; Sendagorta, E.; Alamar, R.; Barranco, R.; Blanca-López, N.; Doña, I.; Fernández, J.; Garcia-Nunez, I.; García-Samaniego, J.; et al. Spanish Guidelines for Diagnosis, Management, Treatment, and Prevention of DRESS Syndrome. J. Investig. Allergy Clin. Immunol. 2020, 30, 229–253.
- Mayorga, C.; Celik, G.; Rouzaire, P.; Whitaker, P.; Bonadonna, P.; Rodrigues-Cernadas, J.; Vultaggio, A.; Brockow, K.; Caubet, J.C.; Makowska, J.; et al. In vitro tests for drug hypersensitivity reactions: An ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2016, 71, 1103–1134.
- Pichler, W.J.; Tilch, J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 2004, 59, 809–820.
- Cabañas, R.; Calderón, O.; Ramírez, E.; Fiandor, A.; Caballero, T.; Heredia, R.; Herranz, P.; Madero, R.; Quirce, S.; Bellón, T. Sensitivity and specificity of the lymphocyte transformation test in drug reaction with eosinophilia and systemic symptoms causality assessment. Clin. Exp. Allergy 2018, 48, 325–333.
- Karami, Z.; Mesdaghi, M.; Karimzadeh, P.; Mansouri, M.; Taghdiri, M.M.; Kayhanidoost, Z.; Jebelli, B.; Foumani, R.S.; Babaie, D.; Chavoshzadeh, Z. Evaluation of Lymphocyte Transformation Test Results in Patients with Delayed Hypersensitivity Reactions following the Use of Anticonvulsant Drugs. Int. Arch. Allergy Immunol. 2016, 170, 158–162.
- Barbaud, A.; Collet, E.; Milpied, B.; Assier, H.; Staumont, D.; Avenel-Audran, M.; Grange, A.; Amarger, S.; Girardin, P.; Guinnepain, M.-T.; et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br. J. Dermatol. 2013, 168, 555–562.
- Ben Said, B.; Berard, F.; Hacard, F.; Pralong, P.; Balme, B.; Nicolas, J.F. Skin tests may induce DRESS relapse. Clin. Transl. Allergy 2014, 4, P136.
- Allouchery, M.; Logerot, S.; Cottin, J.; Pralong, P.; Villier, C.; Ben Saïd, B. Antituberculosis Drug-Associated DRESS: A Case Series. J. Allergy Clin. Immunol. Pract. 2018, 6, 1373–1380.
More
