1. Introduction
The applications of stimulus-responsive polymers in the biomedical and drug delivery fields have been gaining much attention in recent years. Stimulus-responsive polymers are known as smart, intelligent or stimuli-sensitive polymers. They can undergo reversible physical or chemical changes in response to external changes in environmental conditions, and the changes can be observed in properties including surface activity, chain conformation, solubility, configuration and morphology
[1]. The examples of different classes of stimulus responsiveness are shown in
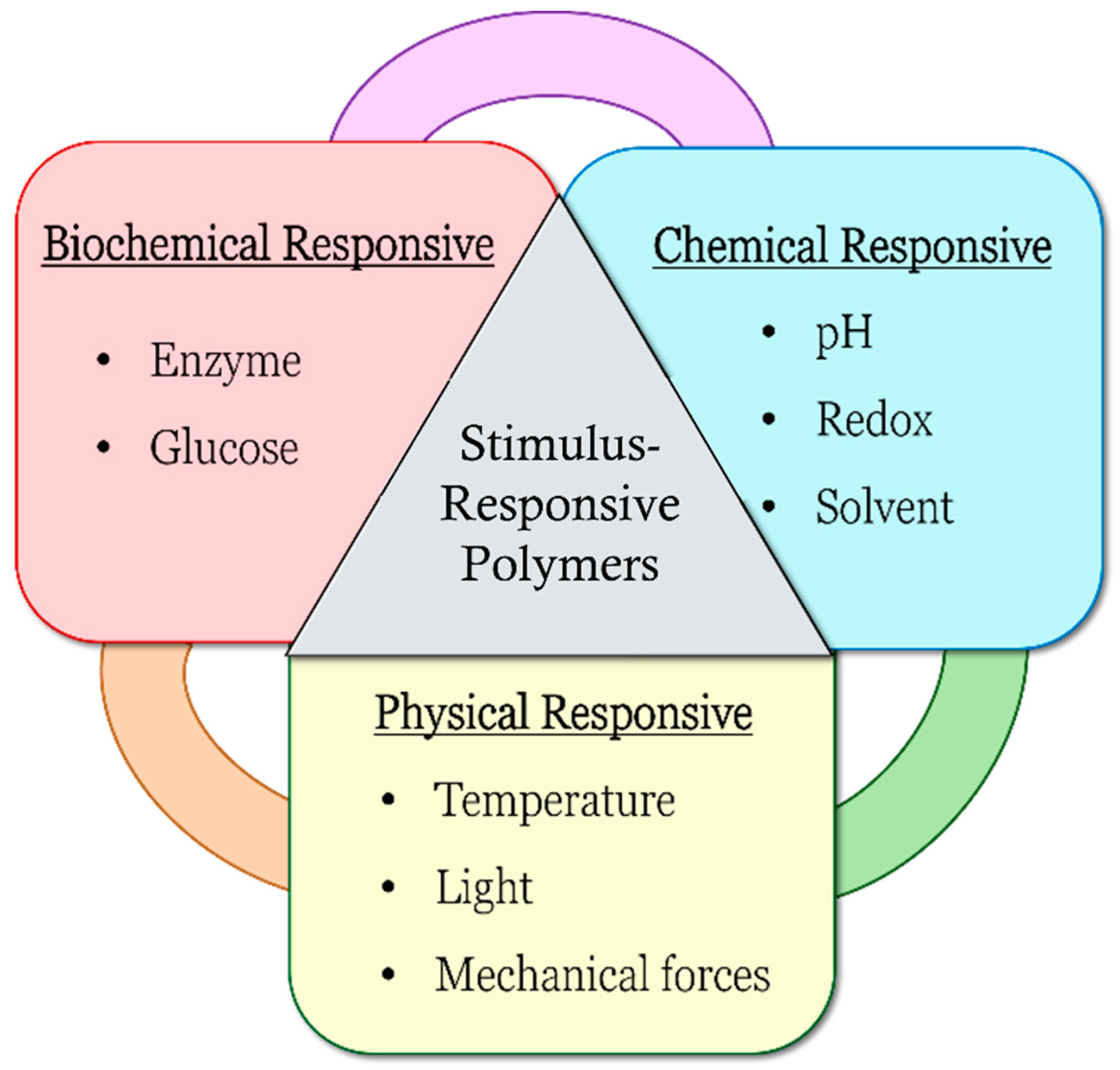
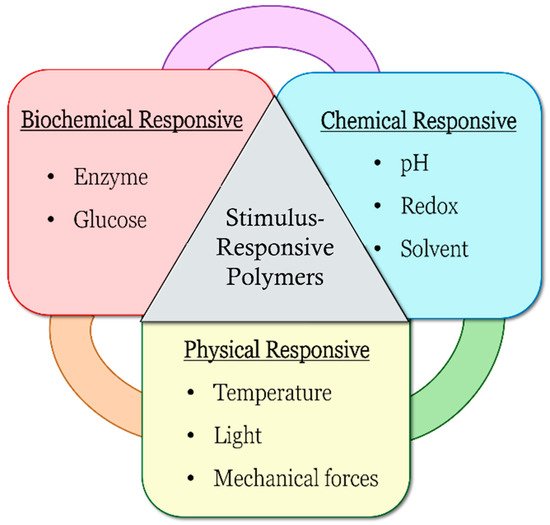
Figure 1, which include biochemical responsiveness such as enzyme- and glucose-responsive, chemical responsiveness such as pH-, redox-, and solvent-responsive and physical responsiveness such as temperature-, light-, and mechanical-force-responsive.
Figure 1.
Types of stimulus-responsive polymers.
Stimulus-responsive polymers are categorized into single-responsive, dual-responsive and multi-responsive properties. Single-responsive polymers can only be responsive to one stimulus, whereas dual- or multi-responsive polymers can respond to several stimuli. With different responsiveness, the polymers can also act differently as a result of changing environment conditions. These polymers have been studied over the years for a range of medical, biomedical and drug delivery applications, such as oral administration drug delivery systems
[2], intravaginal drug delivery systems
[3], anticancer drug delivery systems
[1][4][1,4], tissue engineering
[5], biomaterials such as sensors and actuators
[1][6][1,6], gene delivery systems
[7] and smart coatings
[8][9][8,9].
1.1. pH-Responsive Polymers
pH-Responsive Polymers
pH-responsive polymers are polymers that respond to changes in environmental pH. They can be classified into: (A) polymers with ionizable moieties; and (B) polymers that contain acid-labile linkages.
Polymers containing ionizable functional groups are prepared by introducing pendant acidic groups or basic functional groups during the polymerization reaction
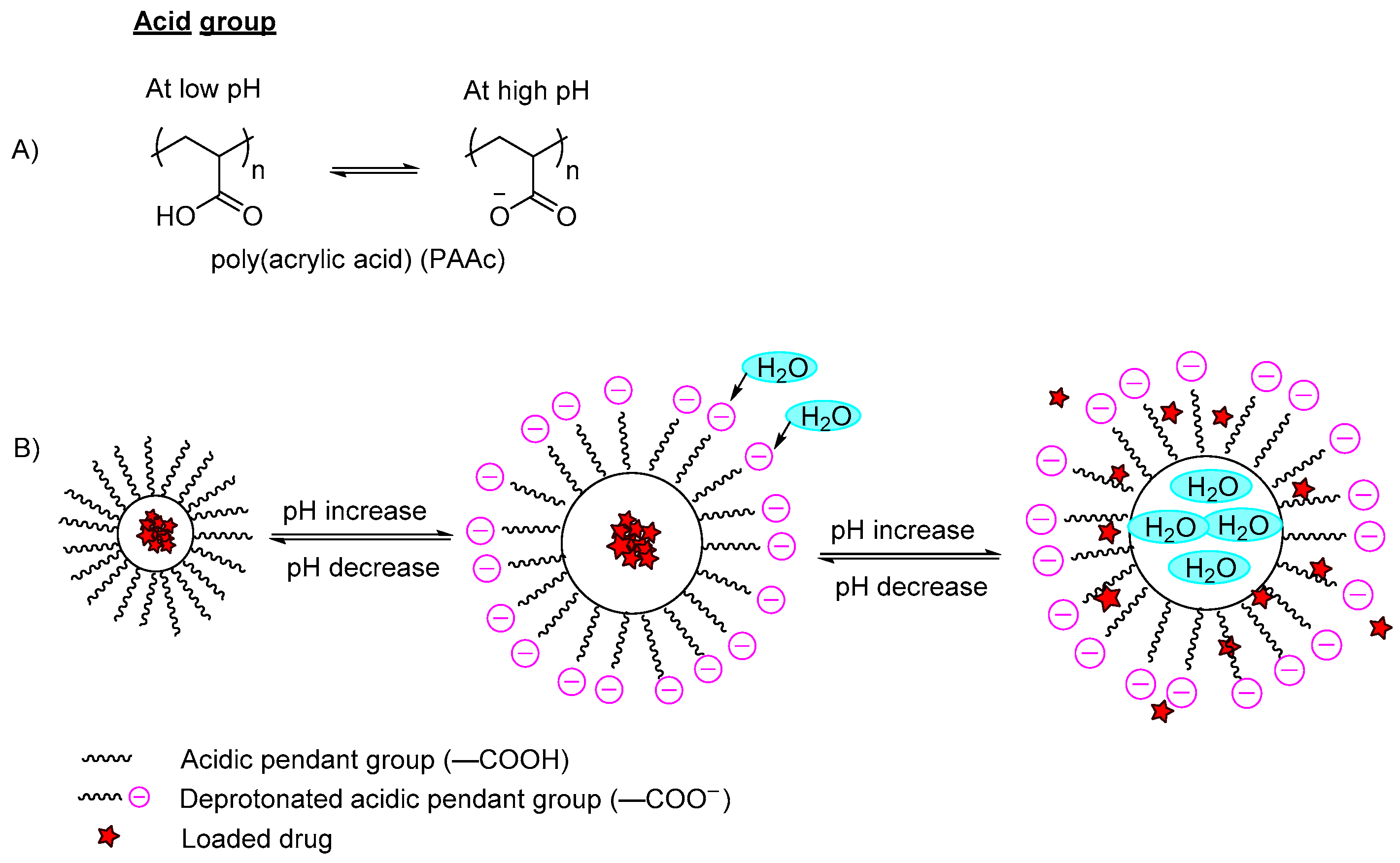
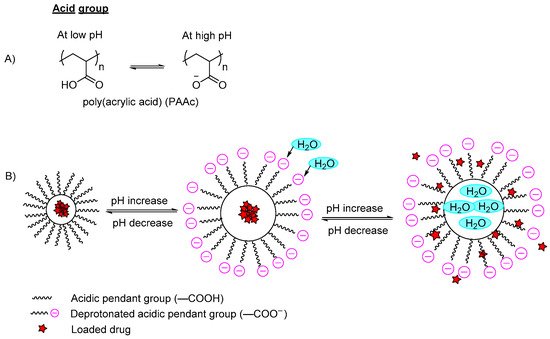
[1][10][11][1,10,11]. The ionizable moieties either protonate or deprotonate at different pH values. For high pH-responsive polymers, acidic functional groups (polyanions) such as carboxylic acid, sulfonic acid or methacrylic acid groups of the polymers are conjugated in the polymer backbone (
Figure 2A). These polymers will dissociate and deprotonate at high pH conditions, resulting in higher charge density due to the electrostatic repulsion between the chains that further leads to absorption of water, hence the polymer swells and the incorporated content is released (
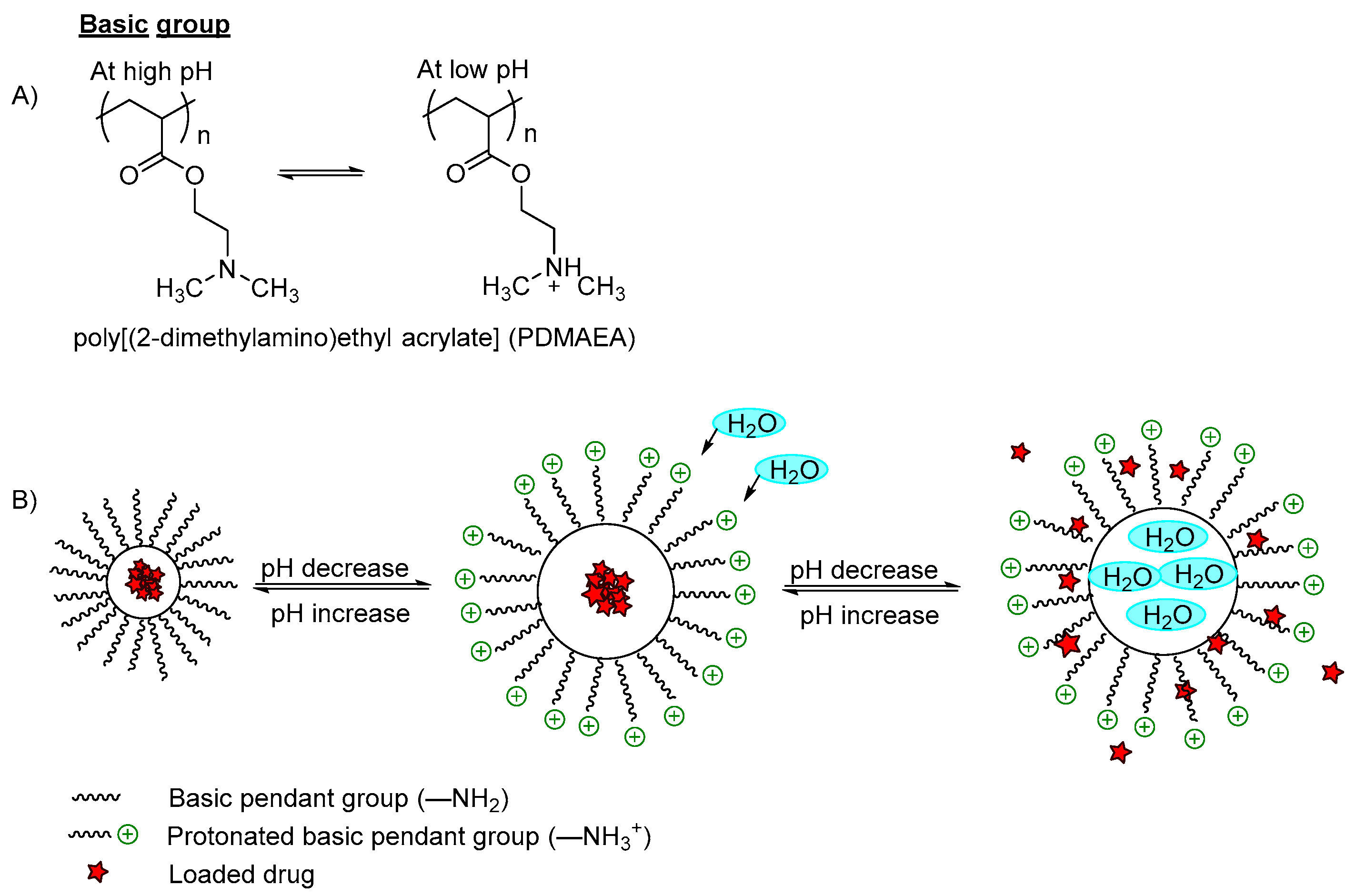
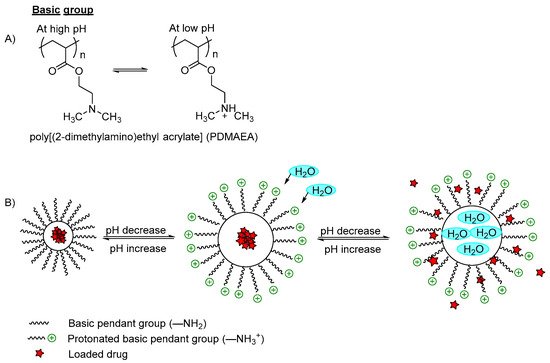
Figure 2B). On the other hand, the low pH-responsive polymers required basic functional groups (polycations) such as pyridine derivatives, piperazines or amino salt groups (
Figure 3A). The basic functional groups are ionized and protonated at low pH conditions, increasing the charge repulsion between neighboring polybasic groups and expanding the network; hence the polymer swells and the incorporated content is released (
Figure 3B).
Figure 2. (A) An example of deprotonation of acidic group in a high pH medium; (B) An example of a polymer containing a carboxylic group (–COOH) with a model drug loaded. In the acidic pH medium, the pendant acidic group remains unionized and retains the drug in the polymer carrier. By increasing the pH, the pendant carboxylic group ionizes and deprotonates, and the polymer swells due to the increase of electron charge density, which induces water diffusion into the polymer network, releasing the incorporated drug.
Figure 3. (A) An example of protonation of a tertiary amine group in a low pH medium; (B) An example of a polymer containing an amino group (–NH2) with a model drug loaded. In a basic pH medium, the pendant amino groups remain ionized and retain the drug within the polymer carrier. However, when the pH is decreased to become acidic, the amino groups were protonated and the polymer networks swelled due to the electrostatic repulsion between the neighboring positive groups, which induced water diffusion into the polymer network, releasing the incorporated drugs.
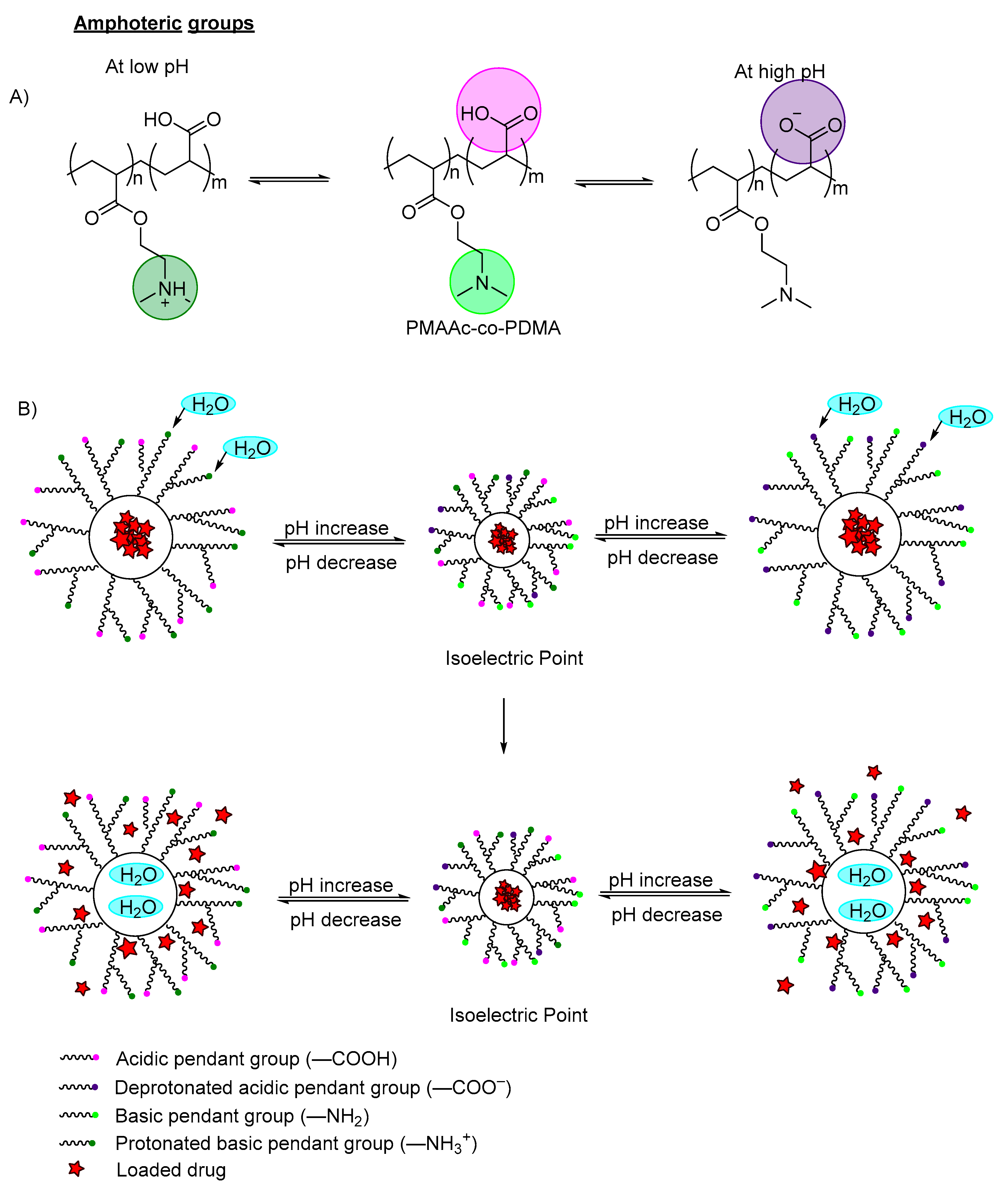
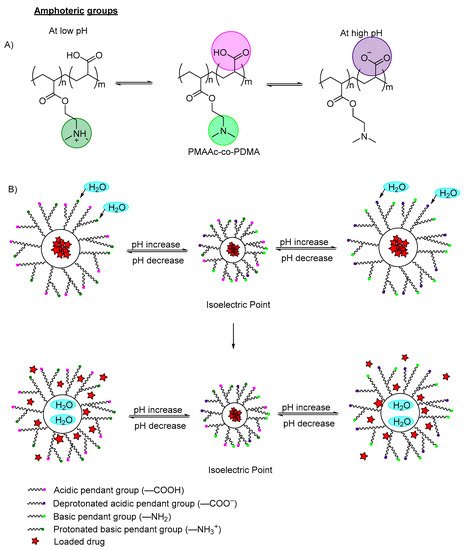
Amphoteric polymers, also known as zwitterionic polymers, are polymers having both acidic pendant groups (anionic) and basic pendant groups (cationic), which are capable of swelling in both acidic and basic media (
Figure 4A)
[12][13][12,13]. The electrostatic interaction can be varied by changing the environmental pH or modifying the ion concentrations of the interfaces. Luo et al., (2010) reported on the synthesis of novel amphoteric pH-sensitive hydrogels derived from ethylenediaminetetraacetic dianhydride, butanediamine and amino-terminated poly(ethylene glycol)
[14]. In the study, both –NH
+ cations and –COO
− anions were at equilibrium at pH 7, which induced electrostatic attractions and ionic cross-linking to the polymer, hence limiting the swelling rate. When the medium was altered to pH 2 or pH 11, there were only –NH
+ cations and –COO
− anions, respectively. The strong electrostatic repulsion induced water diffusion into the network, leading to anomalous diffusion. The mechanism of action was similarly reported by Manal et al., in which a hydrogel containing both methacrylic acid and N,N-dimethyl amino ethyl methacrylate (DMAEMA) was polymerized for insulin delivery
[15]. The hydrogels swelled at pH < 3.0 and pH > 6.0. Between pH 3.0–5.0 (isoelectric point), electrostatic attraction happens between NH
3+ cations and COO
− anions (which dominates over repulsion between unpaired like charges), leading to decreased swelling (
Figure 4B).
Figure 4. (A) An example of deprotonation (at high pH) and protonation (at low pH) of an amphoteric copolymer. The carboxylic groups are labeled in purple, the amine groups labeled in green; (B) An example of a polymer containing zwitterionic ions (both carboxylic group and amino group) with model drug loaded. At the isoelectric point, the acidic pendant group, basic pendant group, cations and anions remain in equilibrium. When the pH is decreased, the amino groups are protonated, and the polymer networks swell due to the electrostatic repulsion between the neighboring positive groups, which induces water diffusion into the polymer network, releasing the incorporated drugs. When increasing the pH, the pendant carboxylic group ionized and deprotonated, and the polymer swelled due to the increase of electron charge density, which induced water diffusion into the polymer network, releasing the incorporated drug.
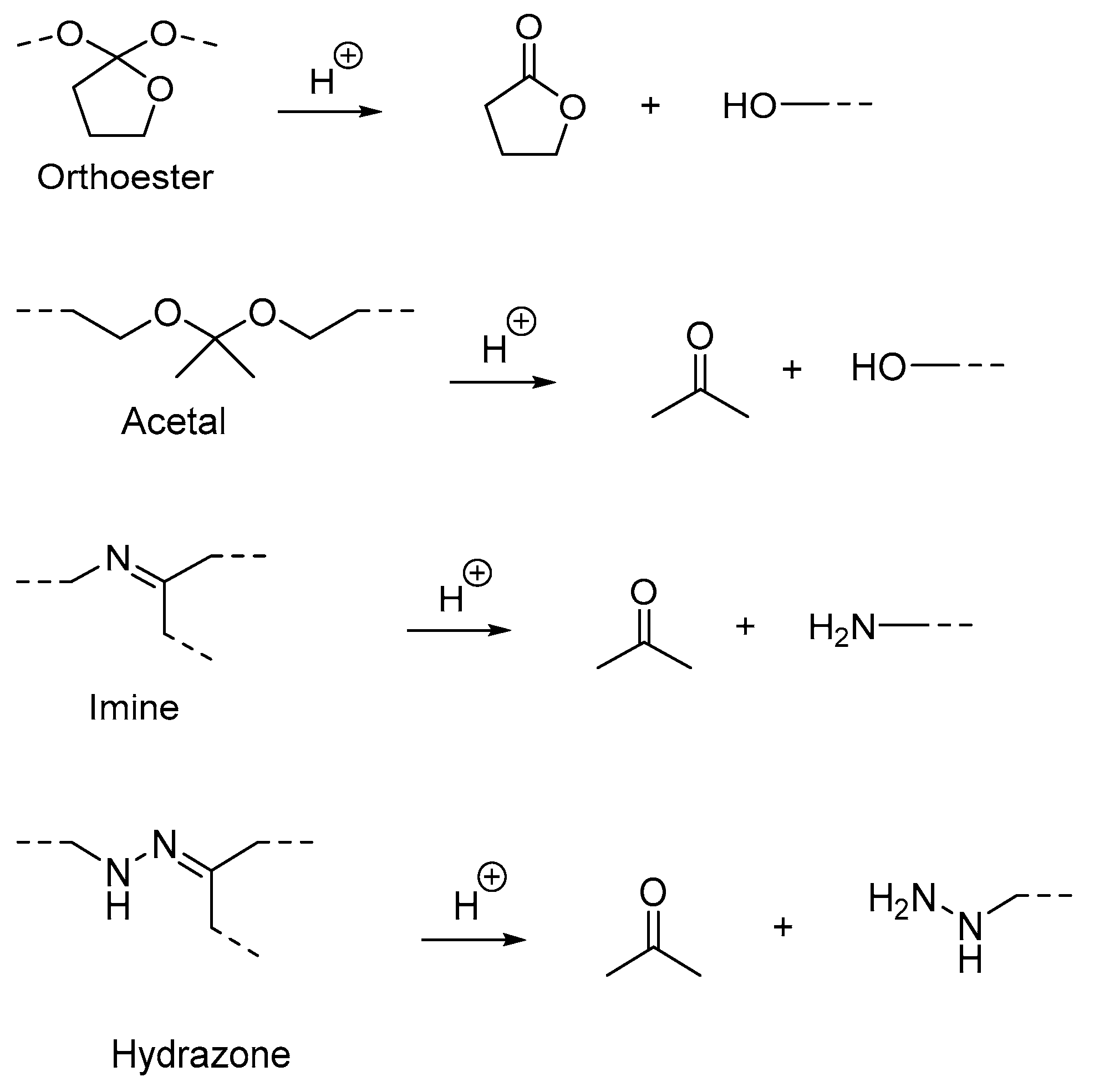
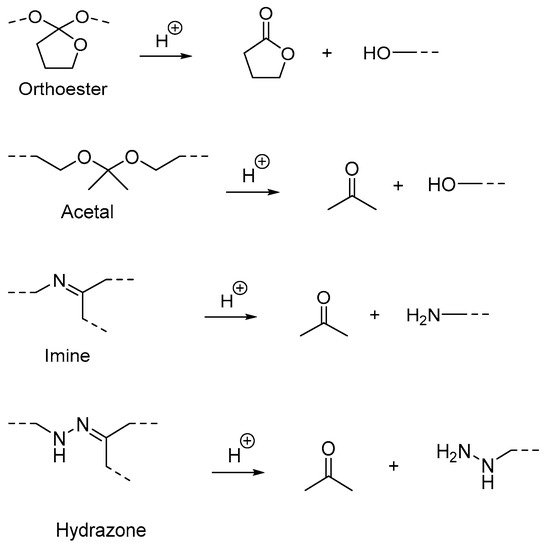
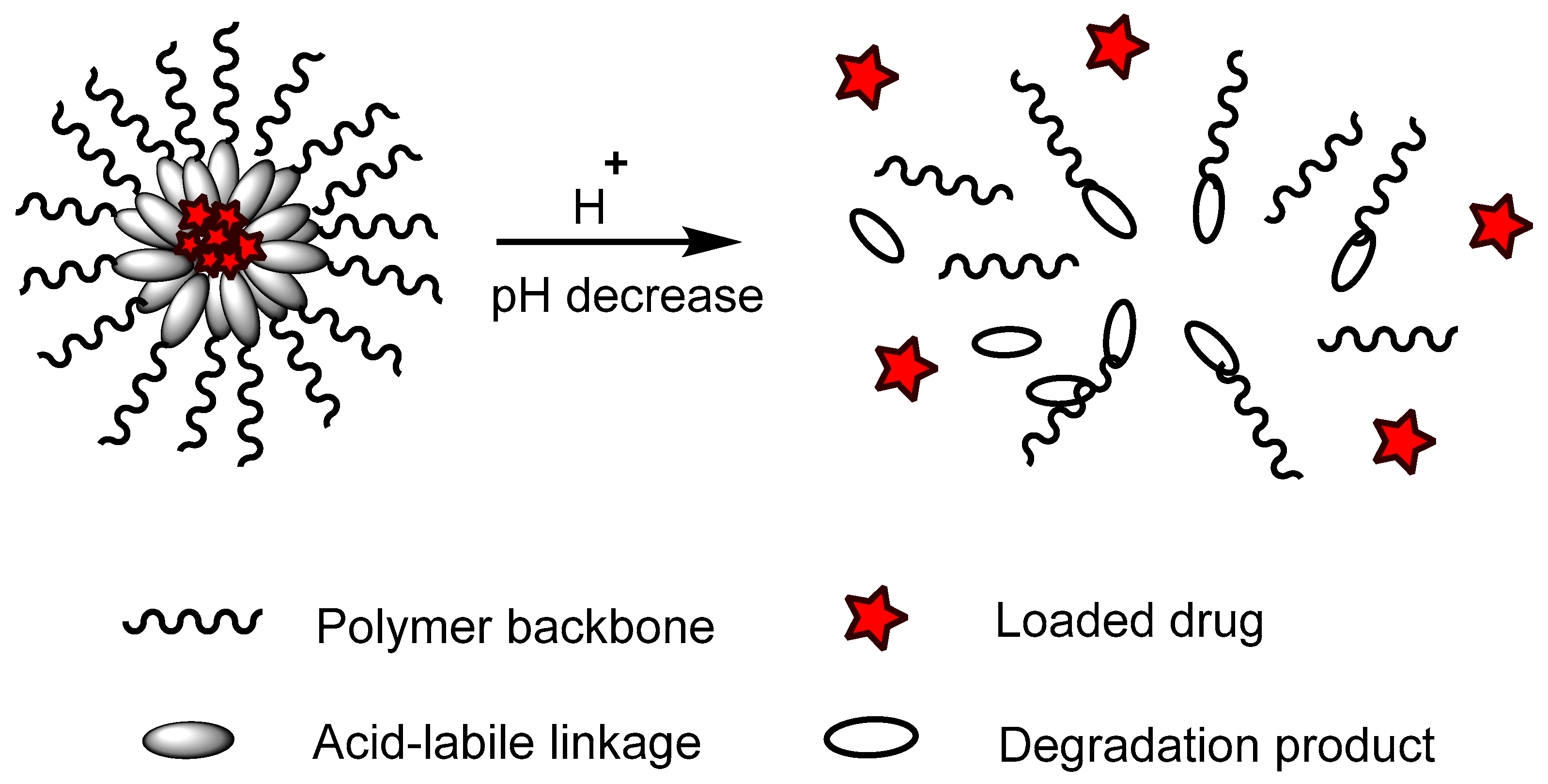
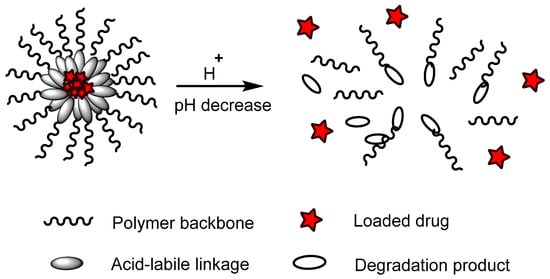
Polymers containing acid-labile linkages (
Figure 5) are prepared by introducing linkers such as hydrazone, acetal, imine and ortho ester into the backbone of the polymer
[16]. By decreasing the environmental pH, the hydrolysis process is triggered and cleaves these bonds, causing degradation of the polymer chain (
Figure 6); the drug is then released. Polymers with acid-labile linkages have been used in developing anticancer drug delivery systems. These polymers are relatively stable in neutral and basic media, but labile in an acid medium due to their covalent linkage, which has slower internal structural transition compared to polymers with ionizable moieties.
Figure 5.
Examples of cleavage of acid-labile linkages and their degradation product.
Figure 6. An example of a polymer containing an acid-labile linkage with a model drug loaded. In an acidic pH environment, the acid-labile linkage hydrolyzed and bond cleavage occurred, releasing the incorporated drugs.
pH-responsive polymers are useful in biological applications due to the variation of pH throughout the body. For instance, the stomach has an acidic condition of around pH 2, while the small intestine has a relatively basic condition, around pH 7.2 to 7.5. In addition, cancer cells have a lower pH value (pH 6.0 to 6.5) compared with normal tissues (pH 7.0)
[17][18][17,18]. This is due to excess accumulation of lactic acid produced by the glycolysis rate in the pathological tissues. Owing to these remarkable variations of pH, along with different tissue sites and sub-cellular spaces, pH-responsive polymers have been used to treat these physiological features in medical and biomedical applications. The mechanism of pH-responsive polymers in drug release systems depends on their swelling and contraction behavior or their degradation properties (cleavage of functional groups). Hence, they have been studied for their applications in oral drug delivery systems of sulforhodamine B
[19], gene delivery systems
[20] and as biosensors for medical diagnostics or health monitoring
[21].
Table 1 categorizes studies that reported on pH-responsive polymers by their mechanism: (A) ionizable moieties; and (B) acid-labile linkages and their applications, for the past two years. Polymers with ionizable functional groups, most of which contained pendant basic groups, especially from amines and their derivatives, are shown in Figure 7. These polymers respond to acidic pH ranging from pH 4.0–6.5; the polymers swell, resulting in enhanced drug release. This group of polymers was used in chemotherapy drug delivery, gene delivery, and certain local therapies. These polymers could respond to an acidic environment, which causes swelling and results in rapid drug release at specific tumor sites. There are also polymers containing pendant carboxylic acid groups (Figure 8), in which the polymers swell and enhance release at pH 7.4.
Figure 7.
Structure of commonly used pendant basic groups.
Figure 8.
Structure of commonly used pendant acidic groups.
On the other hand, polymers containing acid-labile linkage, such as β-thiopropionate, hydrazone linkage, oxazaline and boron-ester linkage, all respond to acidic pH ranging from pH 5.0–6.8, resulting in increased drug release. The applications for this class of polymers were mostly for delivery of anti-cancer drugs, which have non-specific distribution and uncontrollable release. From the results of studying the response to pH value of acid-labile linkages, these polymers aid in the specific target release of anticancer drugs at the tumor cells (pH ranging from 6.0–6.5). Acid-labile linkages are used for their tunable properties, as they can be incorporated into block copolymers at various positions, including the hydrophobic block backbone, pendant chains or at the hydrophilic/hydrophobic block junctions. They remain stable at blood pH, while they degrade upon cleavage under acidic pH conditions (tumor site).
Table 1.
pH-responsive polymers categorized by the mechanism (A) Ionizable moieties and (B) Acid-labile linkages, and their application.
|
Mechanism
|
Pendant Groups
|
Ionizable Group/Linkage
|
Responsive pH
|
Polymer Type
|
Type of Response
|
Biocompatibility Evaluation
Application
|
Ref
|
|
|
|---|
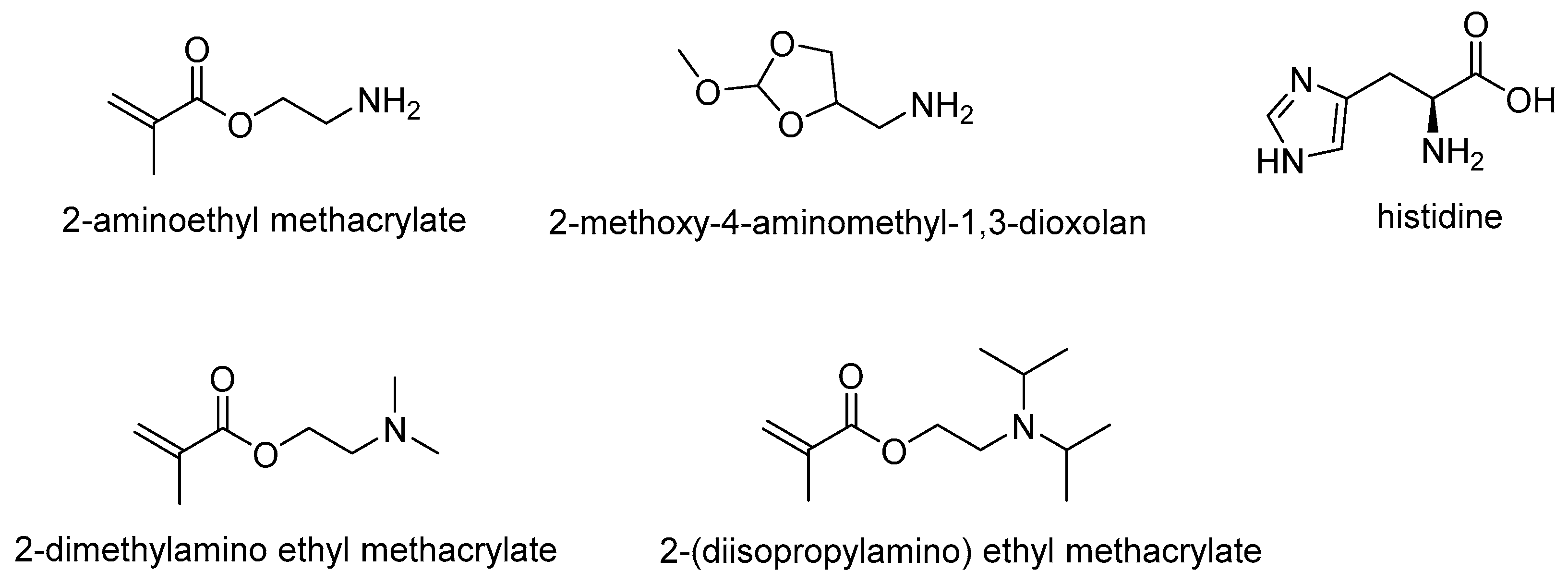
The reported acid-labile linkages include hydrazone and oxazoline linkages; hydrolysis of these linkages was reported to occur at pH ranging from 4.0–6.0. The reported basic ionizable moieties in pH-responsive PUs include 2-(diethylamino) ethyl methacrylate, 2-(diisopropylamino) ethyl methacrylate (DPA), 2-hydroxy ethyl piperazine (HEP), diethanolamine (DEA), N-methyldiethanolamine (MDEA) and pyridine; these PUs with basic ionizable moieties responded to pH environments ranging from 4.0–6.8. The reported acidic ionizable moieties include 2,2-dimethylol propionic acid (DMPA), lactic acid, glycolic acid, mercaptoacetic acid, sodium alginate, lysine, arginine and glutamine; most of these pendant groups respond to pH environments ranging from 7.4–10.4.
The reported dual-stimulus-responsive PUs with pH responsiveness are pH- and thermo-responsive, pH- and photo-responsive, pH- and shape memory-responsive, and pH- and redox-responsive. Within these dual-responsive PUs, pH- and thermo-responsive PUs and pH- and redox-responsive PUs have been reported for application in drug delivery systems. Multi-responsive PUs are introduced due to restriction of stimuli and applications in single-responsive PUs. The advancement of introducing multi-responsiveness is to exploit the maximum number of biological stimuli that occur at the tissue and intracellular level. For instance, the temperature and pH of the cancer tissue environment (40–42 °C and pH ≤ 6.8) are different from normal tissues (37 °C and pH = 7.4)
[58][60]. Thermo-responsive polymeric materials undergo phase separation in the cancer tissue environment; these polymers selectively accumulate in this environment, leading to an increase of concentration. By introducing thermoresponsiveness along with pH responsiveness, finer modulation and specific target drug delivery could be achieved, since the parameters have increased.
Table 3.
Different types of pH-responsive PUs with their attached functional response groups and their different applications.
|
Type of pH-Responsive PU
|
PU System
|
Reactants Used in Synthesis of PU
|
Ionizable Group/Linkage
|
| Biodegradability Evaluation |
|
Applications
|
Ref
|
|
| Additional Stimulus Response |
|
| pH Responsiveness |
|
* Applications
|
Reference
|
Reference Materials
|
Improvement to the Reference Materials
|
|
(A)
Ionizable Moieties
|
|
Single
|
Basic pendant groups, such as amine and its derivatives
|
|
Thermo
2-aminoethyl methacrylate
|
|
|
Single
|
PU micelles
|
A + C
| 6.5 and 6.8
|
-
i.
-
PCL-Hydrazone-PEG-Hydrazone-PCL
-
ii.
-
LDI
|
Poly(ether urethane)
(PEU)
Polyplex Nanoparticles (NPs)
|
Hydrazone linkage
-
i.
-
Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide)
-
ii.
-
HDI
|
Improve cellular uptake and transfection efficiency
|
Ex vivo in rodent model for hydrogel injectability and gelation |
Gene Delivery
|
|
** N/A
|
N/A
|
pH ranging from 4.0–6.0, cleavage of hydrazone bond and degraded
|
A
[7]
|
|
| Controlled and triggered release drug |
|
| [ | 59 | ] | [61][61,63 |
[62][64]
|
] |
|
| N/A |
|
| N/A |
|
2-methoxy-4-aminomethyl-1,3-dioxolan
|
Redox
5.5
|
A + C
|
PU Nanoparticles
|
-
i.
-
PCL
-
ii.
-
HDI |
|
PU micelles
|
-
i.
-
PCL
| -
|
|
-
ii. -
-
PEG
-
iii.
-
N-N-Dimethylacetamide (DMAc)
|
Poly(vinyl alcohol)
PVA
|
Rapid drug release
|
|
Drug carrier for tumor therapy
|
-
i.
|
Hydrazone linkage
-
MTS assay
-
ii.
-
NP (1000 µg/mL): Lung alveolar Type 1 cells (AT1) cell viability >80%
|
[22][24]
|
|
|
| Degrade and reach 50% weight loss (polymers with increase of disulfide bonds) in 10 mM glutathione (GSH) after 14 days |
|
| Chemotherapy drug delivery |
|
[54][56]
|
|
| N/A |
|
| At pH 4.4, particle size increase due to swelling, drug release to 98%
|
A
|
[69][71]
|
Same PU without hydrazone bond
|
N/A |
Histidine
|
Redox
5.0
|
PU/DEA copolymer
A + C
|
PU micelles with mPEG block and PLA block with disulfide bonds |
-
i.
-
IPDI
-
ii.
-
|
Poly(ethylene glycol)-polycaprolactone
PEG-PCL
|
Poly(propylene glycol) diacrylate (PPGDA) -
|
mPEG-PUSS-mPEG
Increase drug release
|
|
|
2-(diethylamino) ethyl methacrylate
-
i.
-
Copolymer PLA
-
ii.
-
Poly (ethylene glycol methyl ester) (MPEG)
-
iii.
-
Isophorone diisocyanate (IDPI)
|
Chemotherapy |
N/A
|
-
i.
-
CCK8 assay
-
ii.
-
Blank micelles (100 mg/L): HepG2 cell viability reduced to 76.3%; HUVEC cell dropped to 65%
|
[23][25]
|
|
|
| Decompose within 24 h in the presence of 10 mM dithiothreitol (DTT) |
|
| Anticancer drug delivery |
|
[ |
At pH 4.0, dynamic swelling and drug release
4]
|
|
| A |
|
| [ | 11 | ][70][11,72]
|
Same PU without DEA monomers
|
N/A
|
2-dimethylamino ethyl methacrylate
|
Redox
4.0 and 7.0
|
|
A + C
Poly(lactic acid) PLA
|
|
PU with disulfide bonds, pendant carboxyl groups, and primary amine group
PU-SS-COOH-NH2 micellesSwells and speeds drug release
|
|
-
i.
-
PCL
-
ii.
-
PEG |
Targeted drug delivery vehicles
|
N/A
|
Drug release at pH 7.0
|
A
|
[74 |
|
-
| L-tyrosine
| -
|
|
-
i.
-
MTT assay
-
ii.
-
WT-MEFs, HeLa, MCF7 cell viability results show non-toxic and biocompatible nature of the polymer
|
|
PU micelles
|
-
i.
-
PEG
-
| -
|
|
|
** N/A
|
Chemotherapy drug delivery
|
[58][60]
|
|
|
ii. -
-
PCL
-
iii.
-
IPDI
|
Diethanolamine
|
-
iii. -
-
HDI -
|
-
i.
-
CCK8 assay
-
ii.
-
Empty PU micelles (1 mg/mL): >80% cell viability for HUVEC and HepG2 cells
|
[24 |
** N/A
|
|
N/A
][26Drug delivery
|
At pH 5.5, highest drug release
]
|
|
| A |
|
| [ | 71 | ] |
[57][59]
|
[ | 73 | ] |
|
| N/A |
|
| N/A
|
2-dimethylamino ethyl methacrylate
|
Light
5.0
|
|
B + C
|
|
PU copolymer
|
-
i.
-
PEG
-
ii.
-
1,6-haxanediol (HD)
-
iii.
-
HDI
-
iv.
-
MDI
| PCL
Serinol-based PU nanoparticles
|
|
|
Swells and enhances drug release
|
-
i.
-
2- amino-1,3-propanediol
-
ii.
-
4,5-dimethoxy-2-nitrobenzyl (4-nitrophenyl) carbonate
|
Drug delivery
|
|
** N/A
|
|
HEP
| [25][27]
|
|
| N/A |
|
|
At pH 4.5, swelled twofold and close to zero drug release; however, sodium diclofenac incorporated release at elevation to pH 7.0
| Nanoparticle count rate decrease ~>30% after 15 min of UV irradiation
|
A
Nanocarrier for controlled drug release
|
[3]
[63][65]
|
[ | 72 | ] | [ | 3 | , | 74]
|
PEG-HD-MDI-HD without HEP monomers
|
Reversible and sharp switch between “on” and “off” drug release, serves as window membrane in reservoir-type intravaginal rings
|
Chitosan
|
Shape memory
|
N/A |
|
PU copolymer NPs
| 5.5
|
|
-
i.
-
MPEG
-
ii.
-
IPDI
|
Shape memory polyurethane (SMPU)
|
| Poly(lactic-co-glycolic acid)
|
-
i.
-
| PLGA
|
HEP -
-
ii.
-
|
Efficient release
|
DMPA
|
Tacrolimus delivery
|
-
Commercial SMPU, MM3520
|
[26][28]
|
|
| N/A |
|
| ** N/A
|
PU containing higher HEP ratio, swelled and highest drug release at pH 5.0
** N/A
|
Endovascular embolization
|
[64][66]
|
|
| A |
|
| [ | 73 | ][75]
|
N/A
|
N/A
|
Chitosan
|
|
Dual
| 5.5
|
Shape memory + water
|
N/A
|
Thermoplastic PU/hydroxyethyl cotton cellulose nanofibers (TPU/CNF-C/CNTs)
|
Commercial TPU, BT-70ARYU
|
** N/A
|
** N/A
|
|
PU copolymer hydrogel
|
-
i.
-
PEG
-
ii.
-
HDI
-
iii.
-
PG
|
Poly(N-isopropylacrylamide)-co-itaconic acid
NIPAM
|
Fast release
|
Local breast cancer therapy
|
[27][29]
|
] | [ | 76 | ] |
|
| Sensors, actuators |
|
|
[65][67]
|
2-(diisopropylamino) ethyl methacrylate |
DMPA |
|
| N/A |
|
| N/A |
|
(DPA)
|
6.5
|
Hydroxyethyl methacrylate-co-DPA copolymers
|
Increased drug release
|
Thermo + light
Ocular drug delivery
|
|
PU hydrogels-
|
-
i.
-
PVA
|
| [ | 28 | ][30]
|
|
| A + C
|
-
ii. -
|
PUA Nanoparticles
|
-
-
i.
-
PCL
-
ii.
-
PLA
-
iii.
-
IDPI
|
-
i.
-
VitaBright-48 (VB-48) assay, CCK8 assay
-
ii.
-
NIH3T3 cell viability ~70%
|
Weight loss of approximately >~10% in 28 days
|
Chitosan -
-
iii. -
-
TDI
|
3D cell-laden bioprinting
|
|
[ |
Poly(azomethine-urethane) (PAMU)
|
N/A
|
Highest swelling degree at pH 3.0; increase of PAMU, swelling degree increase, release of drug increase
|
A
|
[75][77]
66][68]
|
N/A
|
N/A
|
Carboxylic acid pendant groups
|
|
PU nanomicelles |
Methacrylic acid |
|
Thermo + shape memory
|
|
-
i.
-
PEG
-
ii.
| (MAA)
|
-
IPDI -
|
|
A + C
|
-
iii. | 7.4
|
-
-
Poly(neopentyl glycol adipate) diol (PNA-2000) -
-
iv. -
-
HEMA
|
PCL-based PU
(PCLAU/Fe3O4)
Amine-modified bimodal mesopores silica
|
|
-
i.
|
2-[N,N-bis (2-hydroxy-ethyl)] aminoethanesulfonic acid sodium salt (BES-Na)
-
PCL
-
ii.
-
Polytetramethylene ether glycol (PTMEG)
-
iii.
-
HDI
|
Swells and speeds drug release
|
N/A
|
-
i.
-
CCK8 assay
-
ii.
-
L929 fibroblast cell proliferation rate >80%
|
Drug release rate: pH 5.0 > pH 6.8 > pH 7.4
Drug delivery carrier
|
Weight loss of 67% after 13 weeks
|
A
[29][31]
|
|
|
| Vascular stents |
|
| [ | 12]
|
[67][69]
|
N/A
|
N/A
|
Acrylic acid
|
|
Thermo + enzyme
|
7.4
|
|
B + C
Poly(ethylene glycol) PEG
|
Poly(ester urethane) Nanoparticles
Increased drug release
|
Targeted drug delivery
|
[30][32]
|
|
|
| -
| i.
-
1,12-dodecane diol
|
|
PU-sodium alginate (SA) blend
|
-
i.
-
Bis-hydroxyethylene terephthalate (BHET)
-
ii.
-
PEG
-
iii.
-
HDI
|
Sodium Alginate
| -
-
ii.
|
N/A
|
Swelled at pH 7.4, sustained and prolonged release of incorporated protein or insulin
|
A
|
[76][77][78,79]
|
N/A
|
BHET derived from PET waste, biocompatible
|
Mechanism
|
Multi |
Acid-Labile Groups
|
Responsive pH
|
Polymer Type
|
Type of Response
|
Thermo + shape + water
|
A + C
|
PU/nanoporous cellulose gel (PU/NCG)
|
-
i.
-
|
|
Cellulose crosslinked PU
|
-
i.
-
PCL
-
ii.
-
HDI
|
-
i.
-
Lactic acid (LA)
-
ii.
-
Glycolic acid (GA)
-
iii.
-
DMPA
|
N/A
| PEG -
-
ii.
-
TDI
|
Application
|
** N/A
|
|
|
| Ref |
|
|
| ** N/A |
|
|
All 3 PUs swelled and highest release of incorporated drugs at pH 7.4
|
A
|
[78 | Biomaterials, sensors
|
][80]
|
N/A
[68][70]
|
Control release rate by changing chain extender;
Drug release rate LAPU > DAPU > GAPU
|
(B)
Acid-labile linkages
|
β-Thiopropionate |
|
PEG-HTPB (g-COOH)-PEG triblock copolymer
|
|
-
i.
-
Hydroxyl-terminated polybutadiene (HTPB)
-
ii.
-
MPEG
-
iii.
-
HDI
|
5.0
|
|
Mercaptoacetic acid
| PEG
|
N/A
Increased drug release
|
Targeted cancer cell treatment
|
At pH 7.4, micelles swelled rapidly and released drug
[31][33]
|
|
| A |
|
| [ | 79 | ][81]
|
N/A
|
N/A
|
β-Thiopropionate
|
|
PU/cellulose acetate phthalate (CAP) fibers
|
5.0
|
Commercial PU |
Poly(beta-thioether ester)-PEG
|
Rapid drug release
|
Nanocarrier for drug delivery
|
|
CAP
|
N/A
|
Rapid release of Rhodamine B at pH 7.4 within 1 min
[32][34]
|
|
| A |
|
| [ | 80 | ][82]
|
- Pure CAP
- Pure PU fibers
|
Improved tensile strength compared to previously reported CAP fibers
|
Hydrazone linkage
|
|
|
PU films
| 5.5
|
-
i.
-
PEG
-
ii.
-
HDI
|
Lipid Polymer Hybrid NPs
|
|
-
i.
-
Lysine
-
ii.
-
Arginine
-
iii.
|
Fast drug release
|
-
Glutamine -
|
Biomedical and chemotherapy
|
|
N/A
|
PU-Arginine shows highest drug release at pH 4.4; All PU shows average drug release of 64% at pH 10.4
[17][33][17,35]
|
|
| A |
|
| [ | 81 | ][83]
|
N/A
|
N/A
|
Hydrazone linkage
|
|
|
PEG-PU copolymers
| 5.4
|
-
i.
-
PEG
-
ii.
-
HDI
|
N-isopropylacrylamide-co-glycidyl methacrylate
|
-
i.
| (NIPAM-co-GMA)
|
-
HEP
-
ii.
-
DMPA
|
Increased drug release
|
N/A
Chemotherapy drug delivery
|
|
Highest pH buffering capacity 7.02, specific responsiveness not mentioned
[34][36]
|
|
| B |
|
| [ | 82 | ][84]
|
N/A
|
N/A
|
Hydrazone linkage
|
|
Dual
|
5.6
|
|
PU
Poly(β-benzyl malate)
|
-
i.
-
PEG
-
ii.
-
MDI
|
Rapid drug release
|
-
i.
-
DMPA
|
Antitumor drug carrier
|
-
ii.
|
[35][37]
|
-
|
| -
| MDEA -
|
Thermo-responsive
|
PU-MDEA: Swells at pH 4.0–5.5
PU-DMPA: Swells at pH 8.5–10.0
|
N/A
|
[83][85]
|
N/A
|
N/A
|
Oxazoline
|
6.0
|
Poly(lactic acid)-poly(β-amino ester)
|
Increased drug release
|
Colon cancer adjuvant therapy
|
|
|
PU Micelles
|
-
i.
-
PCL
-
ii.
-
MDI
|
-
i.
-
HEP
-
ii.
-
MDEA
-
iii.
-
N-butyl diethanolamine (BDEA)
|
Thermo-responsive
|
HDI-MDEA and HDI-BDEA,
rapid drug release at pH 4.0
| [36][38]
|
|
| A |
|
| [ | 84 | ][86]
|
N/A
|
N/A
|
Boron-ester linkage
|
6.8
|
|
PU/DPA
|
-
i.
-
PPGDA
-
ii.
|
Polymer dots
|
Better fluorescent intensity
|
Bioimaging probe
|
[37][39]
|
|
Borate-ester linkage
|
5.5
|
PNIPAAm
Poly(N-isopropylacrylamide)
|
Rapid drug release
|
Cancer therapy
|
[38][40]
|
|
Others
|
Metal ligand
(Fe3+)
|
5.0
|
PEG-PLGA
|
Rapid drug release
|
MRI-guided therapy
|
[39][41]
|
2. Stimulus-Responsive PU
2.1. Polyurethane
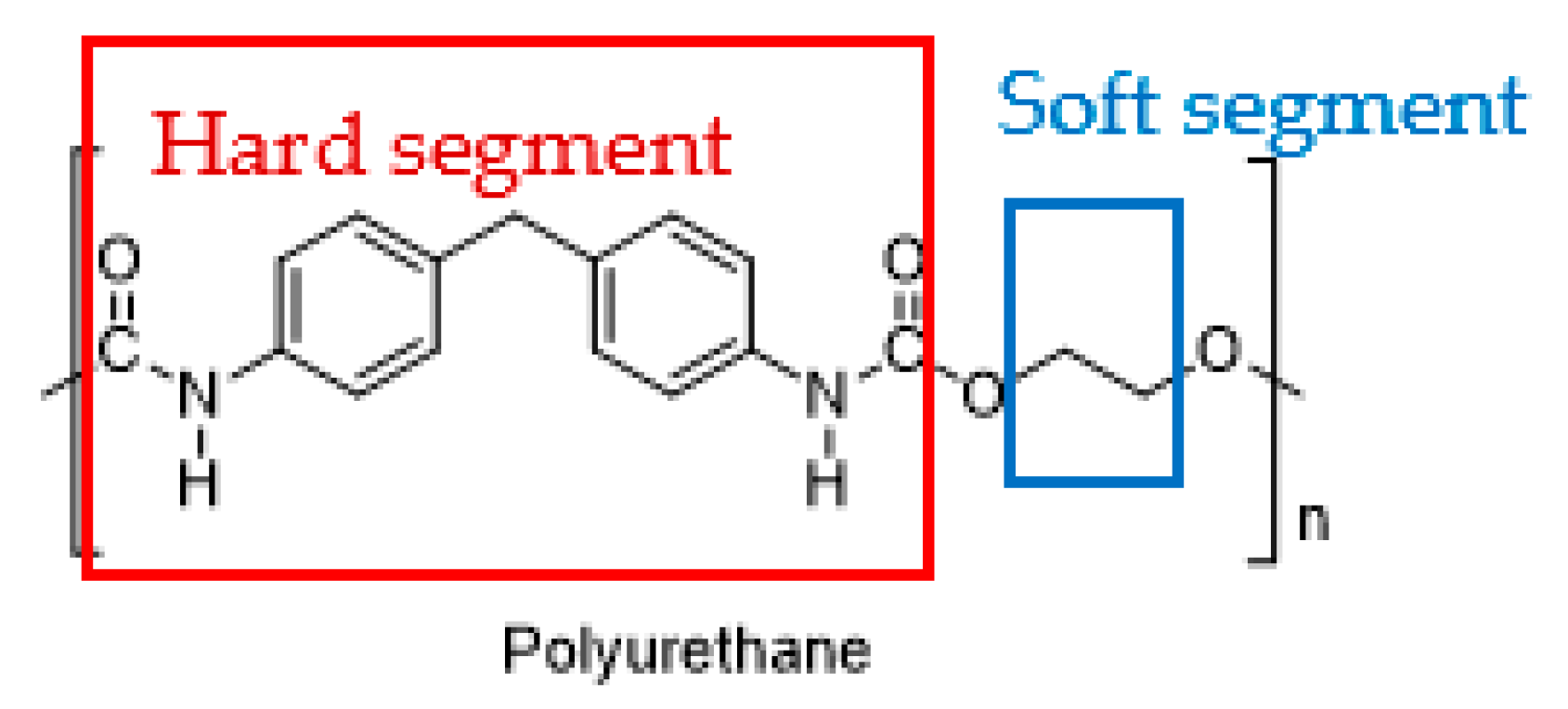
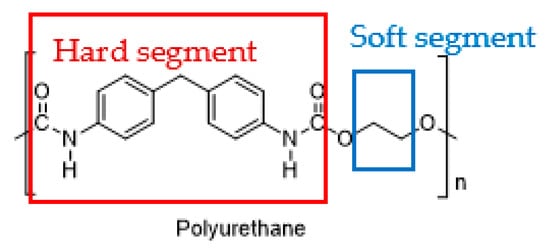
Polyurethane (PU) is a highly utilized synthetic polymer, used to manufacture rigid or flexible foams, coatings, adhesives, sealants, and elastomers. The unique feature of PU is the phase segmentation of hard and soft segments within its structure (
Figure 9), which makes it very versatile. Moreover, it can be synthesized and conveniently modified into different forms of polymeric structures, ranging from elastomeric to porous, by altering the ratio of hard to soft segments. The soft segment is comprised of polyhydroxyl components, while the hard segment is composed of polyisocyanates. PU offers good abrasion resistance and toughness, thus driving its commercial applications in many industries. It is biodegradable, biocompatible and hemocompatible, and demonstrates an accommodative biological response when used in biomedical applications such as bone or tissue implants and controlled drug-release systems
[12][40][41][12,42,43].
Figure 9.
The hard segment (red) and soft segment (blue) of polyurethane.
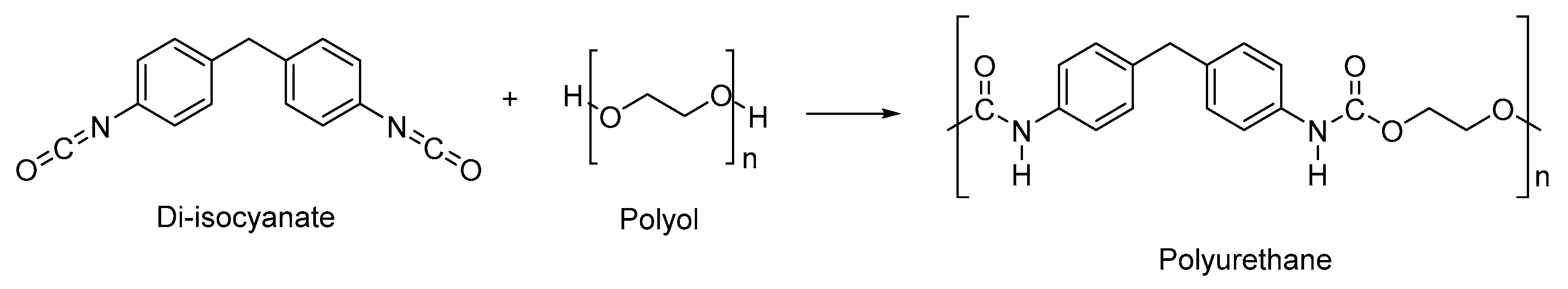
The conventional synthesis route of PU is through reacting polyhydroxyl compounds (–OH group) from non-renewable petrochemicals and polyisocyanates (–NCO group) (
Scheme 1). Polyhydroxyl compounds which have been widely used are polycaprolactone (PCL), polyethylene glycol (PEG), polytetramethylene ether glycol and hexamethylene glycol, which are mostly derived from non-renewable petrochemicals
[42][43][44,45]. Due to the depletion of petroleum, biodegradable and renewable sources were introduced to replace the petrochemicals. Hence, many organizations and researchers are working towards producing bio-based polyurethanes developed from vegetable oils such as soybean oil
[44][46], castor oil
[45][47], sunflower oil
[46][48], and linseed oil
[47][49], for environmentally friendly and biodegradable considerations.
Scheme 1.
Conventional synthesis route of polyurethane.
The most commonly used aromatic diisocyanates are toluene diisocyanate (TDI) and methylene bis-diphenyl isocyanate (MDI), which give better crosslinking; while the commonly used aliphatic polyisocyanates are hexamethylene diisocyanate (HDI) and hydrogenated MDI, which normally act as chain extenders
[48][49][50,51]. Aromatic isocyanates are more reactive, which results in faster reactions. This is due to the presence of an electron-withdrawing group on the isocyanate molecule, which increases the electron density charge, resulting in easier electron transfer from the donor to the carbon
[48][50]. However, aromatic structures are not desired for medical and biomedical applications as they can produce toxic degradation products
[43][45]. In addition, polyisocyanates are classified as a carcinogenic substance by the Occupational Safety and Health Administration (OSHA), and many organizations are working hard to ban isocyanate-synthesized products. Furthermore, during the process, diamine phosgenation is performed in solvents such as chlorobenzene to produce diisocyanates. The solvents used and the phosgene gas produced during this process cause environmental concerns, and create an exposure risk hazard that could affect workers’ health.
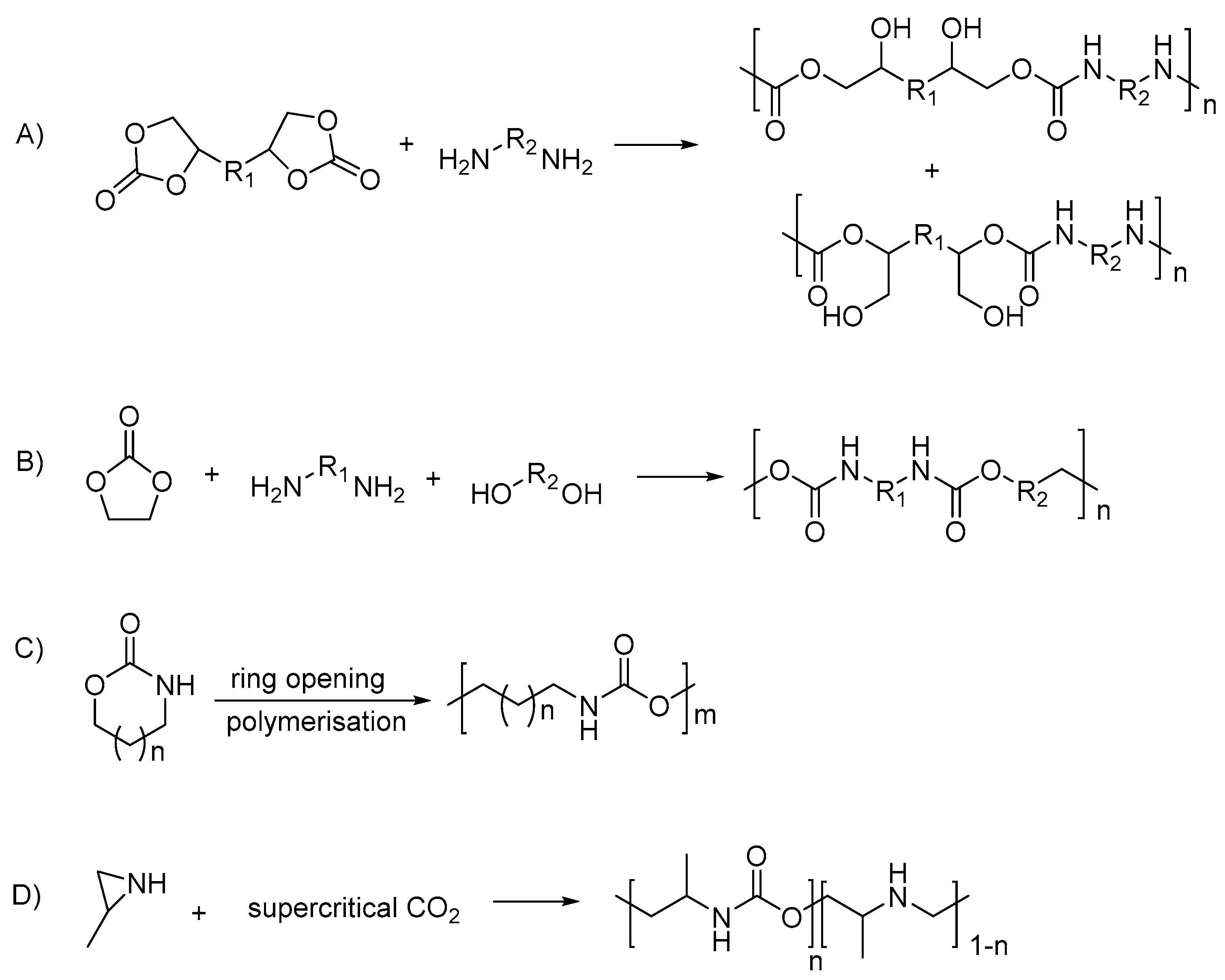
Hence, in recent years, PU synthesis is being classified into isocyanate-based and non-isocyanate-based; while the polyols used can be also differentiated by petrochemical-based and vegetable oil-based. The non-isocyanate PUs were produced from different routes, which include: polyaddition of bifunctional cyclic carbonates with diamines
[50][52]; polycondensation of ethylene carbonate, diamines and diols
[50][51][52,53]; cationic ring-opening polymerization of cyclic urethanes
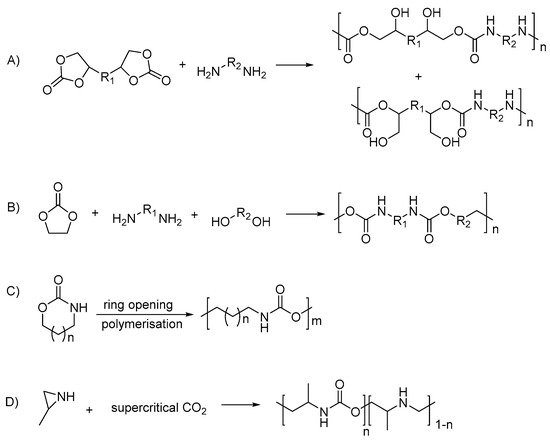
[31][52][33,54]; and copolymerization of substituted aziridines with CO
2 [53][55] (summarized in
Scheme 2).
Scheme 2. Polyurethane synthesis via non-isocyanate routes: (A) polyaddition of bifunctional cyclic carbonate and diamine; (B) the polycondensation of ethylene carbonate, diamines and diols; (C) the cationic ring-opening polymerization of cyclic urethane; (D) the copolymerization of substituted aziridines with carbon dioxide.
2.2. Types of Stimuli/Stimulus-Responsive Polyurethanes Used in Biomedical and Drug Delivery Applications
Polyurethane has been introduced into stimulus-responsive materials; Table 2 summarizes different types of stimulus-responsive PUs that could potentially be applied in the biomedical and drug delivery system in the recent 3 years (database from Web of Science, June 2021). The PU types can be categorized into four types: (A) isocyanate-based PUs and (B) non-isocyanate-based PUs; (C) PUs synthesized with petrochemical-based polyols; and (D) PUs synthesized from bio-based polyols. Table 2 has excluded pH-responsive PUs since they are discussed in the subsequent section. PUs with single-, dual- and multi-responsiveness have been reported. Most of the PUs in the reported works are produced by reacting isocyanates and petrochemical-based polyols (Type A + C), whereby some PUs were purchased commercially, and only two works reported on ring-opening polymerization by reaction of carbonates and tyrosine with petrochemical polyols (Type B + C). There were no studies reporting on non-isocyanate-based responsive PU.
The single-stimulus-responsive PUs reported include shape memory PUs, thermo-responsive PUs, redox-responsive PUs and light-responsive PUs (
Table 2). Thermo-responsive PUs were studied in drug delivery systems due to the variation in temperature between normal cells and inflamed cells. Shape memory PUs have better mechanical properties, force resistance and shape recovery properties, which are suitable for biomaterials applications such as sensors, actuators, vascular stents, and endovascular embolization. Redox-responsive PUs are more likely to be applied in drug delivery. This is due to the fact that within the body, there are different compositions of proteins and enzymes at specific sites which can create reduction or oxidation environments, which further lead to dissociation and drug release. For instance, glutathione (GSH) concentration is higher in tumor cells. By incorporating drugs into a redox-responsive polymeric system with disulfide bonds, the system can easily break down and reduce GSH into sulfhydryl groups, which causes self-degradation and release of the drug
[54][56]. As for photo-responsive PUs, they are mostly designed for application as biosensors or drug carriers for cancer therapies.
The dual-stimuli-responsive PUs mainly consist of the combination of two stimuli to optimize the formulation and increase the effectiveness of its biomedical applications. Most of the studies reported for dual stimulus response come with a combination of thermo- and photo-responsive, thermo- and shape-memory-responsive, thermo- and enzyme- responsive or shape-memory-and water-responsive PUs (Table 2).
Biocompatibility and biodegradation evaluations are important for biomaterials used in medicine and drug delivery. A biodegradable polymer degrades into normal metabolites of the body or is eliminated from the body with or without further metabolic transformation. Biodegradation tests were conducted to monitor the breakdown status of the polymers and the breakdown rate of materials mediated by biological activity
[55][57]. For instance, when exposed to bodily fluids, the materials may undergo chemical, physical, mechanical or biological changes, which may lead to different results in the body. By knowing the rate of degradation, the duration of the polymers persisting inside the body could also be estimated. The biodegradation test is important to achieving desired degradability, along with suitable sustainability
[56][58]. The common biodegradation test was conducted by immersing polymers into a phosphate buffer solution (PBS) containing enzymes (lipase) at a certain pH value for hours to days.
The biocompatibility test confirms the suitability of a polymer when exposed to bodies or bodily fluids. A biocompatible polymer would not trigger any allergies or have side effects when used within the body
[55][57]. The ideal biological research methodology consists of both in vivo and in vitro studies. For in vivo tests, the materials must be exposed to hemocompatibility, cytotoxicity, mutagenicity and pyrogenicity tests. However, due to ethical aspects, in vitro cytotoxicity tests were widely used to determine whether the material contains significant quantities of biologically harmful components. CCK8 assays, MTT assays and MTS assays were used in the reported studies for in vitro cytotoxicity tests.
Most of the studies included in
Table 2 have conducted in vitro cytotoxicity and biodegradation studies to evaluate the PUs’ biocompatibility and biodegradability, except for the shape-memory-responsive PUs. For shape memory PUs, most of the studies conducted were mainly focusing on the polymers’ shape memory behavior and their tensile and mechanical ability to withstand force. For the biocompatibility evaluation, most of the reported studies showed high cell viability of more than 65%, which can be concluded as potentially biocompatible polymeric materials and safe to be used as biomaterials
[57][58][59,60]. Other than interactions between the polymers and cells, the possible effect caused by toxicity of the degradation products must be evaluated. Although some of the studies claimed that PU degraded into non-toxic products or metabolites
[59][61], the biocompatibility after the release of incorporated substances has to be taken into consideration. Hence, while conducting the biocompatibility studies, the biological and chemical properties as well as cytotoxicity of the degraded species should be investigated
[60][62]. Furthermore, histological studies of in vivo implant sites could be conducted to determine the cytotoxicity of degraded stimulus-responsive PUs toward the surrounding tissues
[61][63]. For biodegradability evaluation, weight loss of the PU polymers could be observed within days to weeks. pH-responsive PUs will be further discussed in the following section.
Table 2. Types of stimulus-responsive polyurethanes used in biomedical and drug delivery applications, and their biocompatibility and biodegradability evaluation (excluding pH-responsive).
|
Type of Stimuli/Stimulus-Responsive PU
|
Type of Stimuli/Stimulus
|
* PU Type
|
PU Name
|
Reactants for Synthesis of PU
|
* PU Type: (A) isocyanate-based PU, (B) non-isocyanate-based PU, (C) PU synthesized with petrochemical-based polyols and (D) synthesized from bio-based polyols. ** N/A: information not available.
3. pH-Responsive Polyurethane
Table 3 summarizes all pH-responsive PUs reported in the past 10 years (Web of Science database, June 2021). The pH-responsive PUs are categorized into different applications: (A) biomedical and drug delivery; (B) optical imaging; and (C) biomaterials such as biosensors and bioactuators. pH-responsive PUs are favorable in drug delivery systems, particularly for the delivery of chemotherapy drugs, in which the pH-responsive PU carrier helps to modulate and improve the drug delivery by on-site targeting. The reported chemotherapy drugs used in pH-responsive PU systems are doxorubicin, paclitaxel and 5-fluorouracil, in which the PUs swelled at acidic pH (mimicking the environment of tumor pH) and demonstrated excellent drug release. The detailed applications and the mechanism of pH-responsive PUs reported will be further discussed in the following section.
Referring to Table 3, most of the reported pH-responsive PUs were produced by diisocyanates and polyols derived from petrochemicals. There were no studies reporting on non-isocyanate-based pH-responsive PUs created utilizing renewable resources, such as vegetable oil. As mentioned in the previous section, bio-based PUs have the advantage of protecting the environment and avoiding health concerns.
-
|
PEGMA
|
-
|
|
-
|
iii.
|
-
|
|
-
|
IPDI
|
-
|
|
|
|
|
|
DPA |
|
|
|
| Thermo-responsive |
|
| Increase of DPA, results in highest swelling degree at pH 4.0
|
A
|
[85][87]
|
Same PU without addition of DPA/PPGDA/PEGMA mixture
|
N/A
|
|
PEG-PCL based PU blend with cellulose nanocrystals (CNC)
|
-
i.
-
PEG
-
ii.
-
PCL
-
iii.
-
IPDI
|
-
i.
-
Pyridine-4-carbonyl chloride (-C6H4NO2)
-
ii.
-
2,2,6,6-tetramethyl-1-piperidinyloxy (-COOH)
|
Shape memory
|
CNC-COOH; At pH 4.0, folded strip of
CNC-C6H4NO2 recovers to straight
|
C
|
[86][88]
|
N/A
|
N/A
|
|
PU
|
-
i.
-
PPG
-
ii.
-
IPDI
|
Pyridine
|
Shape memory
|
Swells at pH 1.3, drug release and shape recovers
|
A, C
|
[10]
|
N/A
|
N/A
|
|
Azo-cationic waterborne polyurethane (CWPU)
|
-
i.
-
PEG
-
ii.
-
PCL
-
iii.
-
MDI
|
-
i.
-
MDEA
-
ii.
-
Azobenzene
|
Photo-responsive
|
Shows different color in different pH medium
|
B
|
[87][89]
|
N/A
|
N/A
|
|
PU micelles with disulfide linkage
|
-
i.
-
PEG
-
ii.
-
PCL
-
iii.
-
LDI
|
MDEA
|
Reduction-responsive
|
Rapid drug release at pH 5.5
|
A
|
[88][90]
|
N/A
|
N/A
|
|
PU with disulfide bonds
|
-
i.
-
PCL
-
ii.
-
HDI
-
iii.
-
Bis (2-isocyanatoethyl) disulfide (CDI)
|
Poly(2-ethyl-2-oxazoline)(PEOz)
|
Reduction-responsive
|
Drug release rate higher at pH 5.0
|
A
|
[89][91]
|
- End-group-carboxylated PEOz-PLA
- PEOz-hydrazone-DOX
|
Cumulative drug release increase with presence of 1, 4-dithio-D, L-threitol (DTT)
|
|
MPEG/PU triblock copolymers with disulfide linkage
|
-
i.
-
MDI
-
ii.
-
HDI
-
iii.
-
MDEA
-
iv.
-
BDEA
|
Bis-1,4-(hydroxy-ethyl) piperazine (HEP)
|
Reduction-responsive
|
pH 5.5 and 6.8, swells and faster drug release
|
A
|
[90][91][92,93]
|
N/A
|
N/A
|
|
Multi
|
PU
|
-
i.
-
PEG
-
ii.
-
MDI
|
DMPA
|
Thermo-responsive and
shape memory
|
For PEG-30%-MDI-DMPA, fixes deformed shape at pH 2.0, recovers shape at pH 9.0
|
N/A
|
[92][94]
|
N/A
|
N/A
|
* Applications: (A) Biomedical and drug delivery, (B) optical imaging, and (C) biomaterials.