Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sharanabasava Ganachari and Version 2 by Conner Chen.
Nanotechnology is an innovative developing science in the area of civil engineering that is still in its early stages. Extensive attempts were made to integrate nanomaterials into traditional cementitious pastes to improve performance. Previous research has shown that incorporating nanomaterial into geopolymer concrete (GPC) enhances the geopolymerization and microstructure [6]. As a result of their exceptional and intelligent characteristics, cementitious materials mixed with nanomaterials led to high-performance structural components for various purposes in the building sector.
- nanomaterials
- geopolymer concrete
1. Introduction
Concrete is the second most often used substance on the planet after water, and it necessitates vast volumes of Portland cement (OPC). The manufacturing of OPC not only requires a large quantity of natural resources, such as limestone and fossil fuels, but also emits around 0.8 tonnes of CO2 for every tonne of cement clinker produced [1]. The cement sector ranks second in terms of greenhouse gas emissions, particularly CO2. CO2 emissions are predicted to reach a peak of 100% by 2020, compared to the present levels of output. The annual global cement output will be around 4.38 billion tonnes by 2050, with a 5% rise every year [2]. As a result, finding an alternate material to the current most costly, most resource, and energy demanding Portland cement is unavoidable. Geopolymers are gaining popularity as an alternative to Portland cement due to their reduced carbon footprint [3]. Geopolymers are formed by a polymerization process that involves a chemical reaction of alumina-silicate minerals in the presence of an alkaline media, resulting in the development of a three-dimensional polymeric chain. Geopolymers offer various benefits as binders, including strong mechanical strength, greater chemical resistance to corrosive environments, reduced creep and shrinkage, and resilience to high raised temperatures [4][5][4,5].
Nanotechnology is an innovative developing science in the area of civil engineering that is still in its early stages. Extensive attempts were made to integrate nanomaterials into traditional cementitious pastes to improve performance. Previous research has shown that incorporating nanomaterial into geopolymer concrete (GPC) enhances the geopolymerization and microstructure [6]. As a result of their exceptional and intelligent characteristics, cementitious materials mixed with nanomaterials led to high-performance structural components for various purposes in the building sector. In current years, multidisciplinary study emphasis has shifted to nanomaterials in construction applications [7]. The sol–gel technique to NS-solution may minimize the agglomeration of nanoparticles usually found in dry mixes, and the geopolymers (GP)s produced using this method demonstrated decreased micro-porosity and improved fire resistance performance [7][8][9][7,8,9]. The nanomaterials are extremely strong and have excellent physical and chemical characteristics. Several researchers have discovered several ways for producing nanomaterials. When nanomaterials are added to the polymer matrix, accessible alkaline solution is immobilized. Nanoparticles substantiate the gaps between binder grains, known as the filler effect [10]. The nanoparticles participate in pozzolanic processes, resulting in calcium silicate hydrates (C–S–H and N–A–S–H). Nanoparticles strengthen the connection among binder and aggregates at the interfacial transition zone [11]; ultimately, enhancing the bond strength properties of the mix. Nanoparticles enhance the flexural and tensile strength shear, providing crack arrest and a better interlocking bond among slip planes. The inclusion of ultrafine particles into Portland-cement paste and mortar produces properties that differ from ordinary materials [12]. The presence of nanoparticles in geopolymer concrete (GPC) leads to nanosized porosity at the interfacial transition zone (ITZ) among aggregate and cement matrices that significantly impacts performance. The nanosized particles significantly impact the macro and microstructure of GPC. Because of their high surface area to volume ratio, nanomaterials can operate as a pozzolanic and a nano-filler by filling the gaps among particles in C–S–H gel [13]. The large surface area of nanoparticles is critical for hydration. The nanomaterial improves early hydration and speeds up the creation of the hydration process [14][15][14,15]. Nanotechnology is an emerging discipline that aims to create new materials with improved characteristics and performance from a construction point of view. The main objective of this review is to study the different types of nanomaterials existing so far, and their performances in the strength development of geopolymer concrete (GPC). The synthesis of nanomaterials and their behavior to enhance compressive, tensile, and flexural strength is studied using different plots. In the following sections, attempts have been made to study the impact of nanomaterials on the matrix due to their partial replacement of industrial waste.
2. Nanofabrication Technique
Environmentally friendly, nontoxic, and safe chemicals are used in the “green synthesis” of nanoparticles. Nanoparticles made using biological or green technology have many properties, including high stability and enormous diameters [16]. Nanoparticles may be made using various methods, including chemical, physical, and biological methods. Chemical and physical techniques utilize many radiations, specific reductants, and potentially hazardous substances to the environment and human health. Nano concrete is defined as a nanomaterial or concrete containing nanomaterials with a particle size of less than 500 nm [15][16][15,16]. The addition of nanoparticles to concrete was thought to increase the strength of ordinary concrete. Nanoparticles improve the bulk characteristics of concrete, commonly known as the packing model structure. By refining the intersectional zone in cement and generating higher density concrete, ultrafine or nanoparticles may produce a fantastic filler effect. Their manipulation or change in the cement matrix system happens due to their function as an excellent filler, resulting in a new nanoscale structure [17][18][19][17,18,19]. The concrete microstructure removes micro-voids, porosity, and degradation due to the alkali–silica reaction. Nanomaterials then begin to emerge as a new binding agent smaller than cement particles. This enhances the hydration gel structure, resulting in a clean and stable hydration structure [20].
In addition, a novel concrete called nano concrete has been created using a mix of filler and a different chemical reaction in the hydration system. Nano concrete is durable and has improved performance [21]. Nanotechnology has been implemented in concrete since the early millennium. The use of silica fumes in traditional mix formulas improves the durability and strength of the material. Since then, nanotechnology has been developing to create a viable alternative to silica fume [22].
A common nano substance that replicates the effect of silica fume has been created using the nanomanufacturing idea. Nano silica is a relatively recent nanotechnology that has been utilized as a replacement for silica fume [23]. Many nano-based particles have been created for use in concrete since the discovery of nano silica. Nanomaterials utilized in nano concrete include nano alumina, titanium oxide, carbon nanotubes, and polycarboxylates. To detail an understanding of the effect of nanomaterials, one needs to study the synthesis of nanomaterials, their structural performances such as setting time, flow, compressive, tensile, flexural strength, water absorption, and bulk density and application of nanomaterials in practice.
3. Production of Nanomaterials
Since the advent of nanotechnology in the late 1960s, the notion and concept of manufacturing nanomaterials have evolved. Compared to micro-based materials, the nano size of nanoparticles has a more substantial influence on filler [24]. It is considered successful when nanoparticle production impacts parent material purity or basic chemical composition. The first is a top-to-bottom method, and the second is a bottom-to-top approach. The two techniques were chosen based on their applicability, affordability, and knowledge of nano behavior [10][25][10,25]. Milling is a method used in the top-to-down approach. The milling approach was chosen due to its availability and feasibility since any change may be made directly without the need for any chemical or electrical equipment. The top-down technique states that enormous structures can be reduced in size to nanoscale while retaining their original characteristics or chemical composition with no change in atomic-level control [23][26][23,26]. In other words, mechanical attrition and etching methods break down bulk materials into nanoparticles. This approach is often used in large enterprises. Nanoparticles are generated in large quantities using the milling process because it is cost-effective and straightforward to maintain due to more mechanical instruments and more negligible chemical modification [27]. Another phrase for the top-down technique is the modern method in nanomanufacturing. However, with a top-to-bottom method, the consistency and quality of the end output are uneven.
Although there are drawbacks to the top-down method, the quality of nanoparticles may be enhanced by modifying milling procedures such as the number of balls used, the kind of balls used, the speed of milling, and the type of jar used. High energy ball millings are frequently used to produce nanomaterials, nano grains, nano alloys, nanocomposites, and nano quasi-crystalline materials [28][29][28,29]. John Benjamin was the inventor of the milling process for generating oxide particles in nickel superalloys (1970). His first milling effort was when he changed and strengthened an alloy component for high-temperature construction. Plastic deformation, cold welding, and fracture are variables that influence the deformation and transformation of materials during milling [29].
Milling is the process of mixing numerous particles or materials and converting them into new phases of material composition, in addition to breaking them into smaller bits. The final output of the milling process is usually flaked in shape. However, refining can be performed based on the ball selection and milling type. However, most nanomaterials utilized in concrete, such as nano silica, nano alumina, and nano clay, are generated from the bottom up [2][30][2,30]. A bottom-to-top method is used when materials are created from atoms or molecular components by assembly or self-assembly. It is also referred to as molecular nanotechnology or the molecular manufacturing process, and it has more indirect uses such as synthesis and chemical formulation. The size and form of nanoparticles generated by a bottom–up method may be defined and controlled using a chemical synthesis process. The difference between this top-down strategy is that the bottom–up approach produces more homogeneous and tidy nanoparticle structures. In other words, because the atoms or molecules are precisely organized or crystalline, bottom to up also generates new nanocrystals [31][32][31,32]. Electronic conductivity, optical absorption, and chemical reactivity are the approaches used. The bottom to up method allows for size reduction and tidy surface atom creation, which results in a significant shift in surface energies and morphologies. Typically, using this approach, nanomaterials may be broadly adapted in the circumstances such as enhancing catalytic capability, detecting wave ability, and new pigments and paint with self-healing and cleaning characteristics, and so on. However, the drawback of the bottom to up method is its high operational expense, the need for knowledge in chemical applications, and its restricted applicability since it is intended primarily for laboratory use [8][24][33][8,24,33]. However, nanoparticles produced using this process are ideal for advanced applications such as electrical components and biology. Finally, in addition to assessing the impacts of nanomaterials, distinct techniques to produce nanomaterials for use in ultra-high-performance concrete were described [17][33][17,33].
Sol–Gel Technique
Creating nano metal and metal oxide (MO) materials necessitates a high level of synthetic inventiveness. Although a balanced synthesis technique can be developed, there is a constant element of serendipity in nanomaterials. In the last few decades, a wide range of nanomaterials has been created using this traditional method [1][34][1,34]. The ceramic technique entails combining and crushing component granules, oxidizing, carbonating, and heating other chemicals at high temperatures. When necessary, transitional grinding is used. Precipitation and precursor methods, ion exchange and sol–gel techniques (Figure 1), topochemical methods, and others are all effective chemical methods for synthesizing oxides.
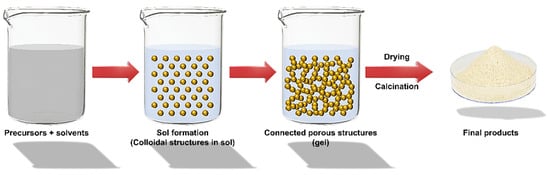
Figure 1.
Scheme of Sol–gel synthesis.
The relevance of the sol–gel method and chemistry in materials production has been steadily increasing [35]. The chemical reactions of volatile metal precursors, generally alkoxides in conjunction with alcoholic solution, sequent inside the conforming hydroxide, are commonly employed to create metal oxide. Hydroxide molecules condense and are linked to eliminating water, which helps organize a robust base network. Gelation occurs when hydroxide molecules create a network-like structure, resulting in a thick porous gel. A chemical compound shows the gel with a three-dimensional skeleton close to the reticular pore. The evaporation of solvents during the drying of the gel results in the creation of ultrafine metal OH powder, which contributes to the conforming ultrafine powder of the MO [36][37][36,37].
Because this technique starts with a nano-sized material and reacts on a nanoscale scale, nanometer material creation is a virtual certainty. Because of the existence of metal-oxygen linkages matching to alkoxide precursors, sol–gel methods have proved appropriate for generating only MO, and the required gels are essentially metal hydroxides or oxides [38]. A sol–gel processing approach was created in a recent study to generate a vast range of ceramic materials, including Al2O3, Fe2O3, SiO2, TiO2, and others. However, several investigations have demonstrated that non-oxide powders may be made from organometallic precursors other than alkoxides via sol–gel processes. For manufacturing nano-powders of MO ceramics, sol–gel techniques provide several advantages over other approaches [39][40][41][39,40,41]. As a result, the invention’s repeatability results from the stable molecular amalgamation of the starting components. The sol–gel method also has a strong potential for generating employment in sectors at more excellent rates. The scaling up of several industrially relevant MO nanoparticles has been successfully developed [42][43][42,43].
4. Structure Interaction of Nanomaterials in GPC
Nanomaterials such as nano-SiO2, CNTs, and nano-TiO2 can positively impact the polymerization processes and the physical structure of N–A–S–H in GPC. Nano-SiO2 can modify the shape of GPC by creating more N–A–S–H gel and fewer ettringite crystals, in addition to being a dense substance [10][25][40][44][10,25,40,44]. Surface energy, morphology, and chemical reactions in GPC can all be affected by the dramatic increase in surface area of nano-SiO2. Nano-SiO2 helps form tiny-size crystals and clusters of N–A–S–H during the pozzolanic reaction due to its small particle size and high surface fineness [45]. The relatively small particle size of nanoparticles compared to traditional concrete cementing ingredients may allow for more excellent void filling and other beneficial filler effects; the filler effects generate a geopolymer microstructure with enhanced density and reduced density porosity [46][47][46,47].
Nanoparticles arrange themselves in an efficient close-packed form. A dense collection of congruent spheres in an endless and regular configuration is known as close-packing comparable spheres in geometry. Nanomaterials can function as fillers to create a dense and less permeable mortar microstructure; they can also operate as nuclei to aid the development of polymerization products and, therefore, encourage the construction of high-density sodium alumina silicates hydrate (N–A–S–H) structures, according to the researchers [15][31][48][15,31,48]. Nano-SiO2 in concrete can make the microstructure more homogeneous and compact than regular cement. Nano-SiO2 improves concrete microstructure in four ways: (a) as a nucleus, (b) by producing improved calcium silicate hydrate (C–S–H), (c) via regulated crystallization, and (d) by filling micro-voids. When nano-TiO2 is combined with cement, the porosity of the concrete is reduced as well. Nano-TiO2 can change the pore size distribution and reduce overall pore volume by progressively filling up the pore space surrounding them as hydration continues [6][49][6,49]. Concrete having nano-TiO2 has a finer pore structure than concrete with nano-SiO2. As a result, geopolymer concrete having nano-TiO2 may be more resistant to the entry of harmful chemicals than GPC containing nano-SiO2.
