Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Fatma Haddad and Version 3 by Vivi Li.
Multiple sclerosis (MS) is known as an autoimmune disease that damages the neurons in the central nervous system. MS is characterized by its most common symptoms of spasticity, muscle spasms, neuropathic pain, tremors, bladder dysfunction, dysarthria, and some intellectual problems, including memory disturbances. Several clinical studies have been conducted to investigate the effects of cannabis on the relief of these symptoms in MS patients.
- Cannabis sativa
- marijuana
- cannabinoids
- 9-tetrahydrocannabinol (THC)
- multiple sclerosis (MS)
1. Introduction
MS is a neurological disease with an autoimmune origin that affects and damages the central nervous system and affects 2.3 million people worldwide [1][2]. This demyelinated disease leads to severe impairment of nerve signal transmission between the brain and spinal cord that causes a loss of myelin sheath [1][2].MS has been characterized by symptoms of spasticity, muscle spasms, tremors, bladder dysfunction, neuropathic pain, dysarthria, and some intellectual problems, including memory disturbances [3][4][3,4]. Some drugs have been licensed to slow down disease progression and reduce relapse frequency [3]. However, more studies are needed to further alleviate the disabling symptoms of MS patients.
In the 21st century, the Sativa plant has become the most widely used illicit drug [5]. It is also known as hemp, cannabis, or marijuana, and comes in a variety of forms, including cigarettes or hash pipes and sweets or brownies [5][6][5,6]. The cannabis plant, which includes over 560 identified components, is primarily composed of phytochemicals [5]. The two prominent species of Cannabis plant, which are mostly used for recreational and medical, are Cannabis indica and Cannabis sativa (C. Sativa) [7][8][7,8]. Both plants are composed of different cannabinoid compositions of THC and CBD [9][10][9,10]. Previous research has shown the different effects of each species due to the different concentrations of the two main components [11]. C. Sativa has a higher ratio of CBD to THC and the reverse is seen for C.indica [10] [10]. There are about 100 cannabinoids in Cannabis sativa [12] [12], the most well-known of which are 9-tetrahydrocannabinol (THC) and cannabidiol (CBD) (Figure 1a,b). The endogenous cannabinoid system, which includes CB1 and CB2 receptors, is where cannabinoids get their effects. The psychotropic effects of THC are primarily mediated by a CB1 receptor agonist actions. CBD, on the other hand, is hypothesized to bind to CB1 and CB2 receptors and act as an antagonist [5][13][5,13].
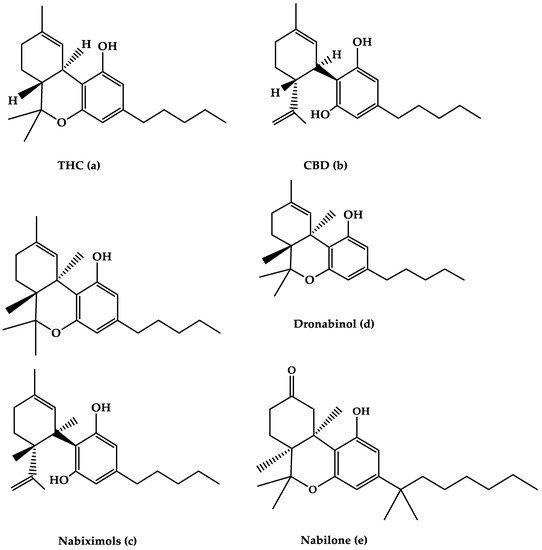
Figure 1. The molecular structures of (a) THC, (b) CBD, (c) Nabiximols, (d) Dronabinol, and (e) Nabilone are depicted in this diagram.
Cannabis has been used for over 5000 years, with the discovery of the endogenous cannabinoid system occurring more than a decade ago. CB1 and CB2 are cannabinoid receptors that are linked to adenylyl cyclase negatively and mitogen-activated protein kinase positively through the Gi/o protein [14][15][14,15]. CB1 and CB2 are distributed in the central and peripheral nervous systems and immune systems [16]. CB1 receptors are found mostly in nerve terminals in the central nervous system and some peripheral tissue and are linked to a specific type of calcium and potassium channel via a G protein. Because it blocks pain pathways in the brain and spinal cord, the CB1 receptor’s main function is to suppress neurotransmitter release, and it plays a crucial role in mediating the pain-relieving effect of cannabis [17].
Phytocannabinoids, endocannabinoids, and synthetic cannabinoids are the three types of cannabinoids. The major chemical components of cannabis are phytocannabinoids, which include a variety of non-cannabinoid C21 terpenes phenolic compounds, or C22 for the carboxylate group, which is largely synthesized in cannabis [18][19][18,19]. Heat can decarboxylate phytocannabinoids, which are biosynthesized in carboxylated form [20].
Cannabis is considered a promising anti-inflammatory and immunosuppressive agent due to its central and peripheral actions on CB1 and CB2 receptors that mediate different intracellular pathways when activated [21]. In addition to the effects of the cannabinoids on CB1 and CB2 receptors, cannabinoids have effects on nuclear receptors and ion channels by their activity on other transmembrane G protein-coupled receptors (GPCRs) and have a modulating activity on opioid and serotonin receptors [16]. THC is a psychoactive component of cannabis with the highest potency. THC can be utilized to treat neuropathic and chronic pain because of its intoxicating, anti-emetic, and anti-inflammatory properties [22]. CBD does not cause psychosis, although it does have pharmacological effects on pain and spasticity [23].
The main psychoactive constituent of the C. Sativa plant, 9-THC, was discovered in the late 1980s and was shown to have activity on a specific cannabinoid receptor in the brain (the cannabinoid CB1 receptor), which had a huge impact on the development of cannabinoid therapeutic drugs and their potential to relieve MS spasticity symptoms [24]. Preclinical animal studies of MS suggested that cannabinoids have anti-septic effects due to the activation of CB1 receptors, which inhibit the release of classical neurotransmitters, such as glutamine, while also decreasing neuronal excitability by activating somatic and dendrite potassium channels [25][26][25,26].
CBD is a key non-psychotropic cannabinoid present in C. Sativa that accounts for up to 40% of the cannabis plant’s extract and binds to a wide range of physiological targets of the endocannabinoid system in the human body. CBD has shown potential in the treatment of MS symptoms. CBD, in particular, has been shown in numerous trials to reduce stiffness, discomfort, inflammation, exhaustion, and depression in MS patients, resulting in increased mobility [27][28][27,28]. CBD also affects the non-cannabinoids receptors GPCRs and ion channels that will lead to pain regulation and anti-inflammatory effects through receptor modulation [16].
Several attempts have been made to determine the primary genetic factor contributing to MS progression and severity [29][30][31][29,30,31]. Although it was demonstrated that genetic effects on MS disease severity and susceptibility are polygenic with modest influence [30][31][30,31], more studies are needed to further understand the pharmacogenetics of MS disease and to define the molecular target of CBD in MS patients by performing the ex vivo/in vitro research in human immune cells as reported by Furgiuele et al. [31].
The efficacy of oromucosal spray nabixiomol, oral dronabinol, and oral nabilone forms of cannabis on MS-related spasticity, pain, tremor, urine function, sleep disturbances, quality of life, disability, and disability progression is assessed in this entry. The sections below go through the signs and symptoms of MS, as well as the treatment options.
1.1. MS-Related Symptoms
1.1.1. Spasticity in MS
Spasticity is a common symptom of MS, affecting 60–84% of patients, particularly throughout the disease’s progression [32]. Spasticity is caused by injury of the higher corticosteroids motor neurons, as well as aberrant supraspinal driving of spinal reflexes [33], which can exacerbate other MS symptoms and harm the patient’s quality of life [34][35][36]. MS spasticity is a complicated condition characterized by the most prevalent symptom, muscle rigidity, which is induced by the stretch reflex’s hyperexcitability. Fatigue, discomfort, and bladder dysfunction are among the more prevalent and troubling symptoms [37]. Combining nonpharmacological and pharmacological therapies is the most commonly employed treatment [38]. Increasing severity of MS spasticity leads to decreased patient satisfaction, with limited and ineffective oral pharmacotherapy available to treat MS spasticity [39]. MS spasticity therapy is used to improve functional ability, assist rehabilitation, avoid contractures, and relieve discomfort in people with MS. The only use of cannabinoids in neurological disorders and the only complementary medicine intervention with high-level evidence for efficacy in MS is pharmaceutical cannabinoids for spasticity, according to most recent studies [40][41]. The most prevalent and available treatment for MS spasticity is multimodal [38], which combines non-pharmacological and pharmaceutical therapies. The majority of current therapy approaches result in patient dissatisfaction. A new cannabis-based pharmaceutical option with different administration methods has been created. Surprisingly, most studies revealed that topical cannabis was most commonly used for treating MS spasticity because of its pharmacokinetic qualities, although smoking or vaping was the most popular strategy in other studies [42][43]. THC and THC: CBD is the most commonly used cannabinoid to treat spasticity, according to reviews by Ben Amar, Koppel et al., Lakhan, Rowland, and Krast et al. [40][44][45][46].1.1.2. MS-Related Pain
MS is frequently linked to pain [47]. Pain has long been recognized as a significant element in MS patients’ overall health-related quality of life (HRQoL). Patients with MS who have pain have reported decreased HRQoL, as well as physical and emotional dysfunctions [48][49]. Neurological injury, as well as neurological dysfunction and incapacity, might be the source of severe discomfort [50]. Continuous central neuropathic pain, intermittent central neuropathic pain, musculoskeletal pain, and mixed neuropathic and non-neuropathic pain are all examples of pain associated with MS [49]. Despite significant advancements in providing novel therapeutic techniques for the symptomatic treatment of MS, such as pain, the present therapeutic modalities are insufficient to meet the needs of patients [50][51].1.1.3. MS-Related Tremor and Ataxia
MS affects the cerebellum and its efferent and afferent pathways, causing tremors, ataxia, and dysarthria in both acute and chronic symptoms [52]. More than 80% of MS patients have tremors or ataxia at some point during their illness [53]. Several pharmacotherapies for the management of tremors or ataxia associated with MS are available, but they are often ineffective [52][54]. Because of the absolute and comparative efficacy and acceptability of pharmaceutical treatments are inadequately recorded, a Cochrane review of six randomized controlled studies for the management of ataxia in MS was unable to provide any recommendations [55]. Furthermore, several randomized controlled studies have looked at the effect of cannabis extracts on these symptoms, but they have concluded that cannabinoids are useless in treating MS tremor and ataxia [40][55][56][57]. As a result, it is prudent to seek out novel treatments.1.1.4. Bladder Dysfunction in MS
In MS patients, lower urinary tract dysfunction is a prevalent symptom. It usually appears in the later stages of the disease. In total, 50–90% of MS patients develop this symptom after 6 years of disease [58][59][60], which is primarily attributable to a neurogenic overactive bladder [61]. Urinary urgency and urge incontinence can hurt a patient’s quality of life and cause immobility. Anti-cholinergic and intermittent self-catheterization may be beneficial in the early stages [62]. A total of four reviews looked at the effect of cannabis on MS bladder function symptoms [40][44][46][63].1.1.5. MS-Related Sleep Disorders
One of the most prevalent symptoms among MS patients is sleep disturbances [64]. The most common causes of MS sleep problems are multifunctional, and they are linked to immunotherapy and symptomatic therapies, as well as MS-related symptoms such as pain and exhaustion [65]. Patients with untreated sleep disturbances are at a higher risk of developing diseases, which can have a long-term impact on their health [66][67][68]. Three studies looked into the impact of cannabis on MS sleep problems. However, no conclusion was reached regarding the clinical effects of cannabis in MS patients with sleep difficulties [44][46][63].1.1.6. Health-Related Quality of Life in MS Patients
HRQoL (Health-related Quality of Life) is a multidimensional perception that encompasses physical, emotional, mental, and social functioning [69]. MS patients have been found to have a lower HRQoL than the general population. The detrimental influence of illness symptoms on daily living performance could explain the worse HRQoL associated with MS [70][71]. In MS patients, the influence of cannabis on HRQoL was investigated, with inconsistent results [46][54][72].1.1.7. MS-Related Disability and Disability Progression
Progression MS is a disability-causing disease that is characterized by relapsing-remitting neurological episodes [73]. Progression is linked to inadequate treatment and diagnosis, which results in demyelination, axonal and neuronal loss, and various cognitive, sensory, and motor problems [73][74]. It occurs as a result of central nervous system lesions and is driven by central inflammatory and other neurodegenerative effects that underpin irreversible disability. Many studies looked at the impact of cannabis on MS impairment and signs of disability progression [46][63][72][75]. The majority of these reviews did not focus on MS disability, and it was treated as a secondary result with no conclusions about cannabinoids’ impact on it.2. Cannabinoid Agents for the Treatment of MS
2.1. Nabiximols
Nabiximols is the generic name for Sativex®, an oromucosal spray containing a 1:1 molecular ratio of THC and CBD (Nabiximols (c), Figure 1) [76]. It has been licensed in several countries for the treatment of severe spasticity in MS patients [5]. The THC, CBD, and the small number of other constituents of the plant extract, including other cannabinoids and terpenoids dissolved in ethanol, make up around 70% of the constituents in Nabiximols [76]. There are 2.7 mg, 2.5 mg, and 0.04 g of THC, CBD, and ethanol, respectively, in each dose of oromucosal spray. Its administration has a favorable pharmacokinetic profile, with fewer first-pass effects and a low plasma concentration, resulting in the avoidance of psychoactive effects that are caused by smoked cannabis [76][77][78][76,77,78]. Furthermore, its maximum plasma concentration would rise gradually within 2–4 h after administration, while the quick onset effect will appear after 15–40 h, making treatment adjustments easier [77]. The synergistic interaction is based on a low-dose combination of THC and CBD, which results in reduced euphoric effects and improved cannabinoid-mediated anti-spasticity therapeutic benefits [76]. According to much research, starting therapy with a 14-day dose titration period is recommended to reach up to 12 sprays as the highest dose per day [78]. Individual dose distribution across a day, as well as dose change over the treatment course, is possible depending on the intensity of the disease. The recommended maximum daily dose is 12 sprays with at least 15 min between each spray [77]. Nabiximols were tested in MS patients with a variety of symptoms, such as stiffness, pain, tremor, and bladder function, in several clinical investigations [56][79][80][81][56,79,80,81]. Nabiximols were found to have good effects on spasticity, pain, and quality of life in MS patients in the majority of trials (Table 1) [56][79][80][81][56,79,80,81].Table 1. The efficacy of oromucosal spray nabixiomol, oral dronabinol, and oral nabilone forms of cannabis on MS-related symptoms and their adverse effects.
| Cannabinoid Agents | Therapeutic Actions | Adverse Effects |
|---|---|---|
| Nabiximols |
|
|
| Dronabinol |
|
|
| Nabilone |
|
|
2.1.1. Effects of Nabiximols on MS-Related Spasticity
Wade and colleagues reported an open-label extension trial in 2006 that included 137 patients diagnosed with MS after 6-week placebo-controlled research, in which the efficacy and tolerability of Sativex for the management of spasticity and other symptoms were assessed for about 15 weeks [82]. The primary outcome was assessed using a visual analog scale, which revealed a consistent reduction in spasticity. Furthermore, it had unfavorable effects on MS pain, tremor, and bladder symptoms, and 58 patients dropped out due to ineffectiveness [82].
Collin et al. conducted a 6-week, double-blind, placebo-controlled research on 189 patients in 2007, comparing the effects of Sativex between the groups [83]. There was a significant difference in spasticity reduction between the active and placebo groups, with a decrease in the numerical rating scale (NRS) score of p = 0.048 [83]. As a secondary endpoint, however, there was no therapeutic impact among other Ashworth scores [83].
Novotna and colleagues conducted a large multicenter phase III trial in 2011 that included two phases of study design: phase A began with a four-week single-blind treatment phase to identify early responders to oromucosal spray Nabixomols, followed by phase B, which was a 12-week randomized, double-blind study phase [77]. The recommended highest daily dose of nabiximols was 12 sprays. In a study comparing oral mucosal spray to placebo, the oral mucosal spray was linked to a 51% reduction in MS spasticity. In addition, the NRS ratings decreased by 20% [77].
Based on a series of randomized controlled clinical trials vs. placebo, Nabiximols were given and licensed for their therapeutic efficacy and relief of spasticity-related symptoms [56][79][83][56,79,83]. The first line anti-spastic study was recently designed and implemented using an enriched–design methodology as proof of the German authority’s request that add-on Nabixomols were more effective than readjusting the anti-spasticity medication regimen alone in providing symptomatic relief of MS spasticity during a 4-week trial period compared to placebo [84]. SAVANT, a well-designed research of oromucosal Nabixomol as an add-on therapy for the treatment of MS spasticity in MS patients, found that it had a promising effect in reducing moderate to severe spasticity symptoms [85].
Nabiximols are approved and advised as an add-on treatment for adult patients with moderate to severe resistant MS spasticity in several Western countries [86][87][86,87].
2.1.2. Effect of Nabiximols on MS-Related Pain
Nabiximols (oromucosal spray): randomized, placebo-controlled research in which 66 patients were randomly assigned to Nabiximols or placebo found that Nabiximols was effective in alleviating both central pain associated with MS and pain-related sleep disruption [88]. In a different double-blind experiment conducted in 2013, 339 patients with central neuropathic pain associated with MS were randomized to 167 Nabiximols and 172 placeboes, with the Nabiximols showing a statistically significant response rate at week 10 compared to the placebo [80]. The authors explained the findings by stating that their patient population may represent a particularly treatment-resistant group because they had long-standing pain spanning more than 5 years on average, and they demonstrated that those with a less than 4-year history of neuropathic pain were more likely to be resistant to treatment [80]. Nine randomized controlled studies with 1289 individuals were included in a recent systematic meta-analysis of THC: CBD oromucosal spray and placebo for the treatment of chronic neuropathic pain. Nabiximols were found to relieve chronic neuropathic pain more effectively than placebo, with a small effect size [89]. This suggests that further research into the full potential of Nabiximols in these people could be very interesting.
2.1.3. Effects of Nabiximols on MS-Related Tremor
Only 13 of the individuals in a Class I trial using the Nabiximols to see if cannabis extracts had an effect on symptoms in MS had a tremor as their predominant symptom. The Visual Analogue Scale did not change when (THC/CBD) oral spray was used at a dose of 2.5/120 daily in divided doses. Due to the small number of participants, no conclusion or difference was reached [56]. A more recent investigation used a 0–10 numeric rating scale (NRS) to assess the severity of MS symptoms in 337 patients treated with Nabiximols, with an undetermined number of patients rating tremor on the NRS, and found no difference between Nabiximols and placebo [79]. Nabiximols were mentioned in a systemic review published by Koppel et al. in 2014. For treating tremors associated with MS, Nabiximols were deemed “potentially ineffective” [40]. As a result, it appears that Nabiximols is unsuccessful in the treatment of MS-related tremors and ataxia.2.1.4. Effect of Nabiximols on MS-Related Bladder Function
THC: CBD oromucosal spray (Nabiximols) reduced urine frequency of nocturia and improved incontinence-related quality of life measures in Class 1 research [9089]. Nabiximols were found to have a minor effect on the alleviation of bladder dysfunctions in MS patients [90]. The impact of a cannabis-based medicinal extract on MS symptoms was studied in 160 MS patients in a double-blind, randomized, placebo-controlled class 1 study. Sativex’s effect on the symptoms of MS bladder dysfunction was investigated [56]. These s suggroups did not experience any improvement in bladder difficulties after using Sativex [56]. Anotsts ther randomized controlled researcht [90] looked at the efficacy and safety of Sativex in treating MS bladder dysfunction symptoms in 135 MS patients. Sativex was utilized to treat detrusor overactivity MS symptoms as an add-on therapyrther [90]. The primary endpoint of this double-blind, randomized, placebo-controlled parallel trial after 10 weeks revealed no significant difference between Sativex and placebo in the mean daily bouts of incontinence (p = 0.056) [90]. Sativex was fsearch intound to be successful in four secondary endpoints out of seven, with a significance of p = 0.00. Sativex appears to have some effect on improving bladder dysfhe function in MS patients; however, the primary endpoint did not reach statistical significance [90]. Giorgia et al. conducted a pilot prospective trial in 2017 to examine the effect of THC/CBD oromucosal spray on bladder dysfunction symptoms in MS patients. A total of 21 MS patients were examined, with 15 of them receiving a detailed clinical evaluation (extensive urodynamic studies) before and after one month of oromucosal THC/CBD treatment [91]. Overactive symptoms were reported to be reduced (p = 0.001), and an oromucosal spray administration was found to be beneficial in treating overactive bladder symptoms. THC/CBD oromucosal spray can improve overactive bladder in people with MS-resistant symptoms, according to a study [91].2.1.5. Effects of Nabiximols on MS-Related Sleep Disturbances
Russo et al. conducted a review of utilizing Sativex to examine sleep disorders and found that there was a sleep improvement with no medication tolerance [92]. Wade et al. assessed the efficacy of potential of Nabixomols on 160 participants in a double-blind, randomized, placebo-controlled, parallel-group research in 2004[56]. The mols in trial lasted 6 weeks, followed by a 4-week active extension period. The administration of Nabixomols resulted in a considerable improvement in sleep difficulties in the study [56]. In 2011, Nse peovotna published the results of a pivotal phase three trial for Nabixomols, which revealed a significant improvement (p-valle coue 0.0001) in MS sleep problems, which were considered a secondary endpoint in the study [77].2.1.6. Effects of Nabiximols on MS-Related HRQoL
The effects of Nad biximols on MS-specific HRQoL were studied for 3 to 4 months in the MOVE 2 research [93]. Although the MOVE 2 trial found a beneficial effect of Nabiximols on physical and mental HRQoL, researchers found no change in HRQoL in MS patients when they assessed them for a year using the same method as the MOVE 2 study [93][94]. In total, 337 MS patients were e valuated in a 15-week randomized controlled trial who reported some favorable but statistically insignificant effects in HRQoL with Nabiximols therapy [79]. Anothry interer 19-week trial that treated 241 MS patients with Nabiximols revealed similar negligible improvements in HRQoL[77]ting.2.1.7. Effects of Nabiximols on MS-Related Disability and Disability Progression
In terms of disability and progression, evaluations found no significant changes in various indexes, such as the Barthel index of daily living activity and walking time of MS disability [56][63][95]. The CUPID experiment assessed the impact and safety of oral cannabis THC to delay progressive inflammatory brain illness in MS patients (CUPID) [96]. It was a randomized, double-blind, placebo-controlled, parallel-group, multicenter trial. A total of 498 participants were randomized (332 to active and 166 to placebo) and 493 (329 actives and 164 placebos) were examined in the study. Patients were given either (9)-THC or placebo in a 2:1 ratio. There was no significant treatment impact in either the primary or secondary outcomes. Furthermore, there were no substantial safety issues, but unfavorable side effects appeared to have an impact on compliance [96].2.2. Dronabinol
The primary THC isomer present in the cannabis plant, (−)-trans-9-THC(Dronabinol(d), Figure 1), is known by the generic name Dronabinol. Dronabinol is available in three strength formulations: oral soft gelatin capsule, oral soft gelatin capsule, and oral soft gelatin capsule (2.5, 5, and 10 mg). It was first used to treat chemotherapy-induced vomiting and nausea, as well as anorexia and weight loss in AIDS patients, and it was tested in ten clinical trials for its efficacy and safety in treating MS symptoms including spasticity, pain, tremor, bladder function, sleep, quality of life, and side effects [57][97][98][99][100][101][102][103][104][105][57,97,98,99,100,101,102,103,104,105]. Dronabinol appears to be highly effective in the treatment of pain in MS patients, according to the bulk of clinical evidence, although other symptoms showed only little improvement (Table 1) [95].2.2.1. Effects of Dronabinol on MS-Related Spasticity
Zajick et al. conducted a multicenter, randomized, placebo-controlled research on 630 patients aged 18 to 64 in 2003 [57]. The effect of orally administered Marinol (Dronabinol) and Cannador on MS symptoms were studied for 15 weeks in this design study [57]. Weight-adjusted 0.25 mg/kg 9-THC (Dronabinol, supplied orally in 2.5 mg capsules), natural Cannabis oil (Cannador), or placebo were used to provide the dose [57]. The study assessed the effects of orally delivered cannabis extract (Cannador), Dronabinol, or placebo on spasticity following 28 days dose-titration phase using the Ashworth scale. Over a year, 80% of the subjects demonstrated a moderate reduction in spasticity [57]. There was no significant therapeutic impact between the active and placebo groups in the primary outcome. In comparison to the placebo group, the groups who took Dronabinol or Candor in spasticity demonstrated better improvement in the secondary result. They discovered that subjects who took Dronabinol exhibited a slight improvement on the Ashworth scale (p = 0.003) after 12 months of follow-up, which was not detected in the canned or placebo groups [57].2.2.2. Effects of Dronabinol on MS-Related Pain
Schimrigk et al. published a study that found Dronabinol to be superior to placebo in the treatment of neuropathic pain [106]. They discovered a clinically relevant difference between the treatment and placebo groups without statistical significance. They also discovered that it is a safe long-term management choice for MS with pain, since the number of side effects was comparable to that of normal treatment [106].2.2.3. Effects of Dronabinol on MS-Related Tremor
2.3. Nabilone
A total of 391 individuals in the CAMS study were given THC (Dronabinol), THC/CBD, or placebo orally; however, there was no statistically significant difference in the reaction to tremor as measured by a physician assessment (p = 0.052) or self-report (p = 0.398) [57].2.2.4. Effects of Dronabinol on MS-Related Bladder Functions
The CAMS trial [57] used oral cannabis extract or (Dronabinol) THC to evaluate bladder complaints in 667 participants with MS symptoms as a secondary outcome. There was no improvement in MS bladder symptoms when Dronabinol or oral cannabis extract were used [57].2.2.5. Effects of Dronabinol on MS-Related Sleep Disorders
The CAMS trial looked at the effects of marijuana on 630 MS patients with spasticity. For 15 weeks, MS patients were divided into four groups: oral Cannador, Dronabinol, Candor placebo, or Dronabinol placebo [57]. When compared to other placebo groups, the orally supplied group that took candor or Dronabinol demonstrated a significant improvement in MS sleep problems [57]. To assess the effect of Dronabinol on sleep difficulties, a case study was conducted on 52-year-old women with MS. Dronabinol was given orally at a dose of 2.5 mg BID for one week before being increased to 5 mg BID. The patient recorded the results using visual analog, concluding that the quality of sleep improved during the treatment [107].2.2.6. Effects of Dronabinol on MS-Related HRQoL
A three-week crossover study comparing the effect of Dronabinol on HRQoL in 24 MS patients found no significant differences in HRQoL in the treated patients compared to the placebo group [100].2.2.7. Effects of Dronabinol on MS-Related MS Disability and Disability Progression
Dronabinol’s effect on progression in progressive MS was studied in a randomized, double-blind, parallel, and placebo-controlled experiment [105]. For 36 months, patients were administered Dronabinol at a maximum dose of 28 mg daily or a placebo. In comparison to the placebo group, 35% of the Dronabinol group experienced at least one adverse event. In general, there were no safety issues or side effects associated with taking Dronabinol to reduce the course of MS [105].2.3. Nabilone
Nabilone (Figure 1e) is the generic name for the primary synthetic analog of 9-THC carrying dibenzopyran-9-one structure with a racemic combination of (S,S)-(+)- and (R,R)-(−)-isomers. Nabilone was approved in 1985 for the treatment of chemotherapy-induced vomiting and nausea in individuals who did not respond to standard anti-emetic drugs; however, serotonin 5-HT3 receptor antagonists have partially supplanted Nabilone for this use. Nabilone is also approved for the treatment of neuropathic and chronic cancer pain, as well as MS spasticity. It comes in three dosages in pill form (0.25, 0.5, and 1 mg). The effectiveness of nabilone in the treatment of MS spasticity was investigated in three clinical studies published between 1995 and 2015 [108][109][110] [108,109,110] and reviewed in four reviews published between 2006 and 2015 [40][44][46][111][40,44,46,111]. Nabilone appears to be beneficial in lowering most MS symptoms, including stiffness, pain, bladder dysfunction, and quality of life, according to the majority of trials (Table 1).2.3.1. Effects of Nabilone on MS-Related Spasticity
Martyan et al. studied the effects of Nabilone on MS-related spasticity for four weeks [108]. Their findings indicated that MS spasticity symptoms have improved [108]. A meta-analysis of three parallel groups based on the GRADE rating approach revealed that Nabilone has a moderate effect on MS-related spasticity [75].2.3.2. Nabilone Effects on MS-Related Pain
A meta-analysis of randomized clinical trials found sufficient evidence to support the improved impact of Nabilone on MS-related pain [75]. In 2015, a randomized double-blind study looked at the effects of Nabilone as an adjunctive to gabapentin for neuropathic pain caused by relapsing-remitting MS. It found that Nabilone combined with gabapentin is an effective, novel, and well-tolerated treatment for MS patients with neuropathic pain [110].2.3.3. Effects of Nabilone on MS-Related Tremor
Oral Nabilone was shown to have no meaningful effect on tremors in a class III trial [55]. In a systemic evaluation of 630 individuals, Koppel et al. concluded that oral Nabilone does not affect MS-related tremors [40].2.3.4. Effects of Nabilone on MS-Related Bladder Function
Martyn et al. observed significant relief of MS bladder dysfunction symptoms with oral Nabilone for two periods of four weeks in a double-blind crossover, placebo-controlled research [108].2.3.5. Effects of Nabilone on MS-Related Sleep Disorders
There was no clinical investigation that demonstrated the efficacy of Nabilone on the symptoms of MS sleep problems [72].2.3.6. Effects of Nabilone on MS-Related HRQol
In 2015, a randomized controlled trial looked at the efficacy of combining Nabilone and gabapentin for the treatment of MS-related neuropathic pain [110]. This combination was found to improve the quality of life for this group of people [110].2.3.7. Effects of Nabilone on MS Disability and Disability Progression
There was no clinical investigation that indicated how Nabilone affected MS-related disability and progression [72].3. Adverse Effects
Several studies that employed cannabis in MS patients reported the side effects of the drugs [72][82][112][113][72,82,112,113]. The severity of these side effects was typically described as ‘mild’ to ‘moderate’, depending on a variety of factors, such as the dose, type, and quantities of cannabinoids in the products used, and the individuals [72][82][112][113][72,82,112,113]. Dizziness/light headedness (14–59%), gastrointestinal symptoms (diarrhea, nausea, and vomiting) (13–37% in active groups), dry mouth (4–26% in active groups), urinary tract infections (15.4–34%), and other adverse effects, such as fatigue, headache, attention disturbance, and disorientation, are the most commonly reported adverse effects in MS patients treated with cannabinoids [56][57][77][112][56,57,77,112]. A recent study took advantage of The Epistemonikos database, and analyzed 25 systematic reviews on the use of cannabinoids in MS patients; they concluded that the benefit-–risk ratio of using cannabinoids in these patients is unfavorable, because there is a high level of evidence about the lack of benefits, and adverse effects are common [114]. The majority of assessments of these studies found no evidence that any of these side effects limit clinical use [72].3.1. Nabiximols
In a class I research, 268 (46.9%) of 572 patients treated with Nabiximols reported at least one side effect, the most prevalent of which were dizziness and weariness [77]. The tolerability of Nabiximols was assessed in 325 patients in the MOVE 2 research, with dizziness, fatigue, drowsiness, nausea, and dry mouth reported in 16.6% of patients (54 out of 325 individuals) over three months [93]. The majority of these side effects (47 patients) were modest; however, there were significant side effects associated with Nabiximols, including despair, reduced walking ability, muscle spasms, and urinary tract infection [93]. In 11.4% of cases, the medicine was discontinued (37 subjects who have experienced adverse effects during the study period). The study classified it as well-tolerated [113]. Nabiximols were found to worsen balance control in MS patients in another study [115], particularly in multitasking conditions; however, a recent study found that Nabiximols had a short-term favorable effect on balance and walking abilities in MS patients [116]. In a recent cohort trial with 396 MS patients who were administered Nabiximols, 63 patients dropped out due to tolerance and safety concerns. The most commonly reported side effects were drowsiness (5.2%), vertigo/dizziness (4.8%), and weariness (3.8%). Despite this, no major side effects were noted during the titration and treatment stages of the trial [117].3.2. Oral Cannabinoids and THC
The majority of the side effects were mild to moderate and dose-dependent, according to a review report that covered multiple clinical trials of CBD/THC in MS patients [46]. They stated that at least 10 mg of THC was required to treat spasticity; nevertheless, deleterious effects were identified at doses of 15 mg and higher [46]. While some studies have found that combining oral THC and CBD causes significantly more negative effects [63][97], others have found that the combination of THC and CBD causes fewer negative effects [57][118]. A total of 144 MS patients were treated with oral THC and CBD (titrated to a maximum dose of 25 mg THC daily) in a class I research, while 135 were given a placebo [112]. Adverse effects caused 30 out of 144 patients in the control group to quit out, while adverse effects caused 9 out of 135 patients in the placebo group to drop out [112]. Dizziness, dry mouth, weariness, urinary tract infection, asthenia, and headache were the most common reported adverse effects in the cannabis extract group, and they were all graded as mild to moderate [112]. In a meta-analysis assessment of cannabinoids’ tolerability in MS patients, the active treatment groups with dronabinol and cannabinoids, but not Nabilone, had a greater probability of withdrawal from the studies due to unpleasant effects [119]. Dizziness, dry mouth, and weariness were the most commonly reported side symptoms [119].References
- Trapp, D.B.; Nave, K.-A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269.
- Schwab, N.; Schneider-Hohendorf, T.; Wiendl, H. Therapeutic uses of anti-α4-integrin (anti-VLA-4) antibodies in multiple sclerosis. Int. Immunol. 2015, 27, 47–53.
- Rice, G.P.; Incorvaia, B.; Munari, L.M.; Ebers, G.; Polman, C.; D’Amico, R.; Parmelli, E.; Filippini, G. Interferon in relapsing-remitting multiple sclerosis. Cochrane Database Syst. Rev. 2001, 2001, CD002002.
- Kraft, G.H.; Freal, J.E.; Coryell, J.K. Disability, disease duration, and rehabilitation service needs in multiple sclerosis: Patient perspectives. Arch. Phys. Med. Rehabil. 1986, 67, 164–168.
- Breijyeh, Z.; Jubeh, B.; Bufo, S.; Karaman, R.; Scrano, L. Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses. Toxins 2021, 13, 117.
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315
- Urits, I.; Borchart, M.; Hasegawa, M.; Kochanski, J.; Orhurhu, V.; Viswanath, O. An update of current cannabis-based pharmaceuticals in pain medicine. Pain Ther. 2019, 8, 41–51
- Brunt, T.M.; van Genugten, M.; Höner-Snoeken, K.; van de Velde, M.J.; Niesink, R.J. Therapeutic satisfaction and subjective effects of different strains of pharmaceutical-grade cannabis. J. Clin. Psychopharmacol. 2014, 34, 344–349.
- Kumar, R.; Chambers, W.; Pertwee, R. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia 2001, 56, 1059–1068.
- Hillig, K.W.; Mahlberg, P.G. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am. J. Bot. 2004, 91, 966–975.
- Pearce, D.D.; Mitsouras, K.; Irizarry, K.J. Discriminating the effects of Cannabis sativa and Cannabis indica: A web survey of medical cannabis users. J. Altern. Complementary Med. 2014, 20, 787–791.
- Rudroff, T.; Honce, J.M. Cannabis and multiple sclerosis—The way forward. Front. Neurol. 2017, 8, 299.
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19.
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202.
- Pertwee, R. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997, 74, 129–180.
- Ingram, G.; Pearson, O.R. Cannabis and multiple sclerosis. Pract. Neurol. 2019, 19, 310–315.
- Pertwee, R.G. Cannabinoid receptors, and pain. Prog. Neurobiol. 2001, 63, 569–611.
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364.
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Phytocannabinoids 2017, 103, 1–36.
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 1–13.
- Ożarowski, M.; Karpiński, T.; Zielińska, A.; Souto, E.; Wielgus, K. Cannabidiol in neurological and neoplastic diseases: Latest developments on the molecular mechanism of action. Int. J. Mol. Sci. 2021, 22, 4294.
- Fischedick, J.T. Identification of terpenoid chemotypes among high (−)-trans-Δ9-tetrahydrocannabinol-producing Cannabis sativa L. cultivars. Cannabis Cannabinoid Res. 2017, 2, 34–47.
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ guide to cannabidiol and hemp oils. Mayo Clin. Proc. 2019, 94, 1840–1851.
- Pertwee, R.G. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009, 156, 397–411.
- Kreitzer, A.C.; Regehr, W.G. Retrograde signaling by endocannabinoids. Curr. Opin. Neurobiol. 2002, 12, 324–330.
- Alger, B.E. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog. Neurobiol. 2002, 68, 247–286.
- Rudroff, T.; Sosnoff, J. Cannabidiol to improve mobility in people with multiple sclerosis. Front. Neurol. 2018, 9, 183.
- Zwibel, H.L. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv. Ther. 2009, 26, 1043–1057.
- Jensen, C.J.; Stankovich, J.; Van Der Walt, A.; Bahlo, M.; Taylor, B.V.; van der Mei, I.; Foote, S.; Kilpatrick, T.; Johnson, L.J.; Wilkins, E.; et al. Multiple sclerosis susceptibility-associated SNPs do not influence disease severity measures in a cohort of Australian MS patients. PLoS ONE 2010, 5, e10003.
- Briggs, F.B.S.; Shao, X.; A Goldstein, B.; Oksenberg, J.R.; Barcellos, L.F.; De Jager, P.L.; International Multiple Sclerosis Genetics Consortium. Genome-wide association study of severity in multiple sclerosis. Genes Immun. 2011, 12, 615–625.
- Furgiuele, A.; Cosentino, M.; Ferrari, M.; Marino, F. Immunomodulatory potential of cannabidiol in multiple sclerosis: A systematic review. J. Neuroimmune Pharmacol. 2021, 16, 251–269.
- Hugos, C.L.; Cameron, M.H. Assessment and Measurement of Spasticity in MS: State of the Evidence. Curr. Neurol. Neurosci. Rep. 2019, 19, 1–7.
- Rizzo, M.A.; Hadjimichael, O.C.; Preiningerova, J.L.; Vollmer, T.L. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult. Scler. J. 2004, 10, 589–595.
- Milinis, K.; Tennant, A.; Young, C. Spasticity in multiple sclerosis: Associations with impairments and overall quality of life. Mult. Scler. Relat. Disord. 2016, 5, 34–39.
- Biering-Sørensen, F.; Nielsen, J.B.; Klinge, K. Spasticity-assessment: A review. Spinal Cord 2006, 44, 708–722.
- Haselkorn, J.K.; Richer, C.B.; Welch, D.F.; Herndon, R.M.; Johnson, B.; Little, J.W.; Miller, J.R.; Rosenberg, J.H.; E Seidle, M.; Guidelines, M.S.C.F.C.P. Overview of spasticity management in multiple sclerosis. Evidence-based management strategies for spasticity treatment in multiple sclerosis. J. Spinal Cord Med. 2005, 28, 167–199.
- Flachenecker, P.; Henze, T.; Zettl, U.K. Spasticity in patients with multiple sclerosis–clinical characteristics, treatment and quality of life. Acta Neurol. Scand. 2014, 129, 154–162.
- Lapeyre, E.; Kuks, J.B.; Meijler, W.J. Spasticity: Revisiting the role and the individual value of several pharmacological treatments. NeuroRehabilitation 2010, 27, 193–200.
- Halpern, R.; Gillard, P.; Graham, G.D.; Varon, S.F.; Zorowitz, R.D. Adherence associated with oral medications in the treatment of spasticity. PMR 2013, 5, 747–756.
- Koppel, B.S.; Brust, J.C.; Fife, T.; Bronstein, J.; Youssof, S.; Gronseth, G.; Gloss, D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014, 82, 1556–1563.
- Yadav, V.; Bever, C.; Bowen, J.; Bowling, A.; Weinstock-Guttman, B.; Cameron, M.; Bourdette, D.; Gronseth, G.S.; Narayanaswami, P. Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2014, 82, 1083–1092.
- Chong, M.S.; Wolff, K.; Wise, K.; Tanton, C.; Winstock, A.; Silber, E. Cannabis use in patients with multiple sclerosis. Mult. Scler. J. 2006, 12, 646–651.
- Ware, M.; Adams, H.; Guy, G. The medicinal use of cannabis in the UK: Results of a nationwide survey. Int. J. Clin. Pract. 2005, 59, 291–295.
- Amar, M.B. Cannabinoids in medicine: A review of their therapeutic potential. J. Ethnopharmacol. 2006, 105, 1–25.
- Lakhan, S.E.; Rowland, M. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: A systematic review. BMC Neurol. 2009, 9, 1–6.
- Karst, M.; Wippermann, S.; Ahrens, J.; Karst, M. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs 2010, 70, 2409–2438.
- Hadjimichael, O.; Kerns, R.D.; Rizzo, M.A.; Cutter, G.; Vollmer, T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain 2007, 127, 35–41.
- Forbes, A.; While, A.; Mathes, L.; Griffiths, P. Health problems and health-related quality of life in people with multiple sclerosis. Clin. Rehabil. 2006, 20, 67–78.
- O’Connor, A.B.; Schwid, S.R.; Herrmann, D.N.; Markman, J.D.; Dworkin, R.H. Pain associated with multiple sclerosis: Systematic review and proposed classification. PAIN 2008, 137, 96–111.
- Iskedjian, M.; Bereza, B.; Gordon, A.; Piwko, C.; Einarson, T.R. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr. Med. Res. Opin. 2007, 23, 17–24.
- Solaro, C.; Lunardi, G.L.; Mancardi, G.L. Pain and MS. Int. MS J. 2003, 10, 14–19.
- Wilkins, A. Cerebellar dysfunction in multiple sclerosis. Front. Neurol. 2017, 8, 312.
- Swingler, R.; Compston, D. The morbidity of multiple sclerosis. QJM Int. J. Med. 1992, 83, 325–337.
- Mills, R.J.; Yap, L.; Young, C.A. Treatment for ataxia in multiple sclerosis. Cochrane Database Syst. Rev. 2007, 1, CD005029.
- Fox, S.H.; Kellett, M.; Moore, A.P.; Crossman, A.R.; Brotchie, J. Randomised, double-blind, placebo-controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Mov. Disord. 2002, 17, 145–149.
- Wade, D.T.; Makela, P.; Robson, P.; House, H.; Bateman, C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult. Scler. J. 2004, 10, 434–441.
- Zajicek, J.; Fox, P.; Sanders, H.; Wright, D.; Vickery, J.; Nunn, A.; Thompson, A. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomised placebo-controlled trial. Lancet 2003, 362, 1517–1526.
- De Sèze, M.; Ruffion, A.; Denys, P.; Joseph, P.-A.; Perrouin-Verbe, B.; International Francophone Neuro-Urological expert Study Group (GENULF). The neurogenic bladder in multiple sclerosis: Review of the literature and proposal of management guidelines. Mult. Scler. J. 2007, 13, 915–928.
- Gallien, P.; Robineau, S.; Nicolas, B.; Le Bot, M.-P.; Brissot, R.; Verin, M. Vesicourethral Dysfunction and Urodynamic Findings in Multiple Sclerosis: A Study of 149 Cases. J. Urol. 1999, 161, 2032–2033.
- Giannantoni, A.; Scivoletto, G.; Di Stasi, S.M.; Grasso, M.G.; Vespasiani, G.; Castellano, V. Urological dysfunctions and upper urinary tract involvement in multiple sclerosis patients. Neurourol. Urodyn. Off. J. Int. Cont. Soc. 1998, 17, 89–98.
- Koldewijn, E.L.; Hommes, O.R.; Lemmens, W.A.J.G.; Debruyne, F.M.J.; Van Kerrebroeck, P.E.V. Relationship between lower urinary tract abnormalities and disease-related parameters in multiple sclerosis. J. Urol. 1995, 154, 169–173.
- Andersson, K.-E. Current and future drugs for treatment of MS-associated bladder dysfunction. Ann. Phys. Rehabil. Med. 2014, 57, 321–328
- Zhornitsky, S.; Potvin, S. Cannabidiol in humans—The quest for therapeutic targets. Pharmaceuticals 2012, 5, 529–552.
- Merlino, G.; Fratticci, L.; Lenchig, C.; Valente, M.; Cargnelutti, D.; Picello, M.; Serafini, A.; Dolso, P.; Gigli, G. Prevalence of ‘poor sleep’among patients with multiple sclerosis: An independent predictor of mental and physical status. Sleep Med. 2009, 10, 26–34.
- Brass, S.D.; Duquette, P.; Proulx-Therrien, J.; Auerbach, S. Sleep disorders in patients with multiple sclerosis. Sleep Med. Rev. 2010, 14, 121–129.
- Crayton, H.; Heyman, R.A.; Rossman, H.S. A multimodal approach to managing the symptoms of multiple sclerosis. Neurology 2004, 63, S12–S18.
- Attarian, H. Importance of sleep in the quality of life of multiple sclerosis patients: A long under-recognized issue. Sleep Med. 2008, 10, 7–8. [Google Scholar] [CrossRef]
- Kaminska, M.; Kimoff, R.J.; Benedetti, A.; Robinson, A.; Bar-Or, A.; Lapierre, Y.; Schwartzman, K.; A Trojan, D. Obstructive sleep apnea is associated with fatigue in multiple sclerosis. Mult. Scler. J. 2012, 18, 1159–1169.
- Group, W. The World Health Organization quality of life assessment: Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409.
- Amtmann, D.; Bamer, A.M.; Kim, J.; Chung, H.; Salem, R. People with multiple sclerosis report significantly worse symptoms and health related quality of life than the US general population as measured by PROMIS and NeuroQoL outcome measures. Disabil. Health J. 2018, 11, 99–107.
- McCabe, M.P.; McKern, S. Quality of life and multiple sclerosis: Comparison between people with multiple sclerosis and people from the general population. J. Clin. Psychol. Med. Settings 2002, 9, 287–295.
- Nielsen, S.; Germanos, R.; Weier, M.; Pollard, J.; Degenhardt, L.; Hall, W.; Buckley, N.; Farrell, M. The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: A systematic review of reviews. Curr. Neurol. Neurosci. Rep. 2018, 18, 1–12.
- Gunnarsson, M.; Malmeström, C.; Axelsson, M.; Sundström, P.; Dahle, C.; Vrethem, M.; Olsson, T.; Piehl, F.; Norgren, N.; Rosengren, L.; et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol. 2011, 69, 83–89.
- Marta, M.; Giovannoni, G. Disease modifying drugs in multiple sclerosis: Mechanisms of action and new drugs in the horizon. CNS Neurol. Disord. Drug Targets 2012, 11, 610–623.
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015, 313, 2456–2473.
- Vermersch, P. Sativex®(tetrahydrocannabinol+ cannabidiol), an endocannabinoid system modulator: Basic features and main clinical data. Expert Rev. Neurother. 2011, 11, 15–19.
- Novotna, A.; Mares, J.; Ratcliffe, S.; Novakova, I.; Vachova, M.; Zapletalova, O.; Gasperini, C.; Pozzilli, C.; Cefaro, L.; Comi, G.; et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols*(Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur. J. Neurol. 2011, 18, 1122–1131.
- Perez, J. Combined cannabinoid therapy via an oromucosal spray. Drugs Today 2006, 42, 495–503.
- Collin, C.; Ehler, E.; Waberzinek, G.; Alsindi, Z.; Davies, P.; Powell, K.; Notcutt, W.; O’Leary, C.; Ratcliffe, S.; Nováková, I.; et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol. Res. 2010, 32, 451–459.
- Langford, R.M.; Mares, J.; Novotna, A.; Vachova, M.; Novakova, I.; Notcutt, W.; Ratcliffe, S. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J. Neurol. 2013, 260, 984–997.
- Leocani, L.; Nuara, A.; Houdayer, E. Effect of THC-CBD oromucosal spray (Sativex) on measures of spasticity in multiple sclerosis: A double-blind, placebo-controlled, crossover study. In Proceedings of the Joint Americas Committee for Treatment and Research in Multiple Sclerosis ACTRIMS—European Committee for Treatment and Research in Multiple Sclerosis ECTRIMS Meeting, Boston, MA, USA, 10–13 September 2014.
- Wade, D.T.; Makela, P.M.; House, H.; Bateman, C.; Robson, P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult. Scler. J. 2006, 12, 639–645.
- Collin, C.; Davies, P.; Mutiboko, I.K.; Ratcliffe, S.; for the Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur. J. Neurol. 2007, 14, 290–296.
- Markovà, J.; Essner, U.; Akmaz, B.; Marinelli, M.; Trompke, C.; Lentschat, A.; Vila, C. Sativex® as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: A double-blind, placebo-controlled randomised clinical trial. Int. J. Neurosci. 2019, 129, 119–128.
- Meuth, S.G.; Henze, T.; Essner, U.; Trompke, C.; Silván, C.V. Tetrahydrocannabinol and cannabidiol oromucosal spray in resistant multiple sclerosis spasticity: Consistency of response across subgroups from the savant randomized clinical trial. Int. J. Neurosci. 2020, 130, 1199–1205.
- Potter, D.J. A review of the cultivation and processing of cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test. Anal. 2014, 6, 31–38.
- Rice, J.; Cameron, M. Cannabinoids for treatment of MS symptoms: State of the evidence. Curr. Neurol. Neurosci. Rep. 2018, 18, 1–10.
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005, 65, 812–819.
- Dykukha, I.; Malessa, R.; Essner, U.; Überall, M.A. Nabiximols in chronic neuropathic pain: A meta-analysis of randomized placebo-controlled trials. Pain Med. 2021, 22, 861–874.
- Kavia, R.; De Ridder, D.; Constantinescu, C.; Stott, C.; Fowler, C.; Constantinescu, C. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult. Scler. J. 2010, 16, 1349–1359.
- Maniscalco, G.T.; Aponte, R.; Bruzzese, D.; Guarcello, G.; Manzo, V.; Napolitano, M.; Moreggia, O.; Chiariello, F.; Florio, C. THC/CBD oromucosal spray in patients with multiple sclerosis overactive bladder: A pilot prospective study. Neurol. Sci. 2018, 39, 97–102.
- Russo, E.B.; Guy, G.W.; Robson, P.J. Cannabis, pain, and sleep: Lessons from therapeutic clinical trials of Sativex®, a cannabis-based medicine. Chem. Biodivers. 2007, 4, 1729–1743.
- Flachenecker, P.; Henze, T.; Zettl, U.K. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice-results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur. Neurol. 2014, 71, 271–279.
- Flachenecker, P.; Henze, T.; Zettl, U.K. Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur. Neurol. 2014, 72, 95–102.
- Gado, F.; Digiacomo, M.; Macchia, M.; Bertini, S.; Manera, C. Traditional uses of cannabinoids and new perspectives in the treatment of multiple sclerosis. Medicines 2018, 5, 91.
- Ball, S.; Vickery, J.; Hobart, J.; Wright, D.; Green, C.; Shearer, J.; Nunn, A.; Cano, M.G.; MacManus, D.; Miller, D.; et al. The CUPID trial: A randomised double-blind placebo-controlled parallel-group multi-centre trial of cannabinoids to slow progression in multiple sclerosis. Health Technol. Assess. 2015, 19, 1–187.
- Killestein, J.; Hoogervorst, E.L.; Reif, M.; Kalkers, N.F.; Van Loenen, A.C.; Staats, P.G.; Gorter, R.W.; Uitdehaag, B.M.; Polman, C.H. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002, 58, 1404–1407.
- Clifford, D.B. Tetrahydrocannabinol for tremor in multiple sclerosis. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1983, 13, 669–671.
- Killestein, J. Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. J. Neuroimmunol. 2003, 137, 140–143.
- Svendsen, K.B.; Jensen, T.S.; Bach, F.W. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004, 329, 253.
- Zajicek, J.P. Cannabinoids in multiple sclerosis (CAMS) study: Safety and efficacy data for 12 months follow up. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1664–1669.
- Petro, D.J.; Ellenberger, C.J. Treatment of Human Spasticity with Δ9-Tetrahydrocannabinol. J. Clin. Pharmacol. 1981, 21, 413S–416S.
- Ungerleider, J.T.; Andyrsiak, T.; Fairbanks, L.; Ellison, G.W.; Myers, L.W. Delta-9-THC in the treatment of spasticity associated with multiple sclerosis. Adv. Alcohol Subst. Abus. 1987, 7, 39–50.
- Freeman, R.M.; Adekanmi, O.; Waterfield, M.R.; Waterfield, A.E.; Wright, D.; Zajicek, J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: A multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int. Urogynecol. J. 2006, 17, 636–641.
- Zajicek, J.; Ball, S.; Wright, D.; Vickery, J.; Nunn, A.; Miller, D.; Cano, M.G.; McManus, D.; Mallik, S.; Hobart, J. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): A randomised, placebo-controlled trial. Lancet Neurol. 2013, 12, 857–865. [Google Scholar] [CrossRef]
- Schimrigk, S.; Marziniak, M.; Neubauer, C.; Kugler, E.M.; Werner, G.; Abramov-Sommariva, D. Dronabinol is a safe long-term treatment option for neuropathic pain patients. Eur. Neurol. 2017, 78, 320–329
- Deutsch, S.I.; Rosse, R.B.; Connor, J.M.; Burket, J.A.; Murphy, M.E.; Fox, F.J. Current status of cannabis treatment of multiple sclerosis with an illustrative case presentation of a patient with MS, complex vocal tics, paroxysmal dystonia, and marijuana dependence treated with dronabinol. CNS Spectr. Int. J. Neuropsychiatr. Med. 2008, 13, 393–403.
- Martyn, C.; Illis, L.; Thom, J. Nabilone in the treatment of multiple sclerosis. Lancet 1995, 345, 579.
- Wissel, J.; Haydn, T.; Müller, J.; Brenneis, C.; Berger, T.; Poewe, W.; Schelosky, L.D. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain. J. Neurol. 2006, 253, 1337–1341.
- Turcotte, D.; Doupe, M.; Torabi, M.; Gomori, A.; Ethans, K.; Esfahani, F.; Galloway, K.; Namaka, M. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: A randomized controlled trial. Pain Med. 2015, 16, 149–159
- Centonze, D.; Mori, F.; Koch, G.; Buttari, F.; Codecà, C.; Rossi, S.; Cencioni, M.T.; Bari, M.; Fiore, S.; Bernardi, G.; et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol. Sci. 2009, 30, 531–534.
- Zajicek, J.P.; Hobart, J.C.; Slade, A.; Barnes, D.; Mattison, P.G.; on behalf of the MUSEC Research Group. Multiple sclerosis and extract of cannabis: Results of the MUSEC trial. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1125–1132.
- Gustavsen, S.; Søndergaard, H.; Linnet, K.; Thomsen, R.; Rasmussen, B.; Sorensen, P.; Sellebjerg, F.; Oturai, A. Safety and efficacy of low-dose medical cannabis oils in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 48, 102708.
- Meza, R.; Peña, J.; García, K.; Corsi, O.; Rada, G. Are cannabinoids effective in multiple sclerosis? Medwave 2017, 17, e6865.
- Castelli, L.; Prosperini, L.; Pozzilli, C. Balance worsening associated with nabiximols in multiple sclerosis. Mult. Scler. J. 2019, 25, 113–117.
- De Blasiis, P.; Siani, M.F.; Fullin, A.; Sansone, M.; Melone, M.A.B.; Sampaolo, S.; Signoriello, E.; Lus, G. Short and long term effects of Nabiximols on balance and walking assessed by 3D-gait analysis in people with Multiple Sclerosis and spasticity. Mult. Scler. Relat. Disord. 2021, 51, 102805.
- Carotenuto, A.; Costabile, T.; De Lucia, M.; Moccia, M.; Falco, F.; Petruzzo, M.; De Angelis, M.; Russo, C.V.; Saccà, F.; Lanzillo, R.; et al. Predictors of Nabiximols (Sativex®) discontinuation over long-term follow-up: A real-life study. J. Neurol. 2020, 267, 1737–1743.
- Wade, D.T.; Robson, P.; House, H.; Makela, P.; Aram, J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. J. Clin. Rehab. 2003, 17, 21–29.
- Torres-Moreno, M.C.; Papaseit, E.; Torrens, M.; Farré, M. Assessment of efficacy and tolerability of medicinal cannabinoids in patients with multiple sclerosis: A systematic review and meta-analysis. JAMA Netw. Open 2018, 1, e183485.
More
