Many countries are immersed in several strategies to reduce the carbon dioxide (CO2) emissions of internal combustion engines. One option is the substitution of these engines by electric and/or hydrogen engines. However, apart from the strategic and logistical difficulties associated with this change, the application of electric or hydrogen engines in heavy transport, e.g., trucks, shipping, and aircrafts, also presents technological difficulties in the short-medium term. In addition, the replacement of the current car fleet will take decades. This is why the use of biofuels is presented as the only viable alternative to diminishing CO2 emissions in the very near future. Nowadays, it is assumed that vegetable oils will be the main raw material for replacing fossil fuels in diesel engines.
1. Introduction
Nowadays, most of the countries worldwide are making an unprecedented effort to reduce anthropogenic greenhouse gas (GHG) emissions to carry out a decarbonization process, which significantly affects the energy sources applied. In this sense, the Treaty of Paris
[1] and the European New Green Deal, as well as the REDII, aim to achieve a climate-neutral Europe by 2050
[2]. Therefore, several countries have also implemented their own energy and climate policy framework for 2030 and beyond, advancing in decarbonization and promoting innovation in order to achieve a viable new climate economy low in CO
2 emissions
[3].
Considering that the choice of green hydrogen as the main energy vector for the decarbonization of the planet seems definitive, biofuels should receive a secondary role in the current research and development priorities of transportation, including cars, trucks, ships, planes, etc. However, the transition from current energy sources to this new technology requires a period of several decades, in accordance with the planning carried out by the same countries involved in these international agreements
[4].
Notwithstanding the possibility of building a transport fleet operating with new technologies and being neutral in CO
2 emissions, it is mandatory to consider the temporary rate of the substitution of current vehicles working with internal combustion engines (ICEs) in order to avoid an economic chaos of unpredictable consequences. In this sense, the replacement of the enormous number of vehicles that operate with ICEs needs to be carried out in such a way that they can continue operating throughout their useful life with diesel fuels or, alternatively, with biofuels with similar properties. This fact does not constitute a trivial problem due to the very high number of vehicles currently in use and the fact that those vehicles that are being built now and in the next two or three decades must be added to the list
[5]. Consequently, the reduction in emissions in this long transition period involves a reduction in fossil fuels and increase in other fuels that allow for their operation in ICEs, together with the incorporation of hydrogen-powered engines and other emerging technologies. In this way, a smooth transition to a scenario without fossil fuels could be foreseen
[6].
In this sense, biofuels can be easily integrated into the logistics of the global transportation system. In fact, the goal pursued by EU is that biofuels constitute 30% of all fuels by 2030
[7]. Despite this goal being easy to achieve considering the technical issues, the substitution of fossil fuels with biofuels is considered unattainable in this deadline due to the impossibility of having enough agricultural land to carry out the necessary crops, since bioethanol and biodiesel (the most widely biofuels employed) require enormous agricultural resources to fulfill these purposes
[8][9][10][11][12][8,9,10,11,12].
2. Strengths and Weaknesses of Biodiesel as Renewable Biofuel in Current Diesel Engines
Biodiesel is defined as a mixture of long chain fatty acid methyl esters derived from renewable lipid sources, such as vegetable oil or animal fat, that can be used in compression ignition engines with little or no modifications. Until now, the use of a homogeneous alkaline transesterification chemical process with methanol has been initially chosen to address the biodiesel production
[13][14][15][16][29,30,31,32]. In fact, biodiesel is, to date, the liquid biofuel produced at a greater quantity, due to the simplicity of its chemical process and its rheological properties, like fossil diesel
[17][18][19][33,34,35]. In addition, it can be produced from different feedstocks, depending on the availability of the crop in the region. Among other advantages, biodiesel exhibits biodegradability, non-toxicity, renewability, a high cetane number, a high flash point, and its high oxygen content allows for its complete combustion in engines, reducing the amount of particulate matter, hydrocarbons, and gases, such as carbon monoxide (CO), CO
2, and sulfur oxides (SOx). Furthermore, biodiesel has a very low sulfur content and very low aromatic components, as well as other pollutant emissions. Nevertheless, a slight increase in nitrogen oxide (NOx) emissions is usually described in comparison to diesel fuel
[20][21][22][36,37,38]. Due to the high flashpoint that biodiesel exhibits, at around 150 °C, it is very safe for transportation and storage
[23][24][25][39,40,41]. In addition, biodiesel perfectly fits into existing engines without any modification and it can be used in its pure form or blended with petroleum-based fuels without modification of existing engines or with only minor modifications
[26][27][28][29][30][31][42,43,44,45,46,47]. Moreover, biodiesel exhibits better lubricant properties than fossil diesel, which allow for the extension of the engine life, and also allow for a reduction in carbon dioxide emissions by 78% in comparison with fossil diesel. In addition, the biodegradability of biodiesel is certainly high, ranging from 80.4% to 91.2% after 30 days, whereas the biodegradability of fossil diesel is only 24.5%
[32][48]. Taking into consideration all of the advantages abovementioned, it is understandable that biodiesel has become a research hot spot during the last years, resulting in an increase in scientific publications and patents
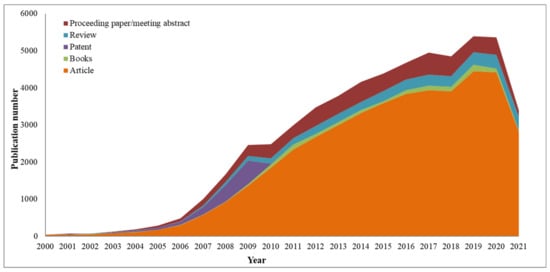
[33][49], as can be seen in
Figure 12. Thus, for only microalgae biodiesel production, more than ten thousand patents have been published in the last 20 years
[34][35][36][50,51,52]. Furthermore, in the last twenty years, almost forty-four thousand articles have been published, producing a growing increase year after year, demonstrating the growing interest in the problem.
Figure 12.
Publications found in the Web of Science database by the keywords “biodiesel” separated by document types from year 2000 to 2021.
Despite these efforts made to achieve better processes, better catalysts, and better sources of raw materials, it is currently concluded that the generation of glycerol represents a barrier that is difficult to overcome for the industrial production of biodiesel
[37][53]. An alternative could be the reduction in the production cost of biodiesel. Nevertheless, the biodiesel industry strongly depends on the cost of the feedstock employed as a raw material. Despite the fact that some feedstock, such as non-edible oil and waste cooking oil, can be obtained at a good cost, they usually need a higher cost in their manufacture processing to produce standard-quality biodiesel
[38][39][40][54,55,56]. The true magnitude of this problem has been proven in all of its consequences when the industrial-scale production of biodiesel has begun in the last three decades. The management of the huge amounts of wastes, where glycerol is the main component, is a problem with a very difficult solution
[41][42][57,58], and there are still no industrial processes capable of integrating the enormous amount of glycerol. Furthermore, this glycerol obtained as a by-product also exhibits a very low quality, since it is in a mixture with other products, such as methanol, water, salts, and some amounts of monoglycerides
[43][59].
Therefore, for being employed as a biofuel, the biodiesel obtained must be cleaned and separated from these by-products. The additional cleaning process is usually carried out by successive washing steps with water, so that a large consumption of water, energy, and time to obtain the glycerol elimination is required in order to obtain the limits established by the quality standard EN 14214 and the ASTM D6751, which are the European and the American ones, respectively
[44][45][46][47][48][60,61,62,63,64]. These limits establish that the amount of glycerol should not exceed the 0.02% in the refined biodiesel in order to prevent its reaction with oxygen at high temperatures inside the engine, which would either produce acrolein or would polymerize generating deposits of carbonaceous compounds on the injector nozzles, pistons, and valves in the engines, consequently reducing the efficiency of the engine and its service life
[49][50][51][65,66,67]. Therefore, it is clear that the industrial production of biodiesel requires a very complex design in order to avoid the presence of glycerol in the final biofuel
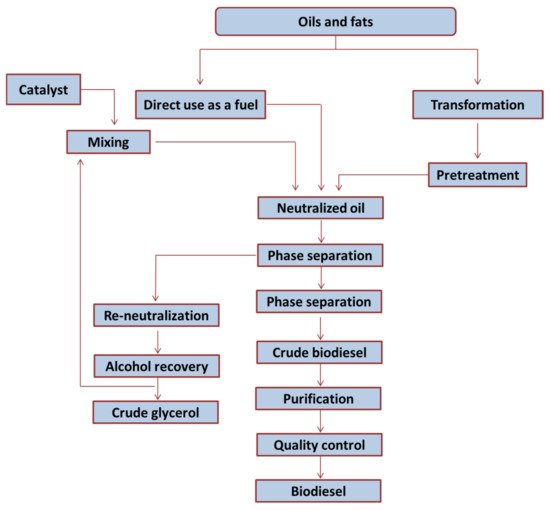
[52][68], as is shown in
Figure 23. In summary, the transesterification reaction is usually carried out in a batch reactor under constant stirring at 60 °C. Then, glycerol is separated together with the excess of methanol by decantation. Then, methanol is recovered by distillation. This crude biodiesel contains catalyst residues that must be neutralized and eliminated.
Figure 23. Standard flowchart of an alkali transesterification process in a conventional biodiesel plant, reproduced with permission of Ref. [52]. Copyright 2019 Elsevier. Standard flowchart of an alkali transesterification process in a conventional biodiesel plant, reproduced with permission of Ref. [68]. Copyright 2019 Elsevier.
As aforementioned, biodiesel must be subjected to several washing steps with water, although the purification process also requires a drying process in an evaporator to remove held residual water
[44][60]. Alternatively, the purification of biodiesel may also be obtained by ultrafiltration and dry washing, employing fumed silica sorbent, molecular distillation, organic resins, and biomass-based adsorbent or starch and cellulose as adsorbents of impurities
[53][54][55][56][57][58][69,70,71,72,73,74]. This vast number of studies devoted to obtaining methodologies that are economically viable show that this step is one of the main factors that lead to an unprofitable biodiesel production
[59][75].
Consequently, there is not a practical solution for the problem associated with the destabilizing glycerol price in the global market, since there are no industrial processes capable of adsorbing the increasing glycerol production
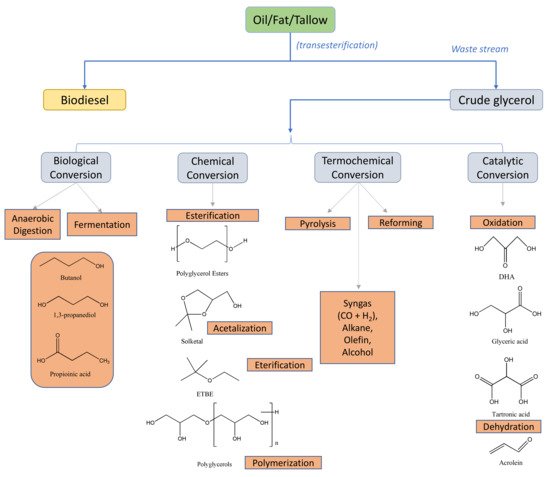
[60][61][76,77]. To minimize this problem, multiple investigations are being carried out in order to valorize this crude glycerol
[62][63][64][78,79,80]; see
Figure 34.
Figure 34. Different chemicals obtained to valorize crude glycerol generated in the industrial production of biodiesel, reproduced with permission of Ref. [62]. Copyright 2020 Elsevier. Different chemicals obtained to valorize crude glycerol generated in the industrial production of biodiesel, reproduced with permission of Ref. [78]. Copyright 2020 Elsevier.
Another element of vulnerability associated with the production of conventional biodiesel is related to the low atomic yield (or atomic efficiency) of the process. The atom yield is an important concept in green chemistry, and is far from the concept of chemical yield. In fact, a high-yielding process can still result in a substantial quantity of by-products, as is the case for biodiesel production. These green metrics are crucial for determining the sustainability and environmental impact of biodiesel production
[65][66][67][81,82,83].
In summary, it is commonly accepted that the greatest contribution to determining the cost of biodiesel is determined by the price of feedstock, which occupies 70% of the biodiesel production cost
[68][69][70][84,85,86]. Therefore, independently of looking at increasing the sources of triglycerides, available at appropriate prices for their transformation into biofuels, by optimizing the parameters influencing the production process of biodiesel, costs could be reduced by up to 30%. In addition, savings could be obtained by avoiding the management of residual glycerol, obtained together with conventional biodiesel, as well as the increase of at least 10% of the final product, if it is no glycerol is generated as a by-product. Thus, the search for different renewable biofuels integrating glycerol is still encouraged, while also avoiding several collective drawbacks, such as being energy-intensive, tedious in recovering glycerol, difficult in removing the acid or base catalyst from the product, the further treatment of alkaline wastewater, and the interference of free fatty acids and water in the reaction
[71][87].