Magnesium is one of the most prominent cations in the human body, with distribution of its concentrations in both extracellular and intracellular areas. Magnesium, in both complex and ionized form, has critical relevance in metabolic functions and homeostasis, serving as a moderator in enzymatic processes. A first important aspect is the role of magnesium in the activation of Adenosine Triphosphate (ATP), which is the primary energy source for cells. Magnesium improves muscle function by competitively binding to calcium sites and ensuring muscle relaxation.
Recently, there is an increasing amount of studies that suggest an important role of magnesium within the gut-brain axis, by modulating the bidirectional balance, alongside the microbiome. this opens a new research direction in both functional gastrointestinal diseases and psychiatric symptoms management.
- magnesium
- orotic acid
- gut-brain axis
- functional gastrointestinal disease
- psychiatry
- microbiome
1. General Aspects of Magnesium
2. Magnesium and Gastrointestinal (GI) Tract Health
3. The Brain–Gut Axis
4. The Gut–Brain Axis and the Microbiome Physiology
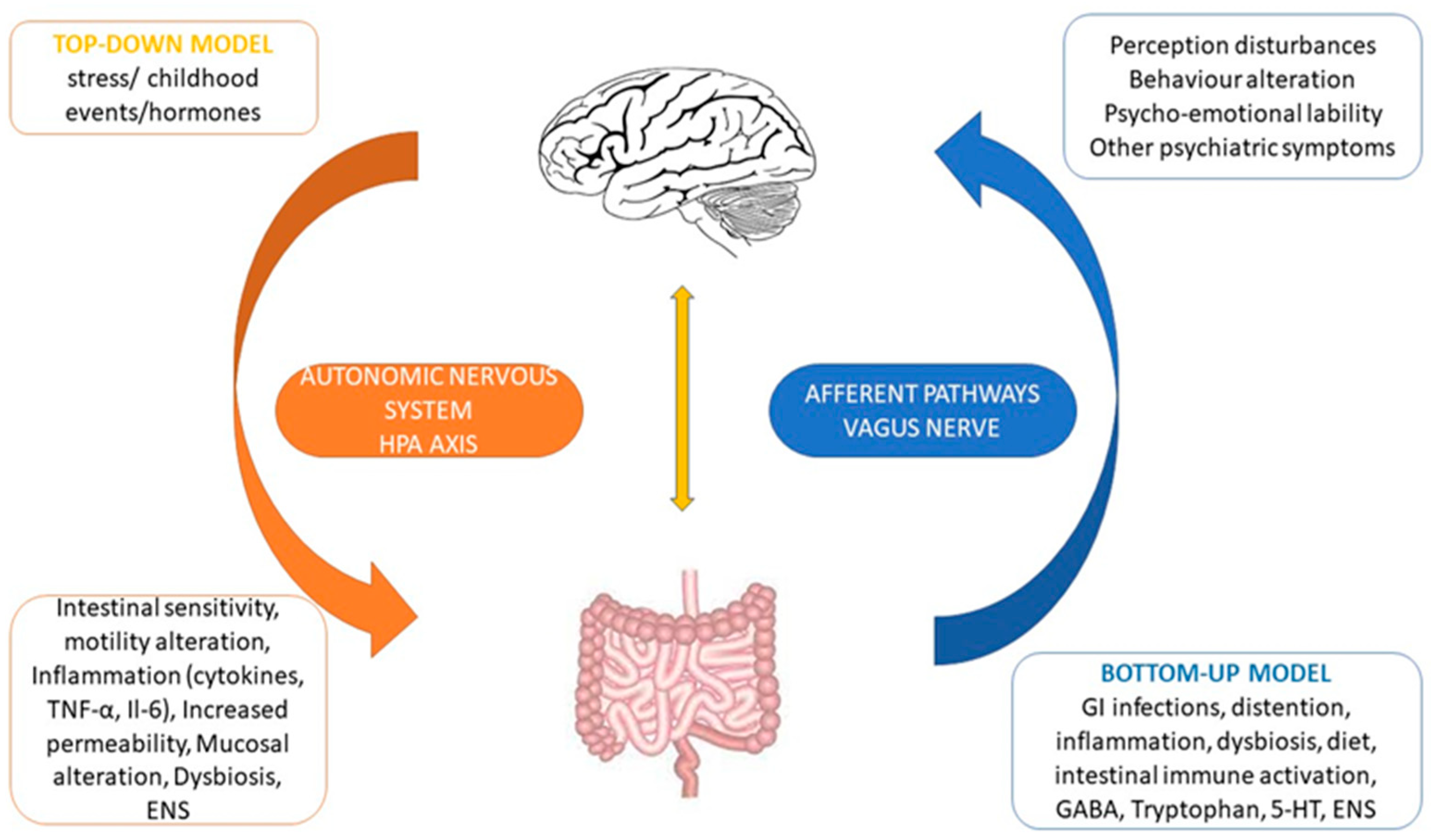
5. Brain to Gut Connection
6. Gut to Brain Connection
| Genera | Neurochemical Involvement | Deficiency | Probiotic Therapeutical Result |
Reference/ Study |
|---|---|---|---|---|
| Lactobacillus | GABA 1, BDNF 2, Vagal stimulation | FGID 3, behavior disorders, affective symptoms | Decrease intestinal distension, excitability and inflammation; decrease visceral pain by expression of opioid/cannabinoid receptors; mood and affective symptoms’ improvement | [24] |
| Bifidobacterium | GABA, 5-HT 4 | Depression, anxiety, cognitive impairment, autism, ADHD 5, FGID | Behavioral symptom resolution, digestive symptoms clearing, neurodegenerative protection, visceral pain modulation | [25] |
| Bacillus | 5-HT | Increased intestinal wall permeability, inflammation, oxidative stress, cognitive impairment, behavior and affective disorders | Decrease gastrointestinal inflammation, mood regulation | [26] |
| Saccharomyces | Myeloperoxidase, acetylcholine esterase | Increases gut inflammation, oxidative stress, neuronal damage | Reduces inflammatory cytokine, neurodegenerative protection | [27][28] |
| Enterococcus, Lactococcus | Dopamine, Histamine | Pathogenic bacteria overgrowth, gut inflammation, eating and affective disorders | Inhibits pathogenic bacteria overgrowth, reduces inflammation, histologic changes’ improvement, visceral pain reduction, mood and eating behavior improvement | [29] |
| Streptococcus | 5-HT | Inflammatory response, depressive/anxiety symptoms, cognitive impairment, Autistic Spectrum Disorder (ASD 6) | Digestive symptoms relief, cognitive and affective improvement | [30] |
| Bacteroides | Currently under study | Apparent role in neurodevelopment disorders (ADHD 5/ASD 6), functional digestive imbalances | Suggested cognitive/behavioral improvement, gastrointestinal function improvement in children with ASD/ADHD. | [31] |
References
- Turner, R.J.; Vink, R. Magnesium in the central nervous system. In New Perspectives in Magnesium Research: Nutrition and Health; Springer: London, UK, 2007.
- Glasdam, S.-M.; Glasdam, S.; Peters, G.H. The Importance of Magnesium in the Human Body: A Systematic Literature Review. Adv. Clin. Chem. 2016, 73, 169–193.
- Van Vuuren, J.J.; Pillay, S.; Van Vuuren, C.J. Relationship between magnesium and lipids in patients with diabetes mellitus. J. Endocrinol. Metab. Diabetes S. Afr. 2019, 24, 46–49.
- Anastassopoulou, J.; Theophanides, T. Magnesium-DNA interactions and the possible relation of magnesium to carcinogenesis. Irradiation and free radicals. Crit. Rev. Oncol. Hematol. 2002, 42, 79–91.
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium deficiency and oxidative stress: An update. Biomedicine 2016, 6, 8–14.
- Schuchardt, J.P.; Hahn, A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium-An Update. Curr. Nutr. Food Sci. 2017, 13, 260.
- Bothe, G.; Coh, A.; Auinger, A. Efficacy and safety of a natural mineral water rich in magnesium and sulphate for bowel function: A double-blind, randomized, placebo-controlled study. Eur. J. Nutr. 2017, 56, 491–499.
- Coffin, B.; Bortolloti, C.; Bourgeois, O.; Denicourt, L. Efficacy of a simethicone, activated charcoal and magnesium oxide combination (Carbosymag®) in functional dyspepsia: Results of a general practice-based randomized trial. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 494–499.
- Omori, K.; Miyakawa, H.; Watanabe, A.; Nakayama, Y.; Lyu, Y.; Ichikawa, N.; Sasaki, H.; Shibata, S. The Combined Effects of Magnesium Oxide and Inulin on Intestinal Microbiota and Cecal Short-Chain Fatty Acids. Nutrients 2021, 13, 152.
- Crowley, E.K.; Long-Smith, C.M.; Murphy, A.; Patterson, E.; Murphy, K.; O’Gorman, D.M.; Stanton, C.; Nolan, Y.M. Dietary Supplementation with a Magnesium-Rich Marine Mineral Blend Enhances the Diversity of Gastrointestinal Microbiota. Mar. Drugs. 2018, 16, 216.
- Jørgensen, B.P.; Winther, G.; Kihl, P.; Nielsen, D.S.; Wegener, G.; Hansen, A.K.; Sørensen, D.B. Dietary magnesium deficiency affects gut microbiota and anxiety-like behaviour in C57BL/6N mice. Acta Neuropsychiatr. 2015, 27, 307–311.
- García-Legorreta, A.; Soriano-Pérez, L.A.; Flores-Buendía, A.M.; Medina-Campos, O.N.; Noriega, L.G.; Granados-Portillo, O.; Nambo-Venegas, R.; Tovar, A.R.; Mendoza-Vargas, A.; Barrera-Oviedo, D.; et al. Effect of Dietary Magnesium Content on Intestinal Microbiota of Rats. Nutrients 2020, 12, 2889.
- Pachikian, B.D.; Neyrinck, A.M.; Deldicque, L.; De Backer, F.C.; Catry, E.; Dewulf, E.M.; Sohet, F.M.; Bindels, L.B.; Everard, A.; Francaux, M.; et al. Changes in Intestinal Bifidobacteria Levels Are Associated with the Inflammatory Response in Magnesium-Deficient Mice. J. Nutr. 2010, 140, 509–514.
- Winther, G.; Jørgensen, B.M.P.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sørensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176.
- Kisters, K.; Gremmler, B.; Schmidt, J.; Gröber, U.; Tokmak, F. Positive Effect of Magnesium Orotate Therapy in Hypertensive Heart Disease. Metabolomics 2017, 7, 195.
- Syrkin, A.L.; Salagaev, G.I.; Syrkina, E.A.; Lysenko, A. Advantages of magnesium orotate for correction of magnesium deficiency in patients with various heart rhythm disturbances. Kardiol. I Serdechno Sosud. Khirurgiya 2019, 12, 308.
- Karachentsev, Y.I.; Kravchun, N.A.; Chernyaeva, A.A.; Dunaeva, I.P.; Kholodny, A.V.; Efimenko, T.I.; Ashurov, E.M. Place of magnesium orotate in the complex therapy of patients with type 2 diabetes mellitus with hyperuricemia. Probl. Endokr. Patol. 2020, 71, 23–29.
- Kalacheva, A.G.; Gromova, O.A.; Grishina, T.R.; Bogacheva, T.E.; Demidov, V.I.; Torshin, I.Y.; Tomilova, I.K. Investigation of the effects of magnesium orotate in a model of primary generalized seizures. Nevrol. Neiropsikhiatriya Psikhosomatika 2017, 9, 61–66.
- Perlmutter, D. The Microbiome and the Brain; CRC Press: Boca Raton, FL, USA; p. 233. Available online: https://www.perlego.com/book/1546614/the-microbiome-and-the-brain-pdf (accessed on 11 December 2021).
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501.
- Ford, A.C.; Lacy, B.E.; Harris, L.A.; Quigley, E.M.M.; Moayyedi, P. Effect of Antidepressants and Psychological Therapies in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2019, 114, 1350–1365.
- Cedeño, M.M.C.; Moreira, J.F.C.; Diaz, M.J.C.; Chavez, P.E.P.; Marquinez, S.P.M.; Espinoza, A.M.F.; Veliz, A.B.B.; Alava, R.A.M.; Mendoza, A.A.G. Use of antidepressant drugs in the treatment of irritable bowel syndrome. Arch. Venez. Farmacol. Ter. 2019, 38, 6.
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 11.
- Gómez-Eguílaz, M.; Ramón-Trapero, J.L.; Pérez-Martínez, L.; Blanco, J.R. El eje microbiota-intestino-cerebro y sus grandes proyecciones. Rev. Neurol. 2019, 68, 111–117.
- de Palma, G.; Collins, S.M.; Bercik, P.; Verdu, E.F. The microbiota-gut-brain axis in gastrointestinal disorders: Stressed bugs, stressed brain or both? J. Physiol. 2014, 592, 2989–2997.
- Cheng, H.-W.; Sha, J.; Jiaying, H. Gut-brain axis: Probiotic, Bacillus subtilis, prevents aggression via the modification of the central serotonergic system. In Oral Health by Using Probiotic Products; IntechOpen: London, UK, 2019.
- Constante, M.; De Palma, G.; Lu, J.; Jury, J.; Rondeau, L.; Caminero, A.; Collins, S.M.; Verdu, E.F.; Bercik, P. Saccharomyces boulardii CNCM I-745 modulates the microbiota–gut–brain axis in a humanized mouse model of Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2021, 33, e13985.
- Roy Sarkar, S.; Mitra Mazumder, P.; Chatterjee, K.; Sarkar, A.; Adhikary, M.; Mukhopadhyay, K.; Banerjee, S. Saccharomyces boulardii ameliorates gut dysbiosis associated cognitive decline. Physiol. Behav. 2021, 236, 113411.
- Villageliú, D.; Mark, L. Dopamine production in Enterococcus faecium: A microbial endocrinology-based mechanism for the selection of probiotics based on neurochemical-producing potential. PLoS ONE 2018, 13, e0207038.
- Srikantha, P.; Mohajeri, M.H. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int. J. Mol. Sci. 2019, 20, 2115.
- Tamana, S.K.; Tun, H.M.; Konya, T.; Chari, R.S.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Moraes, T.J.; Turvey, S.E.; Subbarao, P.; et al. Bacteroides-dominant gut microbiome of late infancy is associated with enhanced neurodevelopment. Gut Microbes 2021, 13, 1930875.
