Hepatitis Delta Virus (HDV) is the cause of hepatitis D, a relatively rare but aggressive form of viral hepatitis developing in patients co-infected with hepatitis B virus (HBV). HDV infection is associated with HBV infection since the defective HDV needs HBV to infect and replicate in the liver. Even if not a frequent cause of chronic liver disease, HDV infection is responsible for an aggressive progression of hepatitis towards advanced liver disease.
1. Introduction
Probably vastly underestimated in HBsAg carriers, the lack of standardized virological methods to diagnose and monitor the infection and effective therapy to treat the liver disease have conditioned the progress in research and clinical management. However, after almost half a century from the initial discovery of the Delta agent, recent developments in understanding and targeting the therapeutic efforts have opened new hope for patients with hepatitis D. In Researchersthis narrative review, we try to summarize and focus on the new opportunities in the management of Delta hepatitis.
2. When All of This Started
Hepatitis Delta virus (HDV) is a defective RNA viral agent associated with HBV infection. In 1977, in Turin, Italy, Mario Rizzetto first detected by immunofluorescence a new antigen, named Delta, in the liver cell nuclei of patients with HBsAg positive chronic liver disease. Corresponding circulating antibodies were similarly found in the serum of chronic HBsAg carriers, especially those with liver damage
[1].
However, it took a few years to understand that the new antigen was not secreted by HBV but represented an independent agent transmitted by superinfection or coinfection of HBsAg carriers. The new agent was characterized as a defective RNA pathogen dependent for replication and infection on helper functions provided by HBV. The virion is a 35–37 nm particle, with the Delta antigen and the RNA genome within a coat made by the HBsAg lipoprotein
[2].
The prevalence of HDV infection is limited to individuals with HBV infection, as coinfection with HBV of normal subjects or superinfection of HBsAg carriers. Parenteral transmission is the route of propagating infection from one individual to another
[3].
Since the 1980s, it was clear that HDV infection had high pathogenic potential, inducing hepatitis in all infected subjects. Hepatitis may be acute or chronic, the former more frequently associated with coinfection, and the latter mainly occurring after superinfection.
The HBsAg carrier state represents the ideal background for infection and replication of HDV, and the subsequent chronic hepatitis is generally active and severe, progressing to more advanced liver disease within a few years
[4].
All of this has been known for decades, and
rwe
searchers are still waiting for significant progress in the clinical management of Delta hepatitis.
3. New Drugs for Delta Hepatitis
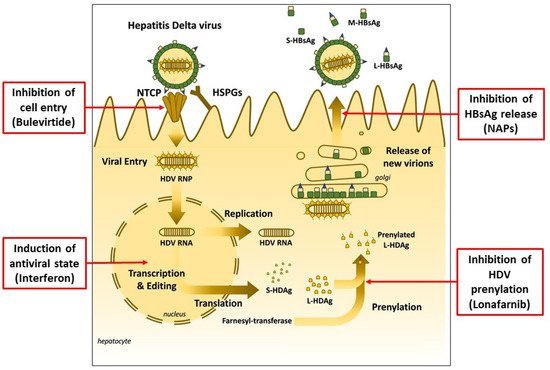
Different approaches have been investigated to interfere and suppress HDV replication during the last years. As a result, three significant therapeutic mechanisms have been identified: (1) inhibition of HDV prenylation, (2) inhibition of HBsAg release and (3) inhibition of cell entry
[5][12] (
Figure 1).
Figure 1. Hepatitis Delta Virus infection and therapeutic targets of currently available and experimental new drugs (adapted from Liver Int. 2021, 41 (Suppl. 1), 30–37, used with permission).
Prenylation involves the covalent addition of a farnesyl or geranylgeranyl isoprenoid molecule to a conserved cysteine residue at or near the C-terminus of a protein. This link promotes membrane interactions with the prenylated protein since the isoprenoid chain is hydrophobic. Lonafarnib, a farnesyltransferase inhibitor, was initially evaluated in a phase 2a study of 14 patients with HDV
[6][13]. Treatment of chronic HDV with oral lonafarnib significantly reduced virus levels, and the decline in virus replication significantly correlated with serum drug levels. In particular, the most effective dose was 200 mg given twice daily. Unfortunately, this dosage was aggravated with significant side effects. For this reason, additional trials were performed using lonafarnib 100 mg twice daily, either ritonavir-boosted or in combination with pegylated interferon. Both strategies were associated with a >2 log drop in HDV viremia, but a significant proportion of patients continued to experience adverse events
[7][14].
Nucleic acid polymers (NAPs) are broad-spectrum antiviral agents whose antiviral activity in hepatitis B virus (HBV) infection is derived from their ability to block the release of the hepatitis B virus surface antigen (HBsAg). This pharmacological activity blocks HBsAg replenishment in the circulation, allowing host-mediated clearance. REP 2139 is a nucleic acid polymer that has been shown to clear HBsAg by blocking the release of subviral particles. This agent was evaluated to treat HDV infection in an uncontrolled phase 2 study
[8][15]. Reported results in this pilot study were encouraging, with HDV suppression rates above 80% during treatment and maintained after treatment in more than 50% of patients. However, additional data to support these promising findings are necessarily needed.
In July 2020, the European Medicines Agency (EMA), Amsterdam, The Netherlands, issued a conditional marketing authorization for a new drug, bulevirtide (previously known as myrcludex-B), with the therapeutic indication for the treatment of chronic hepatitis Delta virus (HDV) infection in HDV-RNA positive adult patients with compensated liver disease. It was the first time a drug was specifically approved to treat Delta hepatitis.
Bulevirtide is an HDV entry inhibitor acting upon the sodium taurocholate co-transporting polypeptide (NTCP), a receptor shared by HBV and HDV, able to block cell entry of HBV and HDV. In an initial clinical study, bulevirtide was administered to 14 individuals, either as monotherapy (2 mg subcutaneously daily) or with pegylated IFN for 24 weeks
[9][16]. Bulevirtide was well tolerated both as monotherapy and in combination with pegylated IFN. After 24 weeks of treatment, HDV-RNA decreased in all patients with chronic hepatitis B and D. Of the 14 patients who received bulevirtide, 13 experienced a >1 log10 reduction in HDV-RNA after 24 weeks of therapy. In addition, two of the seven patients became HDV-RNA negative in the monotherapy arm, compared with five of the seven patients who received combination therapy. However, hepatitis B surface antigen (HBsAg) levels remained unchanged. This initial study was the basis for two following phase 3 studies: Myr204 and Myr301. In the Myr204 study
[10][17], investigators evaluated the safety and efficacy of bulevirtide administered subcutaneously at a dose of 2 or 10 mg daily in combination with pegylated interferon alfa-2a weekly relative to bulevirtide 10 mg monotherapy. Bulevirtide monotherapy and in combination with Peg-IFNα-2a was safe and well-tolerated through 24 weeks of therapy. Combination therapy and bulevirtide monotherapy resulted in high rates of HDV viral decline, well above 70%. In addition, bulevirtide monotherapy resulted in the highest rate of ALT normalization (64%). A combined response (>2 log drop in HDV RNA levels + ALT normalization) was obtained in 24–30% of patients in the combination arms and 50% of patients in the bulevirtide monotherapy arm. In the Myr301
[11][18], investigators evaluated the safety and efficacy of bulevirtide administered subcutaneously for a minimum of 48 weeks, at a dose of 2 or 10 mg daily, compared with no treatment. Patients enrolled to receive the EMA authorized prescription dose of 2 mg daily had a virologic response of 55%, a biochemical response of 53% and a combined response of 37%.
The efficacy of bulevirtide in chronic Delta hepatitis was confirmed in an open-label real-life French study: 2 mg subcutaneously daily induced on treatment virological and combined virological and biochemical response in 79% and 43%, respectively
[12][19]. In addition, despite the EMA authorization being limited to patients with compensated liver disease, real-life experience has confirmed the safety and efficacy of bulevirtide monotherapy even in patients with decompensated liver disease
[13][20]. Finally, and interestingly, the efficacy of bulevirtide does not seem to be influenced by different infecting HDV genotypes
[14][21].