Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Fitri Budiyanto.
Mangroves are halophile plants with vital economic and ecological services. Some mangrove fruits are edible and contain treasury compounds with ethnomedicinal properties. The levels of primary metabolites such as carbohydrates, protein, and fat within mangrove fruit are acceptable for daily intake. The mangrove fruits are rich in phenolic compounds, limonoids, and their derivatives, as the compounds show antimicrobial, anticancer, and antioxidant activity.
- mangrove fruit
- secondary metabolites
- nutrition
1. Introduction
Prolonged drought and other natural disasters drive food shortages, and with the global COVID-19 pandemic over the last two years, global food distribution has been left in disarray. Disruption of the food supply and poverty cause inequity in accessing nutritious food stocks [1,2][1][2]. To the UN report, in 2021, up to 811 million of the world’s population is threatened by undernourishment, which represents an increase from previous years, whilst the production rate and economic aspect continue to disturb the food stock and distribution [3,4][3][4]. Those massive obstacles obstruct the sustainable development program adopted by the UN, especially point 2, to ensure sustainable manufacturer and consumption patterns to negate world hunger [5]. Thus, the search for emerging alternative food sources with a nutrition balance is requested [6]. Even though it is not a staple food for many populations, the consumption of fruit is steadily growing for its health benefit, the daily intake of which can be considered useful in providing nutrition supplementation [7]. Some types of mangroves produce edible fruit. Though it is not categorized as a commonly cultivated plant, the mangrove fruit for many communities is consumed for its ethnomedicinal properties.
Mangroves are halophile plants with vital economic and ecological services [8]. The area is considered the most productive ecosystem, underlying the fisheries’ food web [9]. Mangrove areas are wood/timber producers, feeding–nesting grounds for birds, and consumable fishery commodities such as fish and shellfish [10,11][10][11]. On the global scale, the mangrove area is a prominent natural contributor to managing the climate in complex ways, such as its carbon flux mechanism and sequestration scheme [12,13][12][13]. The mangrove area hides its useful function on a smaller scale, especially for human merit. The mangrove sediments are rich in nutrients due to the rapid decomposition of organic matters [14]. It holds financial worth up to USD 232.49 per hectare when transformed into fertilizer in the agroindustry sector [15,16][15][16]. However, the equilibrium between conservation and exploration is compulsory to contain the sustainability effort [17,18,19][17][18][19].
Various natural treasures are found, from deeply buried in the sediment to high up in the canopy within the mangrove forest. Many microorganisms as micro-producers reside within the ecosystem with their irreplaceable roles [20,21][20][21]. Mangrove plants are hosts for more than 850 fungi, while 38 are classified as endophytic symbionts [22]. Several associated bacteria are also well-recognized for synthesizing phytochemicals, such as compounds from Pseudoalteromonas xiamenensis for its antibiotic properties [23,24][23][24] and Streptomyces euryhalinus for its antioxidant properties [25]. Aside from the symbionts, all parts of mangroves have been used in folklore medicine since time immemorial [26]. The parts of Rhizophora mangle, Avicennia officinalis L., and Xylocarpus granatum J. Koenig are renowned for their pharmacological and ethnomedicinal usage [27,28,29][27][28][29]. Those bioactivities are assumed from the metabolite contents within the plant. Naphthofuranquinone with intense anti-trypanosomal activity is found in the twigs of A. lanata [30], while proanthocyanidins from leaves of Ceriops tagal show solid antioxidant activities [31]. Unfortunately, only 27 species of mangrove have been traditionally used [32]. The fruit is a seducing subject for exploration among parts of the mangrove. The fruit is known for its prowess in traditional medicine, such as treating asthma, bleeding, cough, febrifuge, hemorrhages, intestinal parasites, remedy piles, sprains, swelling, and ulcers [32]. Some mangrove fruits are also edible, assigning their compatibility to traditional food manufacturers with curative properties [33].
2. Nutrition Composition and Bioactivity of Mangrove Fruit Extract
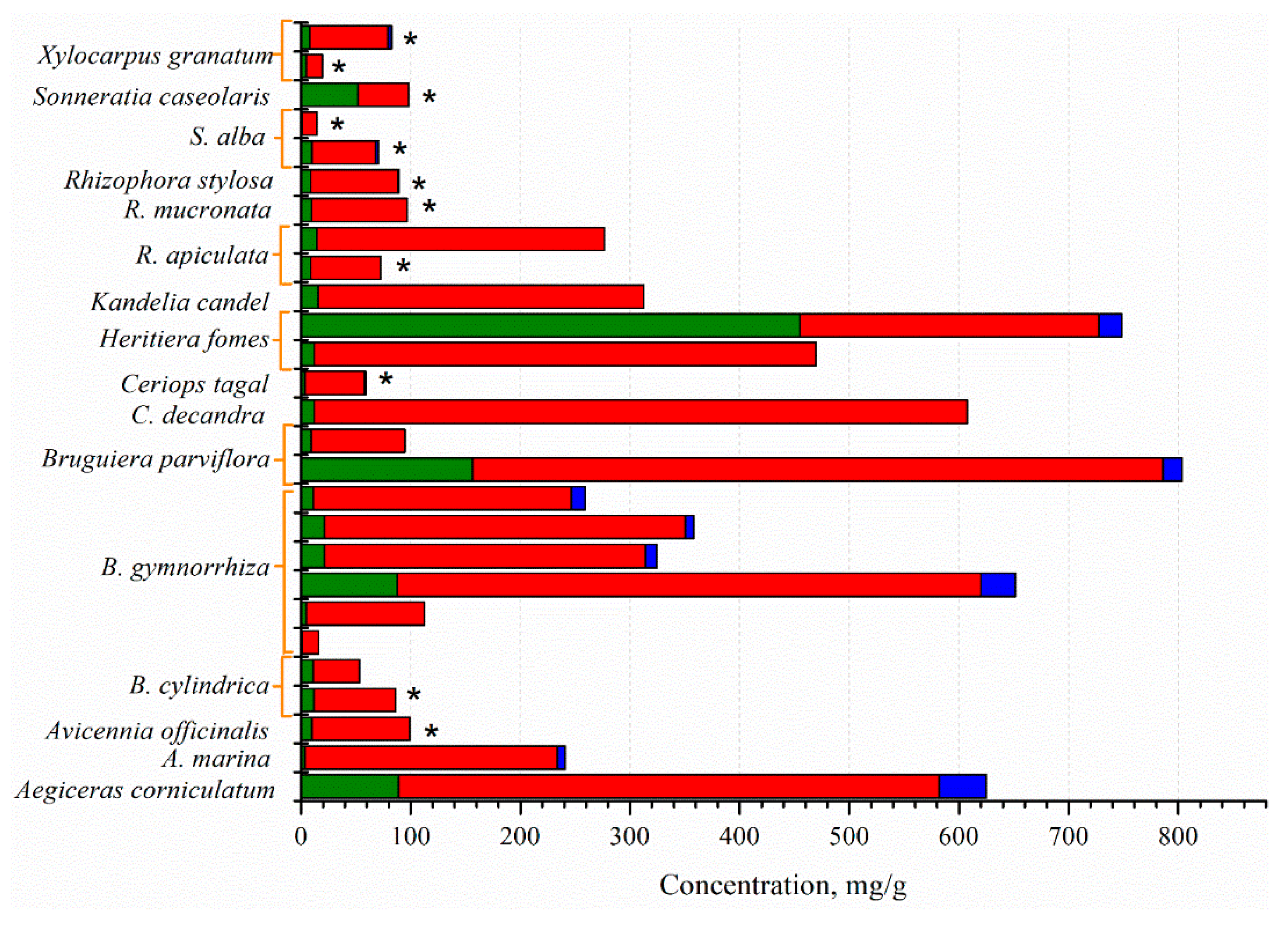
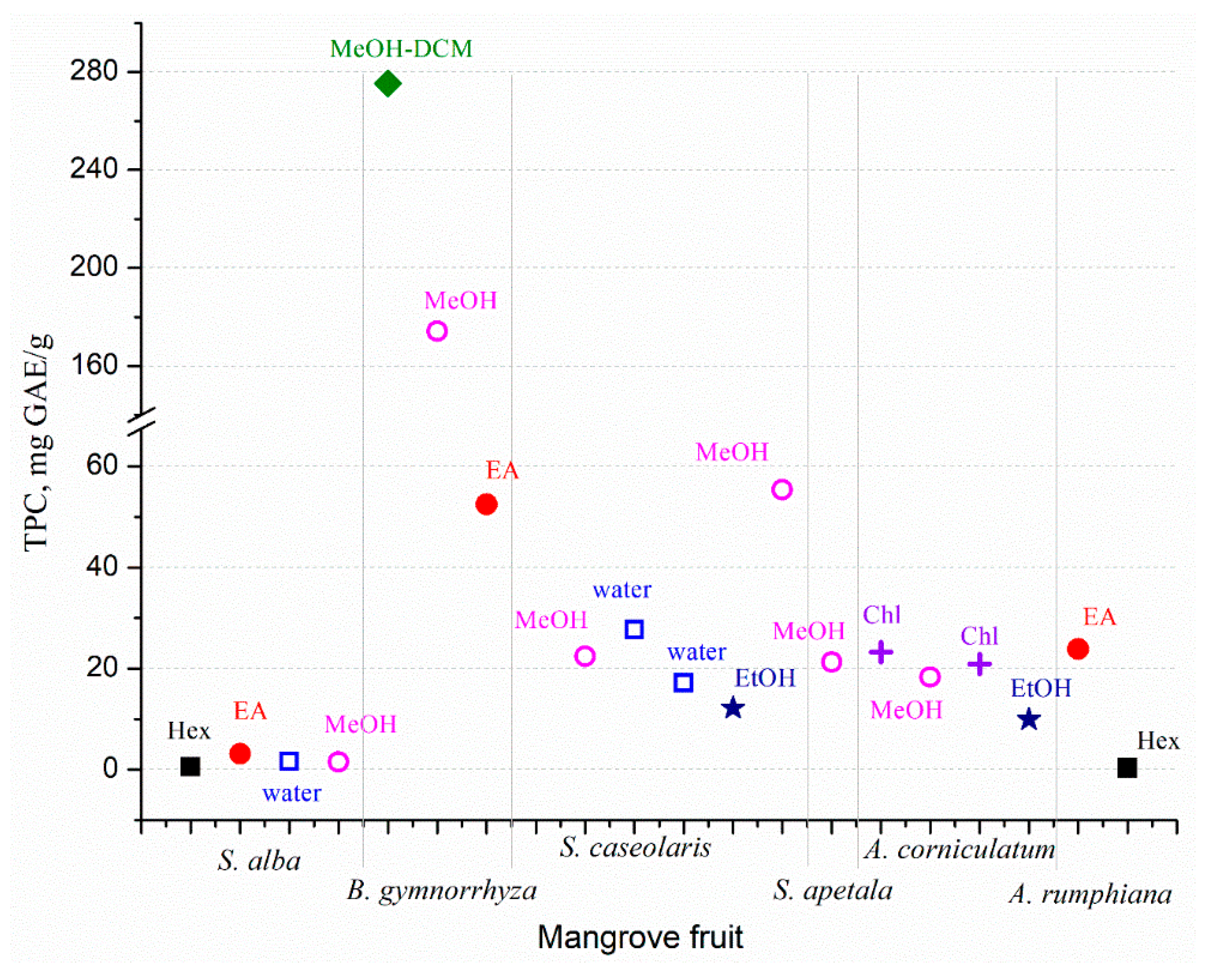
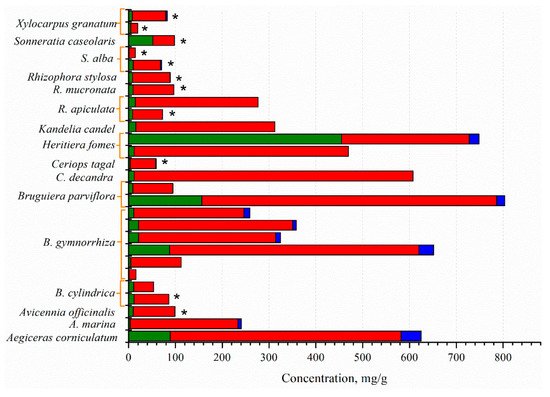
The mangrove fruit contains diverse nutrition compositions concerning the species. Carbohydrate is the dominant nutrition in all mangrove fruit, while the protein and fat contents vary (Figure 1). The total phenolic content (TPC) in the mangrove fruit is also widely investigated and, for most purposes, relates TPC with antioxidant activities [37,38][34][35]. TPC is positively correlated with the antioxidants; thus, the exploration probes for high TPC content in the fruit [39,40][36][37]. However, the evaluation is limited due to the extraction method (Figure 2), which may produce bias in the results. The TPC and other nutrient contents may be distinct among the fruit in the same species. Besides antioxidant activities, the mangrove fruit extract demonstrates other excellent bioactivities.
 The molecular study of the fruit extract of A. officinalis revealed the potentiality of the extract in treating the worldwide emerging coronavirus SARS-CoV-2. Four compounds within the extract—methyl palminoleate, methyl linoleate, hexacosane, and triacontane—showed excellent binding affinity to the virus’ main proteases, such as Arg188, Cys145, Gln189, Glu166, and Met165 [44][41]. However, long clinical steps are still required to produce firm outcomes in administering the mangrove fruit extract to treat SARS-CoV-2.
The antibacterial activity of the mangrove extract comes from the synergistic relationship among secondary metabolites. The extract is rich in steroids, phenolic compounds, alkaloids, flavonoids, and other secondary metabolites. The enrichment of Artemia salina by extract of S. alba enhanced the resistance of giant tiger prawn against Vibrio harveyi. The enrichment of extract into A. salina escalates the steroid and phenol hydroquinone levels [45][42]. Alkaloid disrupts peptidoglycan in bacterial cell walls, leading to the death of cells, while phenol affects the cytoplasmic membrane and breaks the cell nucleus. Flavonoids modify the cell protein and DNA, resulting in the inhibition of the growth of the cell [46][43].
Extract of mangrove fruit exhibited antimicrobial activities (Table 1) due to many phytochemicals, such as the total phenolic content, flavonoid [47][44], saponin, tannin, alkaloids, and saponin [48][45]. The composition of those phytochemicals varies based on the extraction method and solvent used. The composition determines the bioactivity; therefore, selecting extraction procedures is crucial in achieving noticeable positive results. The common antibacterial mechanism is attributed to membrane cell disruption [49][46]. Despite the antibacterial activity, the extract also exhibits antiviral properties. The aqueous extract of B. gymnorrhiza demonstrates potent inhibition of Zika virus (ZIKV) infection on human epithelial A549 cells by preventing the binding of the virus to the host cell surface. The aqueous extract contains polyphenols that disrupt the flavivirus's lipid membrane (outer membrane) [50][47]. The protein for the cell-binding receptor of ZIKV is targeted by cryptochlorogenic acid from the extract, leading to the virus’ death [51][48]. Moreover, the aqueous extract of R. mangle showed activity against various bacteria and depicted the cytotoxicity against human fibrosarcoma cell line HT1080 [52][49].
Various notable compounds are successfully identified from the extract of mangrove fruit. The eminent compound, such as (-)-17β-neriifolin, a cardiac glycoside, is found in the extract of Cerbera manghas with excellent heart stimulation function, effective in curing acute heart failure, at the same time show antiestrogenic, antiproliferative, and anticancer activities [55][52]. The fruit of B. gymnorrhiza contains isopimaradiene and 4-(2-aminopropyl) phenol. Isopimeradiene acts as antioxidative, anti-inflammatory, and antibacterial while 4-(2-aminopropyl) phenol shows high ROS scavenging activity, O2 scavenger, NF-E2-related factor 2 (Nrf2) stimulant, down-regulated cyclooxygenase-2 expression, and lowering of the nitrite level [38][35].
The administration of the mangrove fruit extract to animal models presents multiple vantages such as antioxidant [56][53], anti-atherosclerosis [57][54], antimicrobial [47[44][55],58], anti-diabetes [59][56], and hepatoprotective properties [60][57]. Excessive free-radical levels of diabetes trigger other diseases due to oxidative stress. Synthetic antioxidants are usually administered to reduce the oxidant level in the human body; however, side effects such as carcinogenicity should be addressed [61][58]. Mangrove fruit is a source of bioactive compounds with antioxidant activities. The antioxidant activity of the fruit is commonly related to the high content of vitamin C, anthocyanins, flavonoids, and polyphenols, which have hydrogen donating capabilities against free radicals such as nitric oxide (NO) [56][53]. The administration of methanol extract of the fruit of S. apetala to Long–Evans male rats inhibits the nitrite production in a dose-dependent manner. The extract acts as an insulin-like compound and modifies glucose utilization, enhances the transport of blood glucose to peripheral tissue, and stimulates the regeneration of the pancreas’ cells [40][37].
The molecular study of the fruit extract of A. officinalis revealed the potentiality of the extract in treating the worldwide emerging coronavirus SARS-CoV-2. Four compounds within the extract—methyl palminoleate, methyl linoleate, hexacosane, and triacontane—showed excellent binding affinity to the virus’ main proteases, such as Arg188, Cys145, Gln189, Glu166, and Met165 [44][41]. However, long clinical steps are still required to produce firm outcomes in administering the mangrove fruit extract to treat SARS-CoV-2.
The antibacterial activity of the mangrove extract comes from the synergistic relationship among secondary metabolites. The extract is rich in steroids, phenolic compounds, alkaloids, flavonoids, and other secondary metabolites. The enrichment of Artemia salina by extract of S. alba enhanced the resistance of giant tiger prawn against Vibrio harveyi. The enrichment of extract into A. salina escalates the steroid and phenol hydroquinone levels [45][42]. Alkaloid disrupts peptidoglycan in bacterial cell walls, leading to the death of cells, while phenol affects the cytoplasmic membrane and breaks the cell nucleus. Flavonoids modify the cell protein and DNA, resulting in the inhibition of the growth of the cell [46][43].
Extract of mangrove fruit exhibited antimicrobial activities (Table 1) due to many phytochemicals, such as the total phenolic content, flavonoid [47][44], saponin, tannin, alkaloids, and saponin [48][45]. The composition of those phytochemicals varies based on the extraction method and solvent used. The composition determines the bioactivity; therefore, selecting extraction procedures is crucial in achieving noticeable positive results. The common antibacterial mechanism is attributed to membrane cell disruption [49][46]. Despite the antibacterial activity, the extract also exhibits antiviral properties. The aqueous extract of B. gymnorrhiza demonstrates potent inhibition of Zika virus (ZIKV) infection on human epithelial A549 cells by preventing the binding of the virus to the host cell surface. The aqueous extract contains polyphenols that disrupt the flavivirus's lipid membrane (outer membrane) [50][47]. The protein for the cell-binding receptor of ZIKV is targeted by cryptochlorogenic acid from the extract, leading to the virus’ death [51][48]. Moreover, the aqueous extract of R. mangle showed activity against various bacteria and depicted the cytotoxicity against human fibrosarcoma cell line HT1080 [52][49].
Various notable compounds are successfully identified from the extract of mangrove fruit. The eminent compound, such as (-)-17β-neriifolin, a cardiac glycoside, is found in the extract of Cerbera manghas with excellent heart stimulation function, effective in curing acute heart failure, at the same time show antiestrogenic, antiproliferative, and anticancer activities [55][52]. The fruit of B. gymnorrhiza contains isopimaradiene and 4-(2-aminopropyl) phenol. Isopimeradiene acts as antioxidative, anti-inflammatory, and antibacterial while 4-(2-aminopropyl) phenol shows high ROS scavenging activity, O2 scavenger, NF-E2-related factor 2 (Nrf2) stimulant, down-regulated cyclooxygenase-2 expression, and lowering of the nitrite level [38][35].
The administration of the mangrove fruit extract to animal models presents multiple vantages such as antioxidant [56][53], anti-atherosclerosis [57][54], antimicrobial [47[44][55],58], anti-diabetes [59][56], and hepatoprotective properties [60][57]. Excessive free-radical levels of diabetes trigger other diseases due to oxidative stress. Synthetic antioxidants are usually administered to reduce the oxidant level in the human body; however, side effects such as carcinogenicity should be addressed [61][58]. Mangrove fruit is a source of bioactive compounds with antioxidant activities. The antioxidant activity of the fruit is commonly related to the high content of vitamin C, anthocyanins, flavonoids, and polyphenols, which have hydrogen donating capabilities against free radicals such as nitric oxide (NO) [56][53]. The administration of methanol extract of the fruit of S. apetala to Long–Evans male rats inhibits the nitrite production in a dose-dependent manner. The extract acts as an insulin-like compound and modifies glucose utilization, enhances the transport of blood glucose to peripheral tissue, and stimulates the regeneration of the pancreas’ cells [40][37].
The ethanolic extract of S. apetala shows anti-atherosclerosis in the male Wistar rat model [57][54]. The cholesterol levels, including LDL and HDL, are higher, and the formed foam is lower than the control animal [57][54]. Atherosclerosis is a vascular inflammatory disease characterized by lipid accumulation, fibrosis, and cell death in the arteries. The inflammation is mainly caused by high free-radical concentration ensuing in the incline of plasma lipid levels, such as LDL. LDL infiltrates the vascular sub-endothelium via impaired endothelium, which excites the oxidation process [64][61]. The oxidized LDL activates the endothelial cells expressed by leukocyte adhesion molecules such as vascular cell-adhesion molecule 1 (VCAM-1) on the surface of the artery. The elevated oxidized LDL stimulates the production of ROS via nitric oxide activation. Typically, NO is a protective substance produced by the vascular endothelial cells; however, it enables pro-atherogenesis if produced by macrophages. Moreover, excessive ROS generation stimulates and activates Nf-kB p65 translocation, increasing the expression of monocyte chemoattractant protein (MCP-1) and releasing the pro-inflammatory mediators by macrophages. Monocyte turns phagocytosis and macrophages of oxidized LDL to form foam cells [65,66,67][62][63][64]. Regarding its antioxidant property, the mangrove fruit extract prevents the excessive production of free radicals at the beginning of the mechanism [64][61].
The administration of the ethyl acetate extract of mangrove fruit X. moluccensi presented antidiabetic properties in a male albino Sprague–Dawley rat model; many blood parameters such as the blood glucose, serum fructosamine, serum triglycerides, and serum cholesterol levels declined. The extract improves phosphofructokinase and pyruvate kinase in the liver and kidneys while maintaining body weight [59][56]. The fruit of S. apetala displays hepatoprotective properties of male Kunming mice. The fruit extract’s antioxidative property improves the aspartate aminotransferase level in serum, reduces the alanine aminotransferase, and increases the survival rate. The hepatoprotective ability of the fruit extract in the liver is depicted by the incline in total antioxidant capacity and catalase, improvement in glutathione peroxidase and glutathione, and inhibition of myeloperoxidase, interleukin 6, and tumor necrosis factor-α. The antioxidant properties of the fruit extract are suspected to prevent liver damage caused by oxidative stress from ROS [60][57].

Table 1.
The antimicrobial activities of fruit extract from different types of mangrove species.
| Species | Solvent | Antimicrobial | Ref |
|---|---|---|---|
| Avicennia marina | Ethanol | Aspergillus fumigatus | [58][55] |
| Candida albicans | |||
| A. officinalis | Methanol | Escherichia coli | [47][44] |
| Enterobacter aerogenes | |||
| Klebsiella pneumoniae | |||
| Pseudomonas aeruginosa | |||
| Bacillus subtilis | |||
| Lactobacillus delbrueckii | |||
| Staphylococcus aureus | |||
| Streptococcus pyogenes | |||
| B. gymnorrhiza | Methanol | E. coli | [51][48] |
| P. aeruginosa | |||
| K. pneumoniae | |||
| S. aureus | |||
| Salmonella enteritidis | |||
| Sarcina lutea | |||
| Proteus mirabilis | |||
| Bacillus cereus | |||
| C. albicans | |||
| R. mangle | Ethanol | Enterococcus faecalis | [52][49] |
| Bacillus thuringiensis | |||
| Bacillus cereus | |||
| Streptococcus lactis | |||
| S. aureus | |||
| S. apetala | Methanol | E. coli | [40][37] |
| E. faecalis | |||
| Pseudomonas sp. | |||
| Shigella flexneri | |||
| Staphylococcus epidermidis | |||
| S. caseolaris | Ethyl acetate | E. coli | [48][45] |
| C. albicans | |||
| Ethanol | E. coli | ||
| S. aureus | |||
| C. albicans | |||
| Methanol | S. aureus | [49,62][46][59] | |
| E. coli | |||
| C. albicans | |||
| P. aeruginosa | |||
| Acenobacter baumannii | |||
| Methanol:ethanol | E. coli | [63][60] | |
| Klebsiella sp. | |||
| Shigella boydii | |||
| S. sonnei | |||
| S. aureus | |||
| X. mekongensis | Methanol:ethanol | S. aureus | [63][60] |
References
- Chiwona-Karltun, L.; Amuakwa-Mensah, F.; Wamala-Larsson, C.; Amuakwa-Mensah, S.; Abu Hatab, A.; Made, N.; Taremwa, N.K.; Melyoki, L.; Rutashobya, L.K.; Madonsela, T.; et al. COVID-19: From health crises to food security anxiety and policy implications. Ambio 2021, 50, 794–811.
- Savary, S.; Akter, S.; Almekinders, C.; Harris, J.; Korsten, L.; Rötter, R.; Waddington, S.; Watson, D. Mapping disruption and resilience mechanisms in food systems. Food Secur. 2020, 12, 695–717.
- Baer-Nawrocka, A.; Sadowski, A. Food security and food self-sufficiency around the world: A typology of countries. PLoS ONE 2019, 14, 1–15.
- Del Rio Osorio, L.L.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515.
- da Cunha, J.A.; Rolim, P.M.; Damasceno, K.S.F.d.S.C.; de Sousa, F.C.; Nabas, R.C.; Seabra, L.M.A.J. From seed to flour: Sowing sustainability in the use of cantaloupe melon residue (Cucumis melo L. Var. Reticulatus). PLoS ONE 2020, 15, 1–18.
- Mason-D’Croz, D.; Bogard, J.R.; Sulser, T.B.; Cenacchi, N.; Dunston, S.; Herrero, M.; Wiebe, K. Gaps between fruit and vegetable production, demand, and recommended consumption at global and national levels: An integrated modelling study. Lancet Planet Health 2019, 3, e318–e329.
- Dreher, M.L. Whole fruits and fruit fiber emerging health effects. Nutrients 2018, 10, 1833.
- Chakraborti, U.; Mitra, B.; Bhadra, K. Island Based Assemblage Pattern and Foraging Profile of Insect Flower Visitors on Aegialitis rotundifolia—A Near Threatened Mangrove Plant from Indian Sundarban. Neotrop. Entomol. 2022, 51, 32–42.
- Jänes, H.; Macreadie, P.I.; Rizzari, J.; Ierodioconou, D.; Reeves, S.E.; Dwyer, P.G.; Carnell, P.E. The value of estuarine producers to fisheries: A case study of Richmond River Estuary. Ambio 2022, 51, 875–887.
- He, W.; Nabangchang, O.; Erdman, K.; Vanko, A.C.A.; Poudel, P.; Giri, C.; Vincent, J.R. Inferring economic impacts from a program’s physical outcomes: An application to forest protection in Thailand. Environ. Resour. Econ. 2022, in press.
- Bandaranayake, W.M. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes 1998, 2, 133–148.
- Kacem, H.A.; Bouroubi, Y.; Khomalli, Y.; Elyaagoubi, S.; Maanan, M.; Rhinane, H.; Maanan, M. The economic benefit of coastal blue carbon stocks in a Moroccan Lagoon ecosystem: A case study at Moulay Bousselham Lagoon. Wetlands 2022, 42, 1–15.
- Romero-Uribe, H.M.; López-Portillo, J.; Reverchon, F.; Hernández, M.E. Effect of degradation of a black mangrove forest on seasonal greenhouse gas emissions. Environ. Sci. Pollut. Res. 2022, 29, 11951–11965.
- Nazareth, D.R.; Gonsalves, M.-J. Influence of seasonal and environmental variables on the emission of methane from the mangrove sediments of Goa. Environ. Monit. Assess. 2022, 194, 1–17.
- Hussain, S.A.; Badola, R. Valuing mangrove ecosystem services: Linking nutrient retention function of mangrove forests to enhanced agroecosystem production. Wetl. Ecol. Manag. 2008, 16, 441–450.
- Zhu, D.H.; Song, Q.L.; Nie, F.H.; Wei, W.; Chen, M.M.; Zhang, M.; Lin, H.Y.; Kang, D.J.; Chen, Z.B.; Hay, A.G.; et al. Effects of environmental and spatial variables on bacteria in Zhanjiang mangrove sediments. Curr. Microbiol. 2022, 79, 1–11.
- del Campo, J.T.F.; Olvera-Vargas, M.; Silla-Cortés, F.; Figueroa-Rangel, B.L.; Iñiguez-Dávalos, L.I. Composition and structure of vegetation and tide regulate the occurrence of Oryzomys couesi and Hodomys alleni in mangrove forests of Laguna de Cuyutlán, West-Central Mexico. Wetl. Ecol. Manag. 2022, 30, 67–82.
- Yan, J.; Du, J.; Su, F.; Zhao, S.; Zhang, S.; Feng, P. Reclamation and ecological service value evaluation of coastal wetlands using multispectral satellite imagery. Wetlands 2022, 42, 1–15.
- Thakur, N.K.; Singh, R.; Ojha, A. Dynamical study of harmful algal bloom in Sundarban mangrove wetland with spatial interaction and competing effects. Model Earth Syst. Environ. 2022, 8, 555–577.
- Huang, Z.; Huang, Y.; Lai, Q.; Chen, X.; Dong, C.; Huang, X. Oceanobacter mangrovi sp. Nov., a Novel Poly-β-hydroxybutyrate accumulating bacterium isolated from mangrove sediment. Curr. Microbiol. 2022, 79, 1–6.
- Wei, M.Y.; Li, H.; Zhong, Y.H.; Shen, Z.J.; Ma, D.N.; Gao, C.H.; Liu, Y.L.; Wang, W.H.; Zhang, J.Y.; You, Y.P.; et al. Transcriptomic analyses reveal the effect of nitric oxide on the lateral root development and growth of mangrove plant Kandelia obovata. Plant Soil. 2022, in press.
- Devadatha, B.; Jones, E.B.G.; Pang, K.L.; Abdel-Wahab, M.A.; Hyde, K.D.; Sakayaroj, J.; Bahkali, A.H.; Calabon, M.S.; Sarma, V.V.; Sutreong, S.; et al. Occurrence and geographical distribution of mangrove fungi. Fungal Divers. 2021, 106, 137–227.
- Handayani, D.P.; Isnansetyo, A.; Istiqomah, I.; Jumina, J. New report: Genome mining untaps the antibiotics biosynthetic gene cluster of Pseudoalteromonas xiamenensis STKMTI.2 from a mangrove soil sediment. Mar. Biotechnol. 2022, in press.
- Cheng, M.J.; Wu, M.-D.; Khamthong, N.; Tseng, M. Polar metabolites from the Actinobacterium Isoptericola chiayiensis isolated from mangrove soil. Chem. Nat. Compd. 2021, 57, 1134–1136.
- Biswas, K.; Bhattarcharya, D.; Saha, M.; Mukherjee, J.; Karmakar, S. Evaluation of antimicrobial activity of the extract of Streptomyces euryhalinus isolated from the Indian Sundarbans. Arch. Microbiol. 2022, 204, 1–9.
- Lee, N.L.Y.; Huang, D.; Quek, Z.B.R.; Lee, J.N.; Wainwright, B.J. Distinct fungal communities associated with different organs of the mangrove Sonneratia alba in the Malay Peninsula. IMA Fungus 2020, 11, 1–9.
- Mesquita, L.M.d.S.; Caria, C.R.e.P.; Santos, P.S.; Ruy, C.C.; Da Silva Lima, N.; Moreira, D.K.T.; da Rocha, C.Q.; Murador, D.C.; de Rosso, V.V.; Gambero, A.; et al. Modulatory effect of polyphenolic compounds from the mangrove tree rhizophora mangle L. on non-alcoholic fatty liver disease and insulin resistance in high-fat diet obese mice. Molecules 2018, 23, 2114.
- Dey, D.; Quispe, C.; Hossain, R.; Jain, D.; Ahmed Khan, R.; Janmeda, P.; Islam, M.T.; Suleria, A.R.H.; Martorell, M.; Daştan, S.D.; et al. Ethnomedicinal use, phytochemistry, and pharmacology of Xylocarpus granatum J. Koenig. Evid.-Based Complement. Altern. Med. 2021, 2021, 8922196.
- Das, S.K.; Samantaray, D.; Mahapatra, A.; Pal, N.; Munda, R.; Thatoi, H. Pharmacological activities of leaf and bark extracts of a medicinal mangrove plant Avicennia officinalis L. Clin. Phytosci. 2018, 4, 1–10.
- Mazlan, N.W.; Clements, C.; Edrada-Ebel, R.A. Targeted isolation of anti-trypanosomal naphthofuran-quinone compounds from the mangrove plant Avicennia lanata. Mar. Drugs 2020, 18, 661.
- Zhou, H.C.; Tam, N.F.Y.; Lin, Y.M.; Ding, Z.H.; Chai, W.M.; Wei, S.D. Relationships between degree of polymerization and antioxidant activities: A study on proanthocyanidins from the leaves of a medicinal mangrove plant Ceriops tagal. PLoS ONE 2014, 9, e107606.
- Bibi, S.N.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; Kannan, R.R.R.; Albuquerque, R.D.D.G.; Pandian, S.K. Ethnopharmacology, phytochemistry, and global distribution of mangroves-a comprehensive review. Mar. Drugs 2019, 17, 231.
- Nawar, M.K.; Basyuni, M.; Hanum, C.; Siregar, E.S. Bioprospecting opportunities of mangrove fruits for the coastal community in lubuk kertang and pulau sembilan, north sumatra, indonesia. Asian J. Plant Sci. 2022, 21, 145–153.
- Wonggo, D.; Berhimpon, S.; Kurnia, D.; Dotulong, V. Antioxidant activities of mangrove fruit (Sonneratia alba) taken from Wori Village, North Sulawesi, Indonesia. Int. J. ChemTech Res. 2017, 10, 284–290.
- Riyadi, P.H.; Tanod, W.A.; Dewanto, D.K.; Herawati, V.E.; Susanto, E.; Aisiah, S. Chemical profiles and antioxidant properties of Bruguiera gymnorrhiza fruit extracts from central sulawesi, indonesia. Food Res. 2021, 5, 37–47.
- Abu Bakar, F.I.; Abu Bakar, M.F.; Hassan, S.H.A.; Sanusi, S.B.; Kormin, F.; Sabran, S.F.; Fuzi, F.Z.M. Comparison of phytochemicals, antioxidant and anti-cholinesterase activity of unripe and ripe fruit of Sonneratia caseolaris. Food Res. 2020, 4, 507–514.
- Hossain, S.J.; Basar, M.H.; Rokeya, B.; Arif, K.M.T.; Sultana, M.S.; Rahman, M.H. Evaluation of antioxidant, antidiabetic and antibacterial activities of the fruit of Sonneratia apetala (Buch.-Ham.). Orient. Pharm. Exp. Med. 2013, 13, 95–102.
- Basyuni, M.; Yusraini, E.; Susilowati, A.; Hayati, R.; Siregar, E.S.; Desrita; Susetya, I.E.; Kajita, T. Bioprospecting of selected mangrove fruits based-nutritional, antioxidant, and element properties to support functional food materials for Pulau Sembilan coastal communities, Indonesia. Int. J. Adv. Sci. Eng. Inf. Technol. 2021, 11, 1661–1667.
- Ray, R.; Banerjee, A.; Mullick, J.; Jana, T.K. Nutritional composition of some selected wild mangrove fruits of Sundarbans. Indian J. Geo-Mar. Sci. 2015, 44, 1059–1066.
- Sudirman, S.; Nurjanah; Jacoeb, A.M. Proximate compositions, bioactive compounds and antioxidant activity from large-leafed mangrove (Bruguiera gymnorrhiza) fruit. Int. Food Res. J. 2014, 21, 2387–2391.
- Mahmud, S.; Paul, G.K.; Afroze, M.; Islam, S.; Gupt, S.B.R.; Razu, M.H.; Biswas, S.; Zaman, S.; Uddin, M.S.; Khan, M.; et al. Efficacy of phytochemicals derived from Avicennia officinalis for the management of covid-19: A combined in silico and biochemical study. Molecules 2021, 26, 2210.
- Cahyadi, J.; Satriani, G.I.; Gusman, E.; Weliyadi, E. Inhibiting vibrio harveyi infection in Penaeus monodon using enriched Artemia salina with mangrove fruit Sonneratia alba extract. AACL Bioflux 2020, 13, 1674–1681.
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911.
- Sharief, M.N.; Srinivasulu, A.; Veni, P.S.; Rao, V.U.M. Quantification of phytochemicals and antibacterial activity of fruit extract of Avicennia officinalis. Asian J. Pharm. Clin. Res. 2014, 7, 127–130.
- Pagarra, H.; Hartati; Rachmawaty; Hala, Y.; Rahman, R.A. Phytochemical screening and antimicrobial activity from Sonneratia caseolaris fruit extract. Mater. Sci. Forum. 2019, 967, 28–33.
- Ahmad, I.; Ambarwati, N.S.S.; Lukman, A.; Masruhim, M.A.; Rijai, L.; Mun’Im, A. In vitro antimicrobial activity evaluation of mangrove fruit (Sonneratia caseolaris L.) extract. Pharmacogn. J. 2018, 10, 598–601.
- Sadeer, N.B.; Rocchetti, G.; Senizza, B.; Montesano, D.; Zengin, G.; Uysal, A.; Jeewon, R.; Lucini, L.; Mahomoodally, M.F. Untargeted metabolomic profiling, multivariate analysis and biological evaluation of the true mangrove (Rhizophora mucronata lam.). Antioxidants 2019, 8, 489.
- Sadeer, N.B.; Haddad, J.G.; Ezzat, M.O.; Desprès, P.; Abdallah, H.H.; Zengin, G.; Uysal, A.; El Kalamouni, C.; Gallo, M.; Montesano, D.; et al. Bruguiera gymnorhiza (L.) lam. at the forefront of pharma to confront zika virus and microbial infections—an in vitro and in silico perspective. Molecules 2021, 26, 5768.
- Hicks, M.; Bailey, M.A.; Thiagarajan, T.R.; Troyer, T.L.; Huggins, L.G. Antibacterial and cytotoxic effects of red mangrove (Rhizophora mangle L.Rhizophoraceae) fruit extract. Eur. J. Sci. Res. 2011, 63, 439–446.
- Wetwitayaklung, P.; Limmatvapirat, C.; Phaechamud, T. Antioxidant and anticholinesterase activities in various parts of Sonneratia caseolaris (L.). Indian J. Pharm. Sci. 2013, 75, 649–656.
- Sulmartiwi, L.; Pujiastuti, D.Y.; Tjahjaningsih, W.; Jariyah. Potential of mangrove Avicennia rumphiana extract as an antioxidant agent using multilevel extraction. In Proceedings of the ASEAN-Fen International Fisheries Symposium, Batu, East Java, Indonesia, 7–9 November 2017.
- Deng, Y.; Liao, Y.; Li, J.; Yang, L.; Zhong, H.; Zhou, Q.; Qing, Z. Acaricidal activity against Panonychus citri and active ingredient of the mangrove plant Cerbera manghas. Nat. Prod. Commun. 2014, 9, 1265–1268.
- Parthiban, A.; Sivasankar, R.; Sachithanandam, V.; Khan, S.A.; Jayshree, A.; Murugan, K.; Sridhar, R. An integrative review on bioactive compounds from Indian mangroves for future drug discovery. S. Afr. J. Bot. 2021, in press.
- Masdar, H.; Hamidy, M.Y.; Darmawi; Trihardi, R.; Perwira, A.; Utari, D. Anti-atherosclerotic effects of Sonneratia Alba fruit extract in atherosclerotic-induced rats. Int. J. Appl. Pharm. 2020, 12, 41–43.
- Okla, M.K.; Alatar, A.A.; Al-Amri, S.S.; Soufan, W.H.; Ahmad, A.; Abdel-Maksoud, M.A. Antibacterial and antifungal activity of the extracts of different parts of Avicennia marina (Forssk.) vierh. Plants 2021, 10, 252.
- Srivastava, A.K.; Tiwari, P.; Srivastava, S.P.; Srivastava, R.; Mishra, A.; Rahuja, N.; Pandeti, S.; Tamrakar, A.K.; Narender, T.; Srivastava, M.N.; et al. Antihyperglycaemic and antidyslipidemic activities in ethyl acetate fraction of fruits of marine mangrove Xylocarpus Moluccensis. Int. J. Pharm. Pharm. Sci. 2014, 6, 809–826.
- Liu, J.; Luo, D.; Wu, Y.; Gao, C.; Lin, G.; Chen, J.; Wu, X.; Zhang, Q.; Cai, J.; Su, Z. The protective effect of Sonneratia apetala fruit extract on acetaminophen-induced liver injury in mice. Evid.-Based Complement. Altern. Med. 2019, 2019, 6919834.
- Di Meo, S.; Venditti, P. Evolution of the knowledge of free radicals and other oxidants. Oxid. Med. Cell Longev. 2020, 2020, 9829176.
- Yompakdee, C.; Thunyaharn, S.; Phaechamud, T. Bactericidal activity of methanol extracts of crabapple mangrove tree (Sonneratia caseolaris Linn.) against multi-drug resistant pathogens. Indian J. Pharm. Sci. 2012, 74, 230–236.
- Hosen, M.Z.; Biswas, A.; Islam, M.R.; Hossain, S.J. Anti-bacterial, anti-diarrheal, and cytotoxic activities of edible fruits in the Sundarbans mangrove forest of Bangladesh. Prev. Nutr. Food Sci. 2021, 26, 192–199.
- Hamidy, M.Y.; Masdar, H.; Darmawi. Effect of mangrove (Rhizophora sp.) fruit extract on foam cell formation at the initiation stage of atherosclerosis. Biomed. Pharmacol. J. 2020, 13, 423–427.
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid. Med. Cell Longev. 2020, 2020, 5245308.
- Yan, Y.; Song, D.; Wu, J.; Wang, J. Long non-coding RNAs link oxidized low-density lipoprotein with the inflammatory response of macrophages in atherogenesis. Front. Immunol. 2020, 11, 1–11.
- Hao, W.; Friedman, A. The LDL-HDL profile determines the risk of atherosclerosis: A mathematical model. PLoS ONE 2014, 9, e90497.
More