Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by SITI ZALEHA MAT ISA.
The unique physiochemical properties and biocompatibility of gold nanoparticles have developed a breakthrough in molecular imaging, which allows exploration of gold nanoparticles in drug delivery for diagnostic purpose. The pulsed laser ablation in liquid (PLAL) technique is a versatile synthetic and convincing technique due to its high efficiency, eco-friendly and facile method to produce gold nanoparticle.
- pulsed laser ablation in liquid (PLAL)
- gold nanoparticles
- synthesisation
1. The Pulsed Laser Ablation in Liquid (PLAL) Mechanism
The pulsed laser ablation in liquid (PLAL) mechanism involves multiple physical processes. During PLAL process, photon energy from the laser is absorbed by the metal target and produced the heating and photoionisation at the irradiated area. The ablation laser energy is converted to the excitation of the electron bonding in the metal target and break the bonding at the threshold energy level. Based on the Inverse Bremsstrahlung principle, these free electrons absorb incoming laser photons and induce further ionisation to the target material [19][1]. In addition, boiling, vaporisation and explosive process also occurred simultaneously.
Some of the metal surfaces are extracted as vapours, liquid drops, solid fragmentation, or plasma plume. The amount of ablated area is dependent on the absorbed energy, E. The estimation of the amount is formulated as shown in Equation:
τa >> τ1, La α E2/3, τa α E1/2, τe α E1/3
where τ1 is the laser duration, La is the ablation depth, τa is the duration of the ablation process and τe is the electronic temperature during the ablation process. The absorbed energy by the metal target is not constant in time and not uniform on the metal surface [20][2].
The dense and high energetic nonequilibrium plasma are produced from the atomisation and ionisation process at supersonic velocity. Due to its fast expansion, the plasma acts similar to a piston against the surrounding environment, generating shock waves that travel in two opposite directions, in the solid target on one side and through the liquid on the other. This shockwave has induced temperature and pressure increment within the plume and propagates through the target materials [21][3] The maximum pressure in inward shockwave can be described by the Equation:
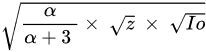
where α is the interaction efficiency, z is the reduced impedance (gcm−2s−1) and Io is the laser irradiance (GW cm−2)
The high temperature and pressure plasma continuously interact with target materials, and it causes another ablation and enhances the plasma formation [20][2]. The shockwave induced by nanosecond pulse, may last for several hundreds of microseconds in water up to a few millimetres in diameter before collapsing during cavitation as shown in A study carried out by A. De Giacomo et al. showed that outward shockwave does not have a significant role in the cavitation effect. This is due to non-transition energy from high temperature shockwave outward to the liquid medium [19][1].
During the expansion, the plasma plume cools down and releases energy to the liquid medium. The plasma plume is commonly extinguished after 10−8 to 10−7 s. This process forms a thin layer of vapour around the plasma volume, generates a cavitation bubble on a time scale of 10−7 to 10−6 and collapses on a time scale 10−4 s [22][4]. The bubble travels in the liquid up to the maximum diameter in millimetre size. S. Ibrahimkutty et al. emphasised that the cavitation bubble is generated due to interaction of ablation plume within liquid medium [21][3].
While travelling, the temperature and internal pressure in the bubble decreases less than the liquid surrounding it. Then, the bubble collapses and releases energy by emitting a second shockwave and possibly affect the phase transition and aggregation of nanoparticles produced. After the collapse of the bubble at a time scale 10−4, the system reaches a steady in physical and chemical state. However, there is a possibility of minimal modification on nanoparticles produced due to the condensation of ablation atoms and clusters existing on surrounding [22][4]. There is also a possibility agglomeration occurred for non-stabile particles produced depending on the particle composition and surface oxidation.
This cavitation bubble acts as a platform for nanoparticle nucleation, growth, coalescence and solidification or more distinct nanoparticles. This bubble plays an important role in confining the primary particles and redeposits to the substrates or nanoparticle [21][3]. The bubble interaction with the enclosed particles is a critical step in defining primary particles size. The nucleation process occurred following by nuclei growth, coalescence and forming the typical polycrystalline structure.
There are several parameters that affect the result of nanoparticle synthesis [8,22][4][5]. Short laser wavelength increases the absorption energy by metal target and increases the nanoparticles produced. However, the effect is not significant due to the reabsorption effect that increases the efficiency of the ablation, especially in noble metal materials such as silver, gold and platinum.
This is due to the plasmon resonance properties in UV visible interval, where the new nanoparticle synthesise in the liquid medium is able to reabsorb incoming laser pulse with the double negative effect that decreases the ablation rate and broadens the size distribution. The plasma plume produced is also able to reabsorb the incoming short wavelength laser and introduced to the nonlinearity among ablation and absorption efficiency or laser fluence. However, this reabsorption effect could be eliminated by using near infrared laser wavelength.
The energy threshold also contributes to the ablation efficiency. Ablation energy thresholds refer to the specific minimum fluences (optical energy per area per pulse) required to remove material from the irradiated area in target metal and generate plasma formation. Higher pulse energy causes larger material to detach from the metal and increase the concentration of the target metal in the plume as well as the energy in the solid and melted fragmentation detachment. This may induce multiple mechanisms simultaneously thermal mechanisms at the edge of the crater and fragmentation at the centre of the spot. Multiple ablation mechanism results in bimodal size distribution produced. Therefore, lower energy is used to produce monomodal size distribution in laser ablation [21][3]. In addition, higher fluences also expand bubble lifetime and reach maximum radius as shown in Equation:
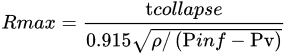 where, Rmax is the radius at the maximum bubble, ρ is density fluid, tcollapse is collapse time and Pin and Pv are the liquid pressure and vapour saturation pressure inside the bubble.
In addition, laser pulse duration also plays and an important role in PLAL results. In femtosecond laser pulse (10−15), laser energy is released to the electron in the metal target faster than the electron-phonon thermalisation process of the target. Compared to the picosecond (10−12) and nanosecond (10−9) laser, by thermal relaxation process where the energy is released to the liquid medium before the end of the pulse. In a few tenths of a picosecond, laser irradiation the plasma is generated and lasted for tens of nanoseconds after the ablation. Therefore, no temporal overlapped occurred between ejected material and laser pulse in picosecond and femtosecond laser. However, in nanosecond laser pulse, there is overlap in ablated material and ablation due to the heat conduction.
A longer laser pulse absorbs the incoming laser energy in the plasma plume and increases the plasma temperature and pressure. Then, the plasma atomises the material contained in the plume. This process homogenises the material ejected from the targets [20][2]. The absorbed energy by the metal target decreases as the plasma plumes produced provided optical shielding around the metal target. However, this phenomenon increases the plasma temperature and enhance the ablation interaction between plasma plume and metal target. For noble material, 105 laser pulses contributed to 1 mg NMPs [20][2].
In addition, physical-chemical properties of solvent and solutes in the liquid medium also contributes to the nanoparticles produced [23][6]. Modification of viscosity, density, and surface tension of the solvent in the liquid affects the cavitation bubble and confinement of the plasma plume on the crater. Increasing the viscosity for example by adding ethanol in the liquid medium increases the ablation efficiency by improving the plasma plume confinement on the crater [22][4] and also increases nanoparticles solution stability [24][7] by reducing the aggregation [25][8]. The bio conjugated of nanoparticles can easily achieved with an additional thiol spacer or NH2 group during the laser ablation in situ [26][9].
The temperature and pressure of liquid medium are used to bring the impact to the laser irradiation efficiency, plasma confinement and the cavitation bubble dynamics. This is due to the changes in liquid medium physical including viscosity, density, refractive index, surface tension and compressibility. Increasing pressure increases the cooling plasma rate and decreases cavitation bubble diameter and lifetime. This might cause a defect to the particles synthesised [22][4].
where, Rmax is the radius at the maximum bubble, ρ is density fluid, tcollapse is collapse time and Pin and Pv are the liquid pressure and vapour saturation pressure inside the bubble.
In addition, laser pulse duration also plays and an important role in PLAL results. In femtosecond laser pulse (10−15), laser energy is released to the electron in the metal target faster than the electron-phonon thermalisation process of the target. Compared to the picosecond (10−12) and nanosecond (10−9) laser, by thermal relaxation process where the energy is released to the liquid medium before the end of the pulse. In a few tenths of a picosecond, laser irradiation the plasma is generated and lasted for tens of nanoseconds after the ablation. Therefore, no temporal overlapped occurred between ejected material and laser pulse in picosecond and femtosecond laser. However, in nanosecond laser pulse, there is overlap in ablated material and ablation due to the heat conduction.
A longer laser pulse absorbs the incoming laser energy in the plasma plume and increases the plasma temperature and pressure. Then, the plasma atomises the material contained in the plume. This process homogenises the material ejected from the targets [20][2]. The absorbed energy by the metal target decreases as the plasma plumes produced provided optical shielding around the metal target. However, this phenomenon increases the plasma temperature and enhance the ablation interaction between plasma plume and metal target. For noble material, 105 laser pulses contributed to 1 mg NMPs [20][2].
In addition, physical-chemical properties of solvent and solutes in the liquid medium also contributes to the nanoparticles produced [23][6]. Modification of viscosity, density, and surface tension of the solvent in the liquid affects the cavitation bubble and confinement of the plasma plume on the crater. Increasing the viscosity for example by adding ethanol in the liquid medium increases the ablation efficiency by improving the plasma plume confinement on the crater [22][4] and also increases nanoparticles solution stability [24][7] by reducing the aggregation [25][8]. The bio conjugated of nanoparticles can easily achieved with an additional thiol spacer or NH2 group during the laser ablation in situ [26][9].
The temperature and pressure of liquid medium are used to bring the impact to the laser irradiation efficiency, plasma confinement and the cavitation bubble dynamics. This is due to the changes in liquid medium physical including viscosity, density, refractive index, surface tension and compressibility. Increasing pressure increases the cooling plasma rate and decreases cavitation bubble diameter and lifetime. This might cause a defect to the particles synthesised [22][4].
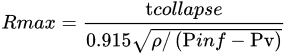 where, Rmax is the radius at the maximum bubble, ρ is density fluid, tcollapse is collapse time and Pin and Pv are the liquid pressure and vapour saturation pressure inside the bubble.
In addition, laser pulse duration also plays and an important role in PLAL results. In femtosecond laser pulse (10−15), laser energy is released to the electron in the metal target faster than the electron-phonon thermalisation process of the target. Compared to the picosecond (10−12) and nanosecond (10−9) laser, by thermal relaxation process where the energy is released to the liquid medium before the end of the pulse. In a few tenths of a picosecond, laser irradiation the plasma is generated and lasted for tens of nanoseconds after the ablation. Therefore, no temporal overlapped occurred between ejected material and laser pulse in picosecond and femtosecond laser. However, in nanosecond laser pulse, there is overlap in ablated material and ablation due to the heat conduction.
A longer laser pulse absorbs the incoming laser energy in the plasma plume and increases the plasma temperature and pressure. Then, the plasma atomises the material contained in the plume. This process homogenises the material ejected from the targets [20][2]. The absorbed energy by the metal target decreases as the plasma plumes produced provided optical shielding around the metal target. However, this phenomenon increases the plasma temperature and enhance the ablation interaction between plasma plume and metal target. For noble material, 105 laser pulses contributed to 1 mg NMPs [20][2].
In addition, physical-chemical properties of solvent and solutes in the liquid medium also contributes to the nanoparticles produced [23][6]. Modification of viscosity, density, and surface tension of the solvent in the liquid affects the cavitation bubble and confinement of the plasma plume on the crater. Increasing the viscosity for example by adding ethanol in the liquid medium increases the ablation efficiency by improving the plasma plume confinement on the crater [22][4] and also increases nanoparticles solution stability [24][7] by reducing the aggregation [25][8]. The bio conjugated of nanoparticles can easily achieved with an additional thiol spacer or NH2 group during the laser ablation in situ [26][9].
The temperature and pressure of liquid medium are used to bring the impact to the laser irradiation efficiency, plasma confinement and the cavitation bubble dynamics. This is due to the changes in liquid medium physical including viscosity, density, refractive index, surface tension and compressibility. Increasing pressure increases the cooling plasma rate and decreases cavitation bubble diameter and lifetime. This might cause a defect to the particles synthesised [22][4].
where, Rmax is the radius at the maximum bubble, ρ is density fluid, tcollapse is collapse time and Pin and Pv are the liquid pressure and vapour saturation pressure inside the bubble.
In addition, laser pulse duration also plays and an important role in PLAL results. In femtosecond laser pulse (10−15), laser energy is released to the electron in the metal target faster than the electron-phonon thermalisation process of the target. Compared to the picosecond (10−12) and nanosecond (10−9) laser, by thermal relaxation process where the energy is released to the liquid medium before the end of the pulse. In a few tenths of a picosecond, laser irradiation the plasma is generated and lasted for tens of nanoseconds after the ablation. Therefore, no temporal overlapped occurred between ejected material and laser pulse in picosecond and femtosecond laser. However, in nanosecond laser pulse, there is overlap in ablated material and ablation due to the heat conduction.
A longer laser pulse absorbs the incoming laser energy in the plasma plume and increases the plasma temperature and pressure. Then, the plasma atomises the material contained in the plume. This process homogenises the material ejected from the targets [20][2]. The absorbed energy by the metal target decreases as the plasma plumes produced provided optical shielding around the metal target. However, this phenomenon increases the plasma temperature and enhance the ablation interaction between plasma plume and metal target. For noble material, 105 laser pulses contributed to 1 mg NMPs [20][2].
In addition, physical-chemical properties of solvent and solutes in the liquid medium also contributes to the nanoparticles produced [23][6]. Modification of viscosity, density, and surface tension of the solvent in the liquid affects the cavitation bubble and confinement of the plasma plume on the crater. Increasing the viscosity for example by adding ethanol in the liquid medium increases the ablation efficiency by improving the plasma plume confinement on the crater [22][4] and also increases nanoparticles solution stability [24][7] by reducing the aggregation [25][8]. The bio conjugated of nanoparticles can easily achieved with an additional thiol spacer or NH2 group during the laser ablation in situ [26][9].
The temperature and pressure of liquid medium are used to bring the impact to the laser irradiation efficiency, plasma confinement and the cavitation bubble dynamics. This is due to the changes in liquid medium physical including viscosity, density, refractive index, surface tension and compressibility. Increasing pressure increases the cooling plasma rate and decreases cavitation bubble diameter and lifetime. This might cause a defect to the particles synthesised [22][4].
2. The PLAL Synthetisation Method
A recent study reported that the most promising and convincing technique in producing gold nanoparticles is by using the PLAL technique [6][10]. Some studies used pulse laser Neodymium-doped Yttrium Aluminium Garnet (Nd:YAG) to produce gold nanoparticles in the PLAL method [18,27,28,29,30,31,32,33][11][12][13][14][15][16][17][18]. The Nd:YAG laser was selected due to its efficiency and wide availability. Green laser with 532 nm wavelength and near-infrared (NIR) laser with 1064 nm wavelength were also used for gold nanoparticles production in the PLAL method. There are several factors that affect the characteristics of gold nanoparticle formation from the PLAL synthetisation method.2.1. Laser Parameters
The laser wavelength, energy, fluence, pulse repetition and time ablation affect the shape, structure, diameter size of synthesised gold nanoparticles and its distribution. The morphology of gold nanoparticles produced by PLAL was in spherical shape. This shape is very important in offering a large surface area for conjugation with other molecules such as peptides, biomarkers and drugs in molecular imaging due to the large surface provided. In addition, the diameter of gold nanoparticle refers to the size of the particles, and the size distribution of gold nanoparticle refers to the amount and pattern of gold nanoparticle in a liquid medium such as uniform or clusters. There are two common types of laser ablation wavelengths used in the previous studies to synthesise gold nanoparticles using the PLAL technique. It includes NIR laser with 1064 nm and green laser with 532 or 355 nm wavelength. Alluhaybi et al. [6][10] reported that laser wavelength plays an important role in controlling the shape, size, stability and growth of gold nanoparticles [12][19]. The absorption energy by metal target is increased with lower laser ablation wavelength. The interband transition in metal targets and strong plasmon (for gold material) from nanocomposites with other particles in the same liquid medium also increases the absorption energy by target material. These will increase large fragmentation from metal targets and produce smaller size distribution with smaller average diameter sizes of the particles [22,32][4][17]. Other than that, the ablation laser at 555 nm wavelength also produced more homogenous erosion on the target surface than the rugged erosion at 1064 nm wavelength at the same fluences. The crater morphology of metal target correlated to the homogeneity product produced which is bring implication to the particles size, size distribution, surface, and inner composition of nanoparticles. The homogenous erosion produced smaller average diameters (3–6 nm) due to the vaporisation process, and the opposite character produced larger size of nanoparticles with size distribution peaks at 10 nm due to the explosive boiling process of the target surface [20][2]. Furthermore, laser energy contributes to the size of the resultant gold nanoparticle and amount of ablated material. The laser energy is measured in mJ. The linear decay of the ablation rate with spot diameter leads to the inverse-proportional increase of the ablation rate with the square root of the laser fluence [6,20][2][10]. An increment in laser energy enhances the number of synthesised gold nanoparticles with larger size distribution, larger nanoparticle sizes and higher solution concentration [33][18]. However, this effects for materials with lower reflectivity [33][18]. Enhancing the power ablation to the gold target material will cause a higher detachment of target material, formation of plume and nanoparticles dissolved in a liquid medium and increase solution concentration. High concentration of gold nanoparticles by the PLAL method visually shows a darker colour due to surface plasmon resonance (SPR) characteristic. Apart from fragmentation, explosive boiling and vaporisation that occur simultaneously, there is high probability occurrence of thermal mechanism at the edge of the crater and fragmentation at the centre part of the laser spot. Due to the multiple ablation mechanism, bimodal size distribution is obtained in high laser energy compared with monomodal in lower energy prospectively.A study shows that higher laser energy at 318 mJ will produce smaller average particle sizes of gold nanoparticle (7.516 nm) than that at 286, 226 and 96.6 mJ with particle sizes at 17.5, 25 and 30 nm, respectively, with the same 1064 nm laser ablation wavelength [6][10].
However, the particle size with a laser energy of 46 mJ is smaller than that of 128 mJ with 83.63 and 106.3 nm, respectively. Hernandez-Maya et al. emphasised that the optimal energy used for laser ablation ranges between 100 and 180 mJ. The reseauthorchers also claimed that it was not possible to ablate the target material with energy less than 100 mJ and difficult to reproduce gold nanoparticles with energy more than 180 mJ [5][20]. The study also reported that saturation tendency might occur in gold nanoparticle solution and a possibility of gold debris present in the solution [5][20].
Another laser parameter that affects the characteristics of synthesised gold nanoparticles in the PLAL technique is laser fluences. Laser fluence refers to the time-integrated flux laser to the target. It corresponds to laser energy and is measured in J cm2. The higher the laser energy, the greater the laser fluences produced with the same size of the targeted area involved. Therefore, higher laser fluences will produce a smaller diameter size of gold nanoparticle produced. Higher fluences ablation laser for green laser and NIR laser wavelengths will produce a rugged crater on the metal target surface. Thus, 60 J cm−1 produced sharper craters, whereas 1000 J cm−1 produced irregular craters. Homogenous craters surface and low fluences of laser ablation produced will produced small size nanoparticles diameters and distribution [20][2].
Furthermore, laser pulse repetition rate showed a significant effect on the development of gold nanoparticles. Repetition rate refers to the number of laser pulse per second that hits the target. Higher pulse repetition will produce more gold nanoparticles. This linear correlation occurred in the range of 10−4–104 s or 103–104 Hz, where the repetition rate is longer than the lifetime bubble cavitation time [22][4]. The bubble cavitation reduces the laser ablation to the metal target and causes scattering of laser light. During the expansion of bubble cavitation, laser ablation occurs in the low density hot gaseous phase. Therefore, confined plasma plume on the crater surface is less efficient on the detached metal target.
A high pulse repetition rate will maintain the heat produced by ablation and reduce energy waste. This situation speeds up aggregation and coalescence and increases the amount and concentration of synthesised gold nanoparticles [6][10]. A study shows that increasing the pulse repetition rate at 8 Hz reduced the average size of particle size to 3.29 nm compared with 17.54 nm at 2 Hz [6][10] Other than that, at higher repetition rate (kHz), the metal target will increase higher (26–27 °C) than the room temperature and reaches the energy threshold for detachment metal surface [22][4].
Other than that, the ablation duration in the PLAL method also plays a role in nanoparticles synthesised. A longer ablation duration will increase the amount of ablated metal target due to the increase of temperature, and the pressure generated in plasma plume. Continues ablation will produces a smaller size of gold nanoparticles and increasing the concentration of gold particles in a liquid medium [7][21].
However, shorter pulse duration minimised the thermal damage that is inflicted on the surroundings, and it has high possibility of performing ablation at maximum peak power.
2.2. Liquid Medium
There are various types of liquid medium used to immerse the target metal (solid gold) during the PLAL method. The selection of the liquid medium depends on the type of gold nanoparticle production to produce either hybrid (gold coating with other metals such as iron oxide nanoparticles) or pure gold nanoparticle [34][22]. Deionised or pure water was used to produce pure gold nanoparticles [6,35,36][10][23][24]. The ionic solvent was used as a capping agent, template and precursor in gold nanoparticle synthetisation [4,37,38][25][26][27]. Compared with other metals, the gold nanoparticles can be synthesised stable either in water or organic solvents without stabilising molecules or ligand using the PLAL method [23][6]. The stability and nanoparticle size in liquid could be controlled by adjusting the ionic strength without compromising the nanoparticle adsorption of the supporting material. A study shows that palladium nanoparticles in pure water have weak stability where the particles were precipitated with mass loss approximately 30% after 50 min of ablation times [23][6]. Extra salt in saline acts as an oxidising agent that could increase the stability by reducing the aggregation effect and preventing particle growth. Therefore, the particle sizes produced are smaller. In addition, the stability of nanoparticles could be increased by ablated material in highly diluted electrolytes medium [23][6]. Meanwhile, the high stability of nanoparticles in deionised water is reported related to the surface charge of the particles causing a zeta potential and electrostatic repulsion of the particles [39][28]. In certain condition, the stabilisers such as thiols, citrate and other ligands were added to the gold particle during the ablation process. This stabiliser will dump on gold nanoparticles surface and caused a negative effect to the gold nanoparticle plasmonic properties. It will decrease the nanoparticle sizes by inhibit agglomeration process [22,23][4][6]. Another study reported that liquid media volume used to immerse the target metal does not affect the ablation duration and physiochemical property of gold nanoparticle with respect to morphology, structure and its function [5,7][20][21]. These findings were proven in the fabrication of gold nanoparticles and other nanoalloys such as argentum (Ag), stannic oxide and silicon [3,40,41,42][29][30][31][32] via the PLAL technique. In addition, with the use of a small amount of liquid media and gold target, the number of gold nanoparticles obtained did not exceed 10−4 g [5][20]. On the other hand, a recent study also reported that stirring the liquid medium during the ablation time or immediately a few minutes after the ablation process produced homogeneous gold nanoparticles without any agglomeration in gold nanoparticle solution [37][26]. There are a few techniques used in stirring by either rotation motor magnetically with constant speed or manual stirring by using a spatula. High concentration of nanosolution or ionic solvent decreases laser energy to target metal exponentially with radiation time. The effect of laser parameters and liquid medium in gold nanoparticle synthesis via the PLAL method is summarised in Table 1.Table 1. A summary of the effect of laser parameters selection on diameter of gold nanoparticles.
| Reference | Laser Wavelength (nm) | Laser Energy (mJ) | Laser Fluence (J/cm | −1 | ) | Repetition Rate (Hz) | Laser Pulse/Time Ablation | Liquid Medium/Depth (mL) | Average Diameter (nm) | Scaling Instruments |
|---|---|---|---|---|---|---|---|---|---|---|
| [43] | [33] | 1064 | – | 23.96 | 1 | 500 pulses | Deionised water/5 mL | 7–10 | TEM | |
| [11] | [34] | 532 | 30 | 10 | 30 min | Distilled water/30 mL | 13 | SEM, TEM | ||
| [9] | [35] | 532 | 318 | – | 40 | 30 min with stirring | THF/20 mL | 6 | HRTEM | |
| [7] | [21] | 1064 | – | – | 10 | 30 min (stop every 3 min) | Deionised water/10 mL | 7.4 | TEM, DLS, zeta potential | |
| [5] | [20] | 532 | 120 | – | 10 | 5 min | Milli-Q water/15 mL | 21 | SEM, XRD, XPS | |
| 10 min | 9 | |||||||||
| 5 min | 15 mL | 2.88 | ||||||||
| 10 mL | 4.80 | |||||||||
| [44] | [36] | 1064 | 950 | – | 5 | 1000 | Distilled water/3 mL | 6.09 | TEM, X-ray diffraction | |
| Ethanol/3 mL | 24.71 | |||||||||
| [41] | [31] | 1064 | 1.5 ns | – | 10 | 5000 | Deionised water | 60 | XRD, TEM | |
| [33] | [18] | 532 | 950 | – | 5 | 1000 | Distilled water/3 mL | 9.738 | TEM | |
| 1064 | 12.09 | |||||||||
| [45] | [37] | 1064 | 2.5 ns | – | – | 20 min | Deionised water/6 mL | <20 | TEM, HRTEM, EDX |
References
- De Giacomo, A.; Dell’Aglio, M.; Santagata, A.; Gaudiuso, R.; De Pascale, O.; Wagener, P.; Messina, G.C.; Compagnini, G.; Barcikowski, S. Cavitation dynamics of laser ablation of bulk and wire-shaped metals in water during nanoparticles production. Phys. Chem. Chem. Phys. 2013, 15, 3083–3092.
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Physic. Chem. Chem. Phys. 2009, 11, 3805–3821.
- Ibrahimkutty, S.; Wagener, P.; Rolo, T.D.S.; Karpov, D.; Menzel, A.; Baumbach, T.; Barcikowski, S.; Plech, A. A hierarchical view on material formation during pulsed-laser synthesis of nanoparticles in liquid. Sci. Rep. 2015, 5, 1–11.
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nano- materials generated by laser ablation in liquid solution? †. Phys. Chem. Chem. Phys. 2013, 15, 3027–3046.
- Letzel, A.; Gökce, B.; Menzel, A.; Plech, A.; Barcikowski, S. Primary particle diameter differentiation and bimodality identification by five analytical methods using gold nanoparticle size distributions synthesized by pulsed laser ablation in liquids. Appl. Surf. Sci. 2018, 435, 743–751.
- Marzun, G.; Nakamura, J.; Zhang, X.; Barcikowski, S.; Wagener, P. Size control and supporting of palladium nanoparticles made by laser ablation in saline solution as a facile route to heterogeneous catalysts. Appl. Surf. Sci. 2015, 348, 75–84.
- Wagener, P.; Jakobi, J.; Rehbock, C.; Chakravadhanula, V.S.K.; Thede, C.; Wiedwald, U.; Bartsch, M.; Kienle, L.; Barcikowski, S. Solvent-surface interactions control the phase structure in laser-generated iron-gold core-shell nanoparticles. Sci. Rep. 2016, 6, 23352.
- Rehbock, C.; Merk, V.; Gamrad, L.; Streubel, R.; Barcikowski, S. Size control of laser-fabricated surfactant-free gold nanoparticles with highly diluted electrolytes and their subsequent bioconjugation. Phys. Chem. Chem. Phys. 2013, 15, 3057–3067.
- Hahn, A.; Barcikowski, S.; Chichkov, B.N. Influences on nanoparticle production during pulsed laser ablation. J. Laser Micro Nanoeng. 2007, 3, 73–77.
- Alluhaybi, H.A.; Ghoshal, S.K.; Shamsuri, W.N.W.; Alsobhi, B.O.; Salim, A.A.; Krishnan, G. Pulsed laser ablation in liquid assisted growth of gold nanoparticles: Evaluation of structural and optical features. Nano-Struct. Nano-Objects 2019, 19, 100355.
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene-gold nanoparticles hybrid-synthesis, functionalization, and application in a electrochemical and surface-enhanced raman scattering biosensor. Materials 2016, 9, 406.
- Patil, M.P.; Kim, G.D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92.
- Menazea, A.A.; Abdelghany, A.M. Precipitation of silver nanoparticle within silicate glassy matrix via Nd:YAG laser for biomedical applications. Radiat. Phys. Chem. 2020, 174, 108958.
- Chen, Q.; Ye, Y.; Liu, J.; Wu, S.; Li, P.; Liang, C. Stability evolution of ultrafine Ag nanoparticles prepared by laser ablation in liquids. J. Colloid Interface Sci. 2021, 585, 444–451.
- Torrisi, A.; Cutroneo, M.; Torrisi, L.; Vacík, J. Biocompatible nanoparticles production by pulsed laser ablation in liquids. J. Instrum. 2020, 15, 03053.
- Torrisi, L. Physical aspects of gold nanoparticles as cancer killer therapy. Indian J. Phys. 2021, 95, 225–234.
- Jamaludin, N.; Chaudhary, K.T.; Haider, Z.; M, D.; Ismail, F.D.; Roslan, M.S.; Amira, N.H.; Ali, J. Effect of laser energy and wavelength on average size of gold nanoparticles synthesized by pulsed laser ablation in deionized water. J. Physic Conf. Ser. 2020, 1484, 012029.
- Mohd Hilmi Tan, M.I.S.; Omar, A.F.; Rashid, M.; Hashim, U. VIS-NIR spectral and particles distribution of Au, Ag, Cu, Al and Ni nanoparticles synthesized in distilled water using laser ablation. Results Phys. 2019, 14, 102497.
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445.
- Hernández-Maya, M.; Rivera-Quintero, P.; Ospina, R.; Quintero-Orozco, J.H.; García-Castro, A.C. Ablation energy, water volume and ablation time: Gold nanoparticles obtained through by pulsed laser ablation in liquid. J. Phys. Conf. Ser. 2019, 1386.
- Kuriakose, A.C.; Nampoori, V.P.N.; Thomas, S. Facile synthesis of Au/CdS core-shell nanocomposites using laser ablation technique. Mater. Sci. Semicond. Process. 2019, 101, 124–130.
- Torrisi, L.; Torrisi, A. Laser ablation parameters influencing gold nanoparticle synthesis in water. Radiat. Eff. Defects Solids 2018, 173, 729–739.
- Lee, S.H.; Jung, H.J.; Lee, S.J.; Theerthagiri, J.; Kim, T.H.; Choi, M.Y. Applied Surface Science Selective synthesis of Au and graphitic carbon-encapsulated Au (Au @ GC) nanoparticles by pulsed laser ablation in solvents: Catalytic Au and acid- resistant Au @ GC nanoparticles. Appl. Surf. Sci. 2020, 506, 145006.
- Ahmed, S.M.; Imam, H. Characterization and photocatalytic activity of Eu:ZnO & Au/Eu:ZnO nanoparticles prepared by laser ablation in water. Mater. Sci. Semicond. Process. 2020, 115, 105128.
- Nancy, P.; Nair, A.K.; Antoine, R.; Thomas, S.; Kalarikkal, N. In situ decoration of gold nanoparticles on graphene oxide via nanosecond laser ablation for remarkable chemical sensing and catalysis. Nanomaterials 2019, 9, 1201.
- John, M.G.; Tibbetts, K.M. One-step femtosecond laser ablation synthesis of sub-3 nm gold nanoparticles stabilized by silica. Appl. Surf. Sci. 2019, 475, 1048–1057.
- Simon, J.; Nampoori, V.P.N.; Kailasnath, M. Facile synthesis of Au-Ag core shell and nanoalloy using femtosecond laser ablation and their optical characterization. Optik 2019, 195, 163168.
- Shin, C.-Y.; Streaubal, R.; Heberle, J.; Letzel, A.; Shugaev, M.V.; Wu, C.; Schmit, M.; Gökce, B.; Barcikowski, S.; Zhigilei, L.V. Two mechanisms of nanoparticle generation in picosecond laser ablation in liquids: The origin of the bimodal size distribution. Nanoscale 2018, 10, 6900–6910.
- Mohazzab, B.F.; Jaleh, B.; Nasrollahzadeh, M.; Issaabadi, Z.; Varma, R.S. Laser ablation-assisted synthesis of GO/TiO2/Au nanocomposite: Applications in K3 and Nigrosin reduction. Mol. Catal. 2019, 473, 110401.
- Mostafa, A.M.; Mwafy, E.A. Effect of dual-beam laser radiation for synthetic SnO2/Au nanoalloy for antibacterial activity. J. Mol. Struct. 2020, 1222, 128913.
- Nasiri, P.; Doranian, D.; Sari, A.H. Synthesis of Au/Si nanocomposite using laser ablation method. Opt. Laser Technol. 2019, 113, 217–224.
- Mahdieh, M.H.; Fattahi, B. Effects of water depth and laser pulse numbers on size properties of colloidal nanoparticles prepared by nanosecond pulsed laser ablation in liquid. Opt. Laser Technol. 2015, 75, 188–196.
- Sadrolhosseini, A.R.; Krishnan, G.; Safie, S.; Beygisangchin, M.; Abdul Rashid, S.; Harun, S. Enhancement of the fluorescence property of carbon quantum dots based on laser ablated gold nanoparticles to evaluate pyrene. Opt. Mater. Express 2020, 10, 2227–2241.
- Riedel, R.; Mahr, N.; Yao, C.; Wu, A.; Yang, F.; Hampp, N. Synthesis of gold-silica core-shell nanoparticles by pulsed laser ablation in liquid and their physico-chemical properties towards photothermal cancer therapy. Nanoscale 2020, 12, 3007–3018.
- Naharuddin, N.Z.A.; Sadrolhosseini, A.R.; Bakar, M.H.A.; Tamchek, N.; Mahdi, M.A. Laser ablation synthesis of gold nanoparticles in tetrahydrofuran. Opt. Mater. Express 2020, 10, 323–331.
- Mostafa, A.M.; Mwafy, E.A. Synthesis of ZnO and Au @ ZnO core/shell nano-catalysts by pulsed laser ablation in different. Integr. Med. Res. 2020, 9, 3241–3248.
- Muniz-miranda, M.; Muniz-Miranda, F.; Giorgetti, E. Spectroscopic and microscopic analyses of Fe3O4/au nanoparticles obtained by laser ablation in water. Nanomaterials 2020, 10, 132.
More
