Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Kavindra Kumar Kesari and Version 2 by Camila Xu.
The term “coronaviruses” was coined in 1968. The term was derived from the Greek word κορώνα, meaning crown for the entire group. However, in our century, researchers have encountered highly pathogenic CoVs such as SARS-CoV and MERS-CoV, causing outbreaks that had originally been initiated in China in 2003 and Saudi Arabia in 2012, respectively. The outbreak soon spread to other countries causing horrible morbidity and mortality. COVID-19 is the third CoV outbreak recorded in the history of human beings. This novel strain of coronavirus (SAR-CoV-2) was first detected in Wuhan in 2019, a city in the Hubei province of China, and has now spread to around 200 countries.
- immunotherapy
- coronavirus
- viral mutations
- interferons
1. Introduction
The history of the novel coronavirus goes back to the nineteenth century when it was infecting cats and causing high fever and a swollen belly, making it the first-ever reported case of infection by a coronavirus. At that time, it was infecting other animals too such as pigs and chickens, but the veterinary doctors were unaware. It was only after the discovery of two viruses in the UK and US, possessing crown-like structures causing the common cold in humans, that established the relationship between the viruses infecting both humans and animals, which had similar structures. These viruses were then studied under electron microscopes and it was concluded that they resemble the “solar corona”. The term “coronaviruses” was coined in 1968. The term was derived from the Greek word κορώνα, meaning crown for the entire group. However, in our century, researchers have encountered highly pathogenic CoVs such as SARS-CoV and MERS-CoV, causing outbreaks that had originally been initiated in China in 2003 [1] and Saudi Arabia in 2012, respectively. The outbreak soon spread to other countries causing horrible morbidity and mortality. COVID-19 is the third CoV outbreak recorded in the history of human beings. This novel strain of coronavirus (SAR-CoV-2) was first detected in Wuhan in 2019, a city in the Hubei province of China, and has now spread to around 200 countries. This outbreak was labelled as a global pandemic by the WHO in 2019. Four coronavirus genera (α, β, γ, δ) have been identified so far, with human coronaviruses (HCoVs) detected in the α coronavirus (HCoV-229E and NL63) and β coronavirus (MERS-CoV, SARS-CoV, HCoV-OC43 and HCoV-HKU1) genera [2][3][4][5][6][2,3,4,5,6]. The coronavirus is now becoming dangerous with time. Mortality due to the virus is at its highest peak, which has led the scientific community to gather as much information as possible on the biology of the virus.
The coronavirus family is a family of deadly pathogenic viruses and has persisted for the past 300 million years with the identification of dozens of coronavirus strains so far as mentioned in Figure 1. However, only seven strains are known to infect humans and four strains are known to cause the common cold. The four strains have originated from rodents (OC43 and HKU1) and bats (229E and NL63). Most recently, it has been reported that in China that more than 500 CoVs have been identified in bats [7][8][7,8]. The three most dangerous strains, namely SARS-CoV, MERS-CoV, and SAR-CoV-2, are giving rise to severe associated diseases inflicting damage to the respiratory tract, namely severe acute respiratory syndrome (SARS), the Middle East respiratory syndrome (MERS), and COVID-19, respectively. SARS-CoV-2 has originated from bats owing to around 80% sequence homology between SAR-CoV-2 and SAR-CoV found in bats. Further, the structural analysis reports state that this RNA virus is much larger than its family members with a size of around 125 nm, and possesses the largest genomes (30 Kb in size) having 32–43% GC content [5][9][10][11][5,9,10,11]. The family of coronaviruses consists of enveloped viruses consisting of nucleoprotein (a positive-sense single-stranded RNA) encased within a capsid which is made up of matrix protein. The envelope consists of club-shaped glycoproteins projections. They possess several small open reading frames (ORFs) intertwined between conserved genes (ORF1ab, spike, envelope, membrane and nucleocapsid) located downstream of the nucleocapsid gene across different corona lineages. The coronavirus family has been classified into three groups based on their antigenic similarities. The first two groups constitute the mammalian coronaviruses, whereas the third group constitutes the avian coronaviruses [12][13][14][12,13,14]. Now in the current scenario, the world has witnessed the fastest technical assistance and alternative, provided by bioinformatics (BI) tools and programs in not only identifying the characteristics and probable existence of the deadly coronavirus but also playing an essential role in directing the theranostics approaches against COVID-19 infections. Subsequently, the CADD (Computer-Aided Drug Design) approach has shown remarkable development in the drug repurposing of various existing drugs in the market as well as identifying new antiviral compounds. Additionally, BI tools immensely helped in locating the viral genome data, prediction for protein–peptide, protein–drug, protein–protein docking, identifying the antigenic epitopes and antibody structures along with vaccine development, etc.
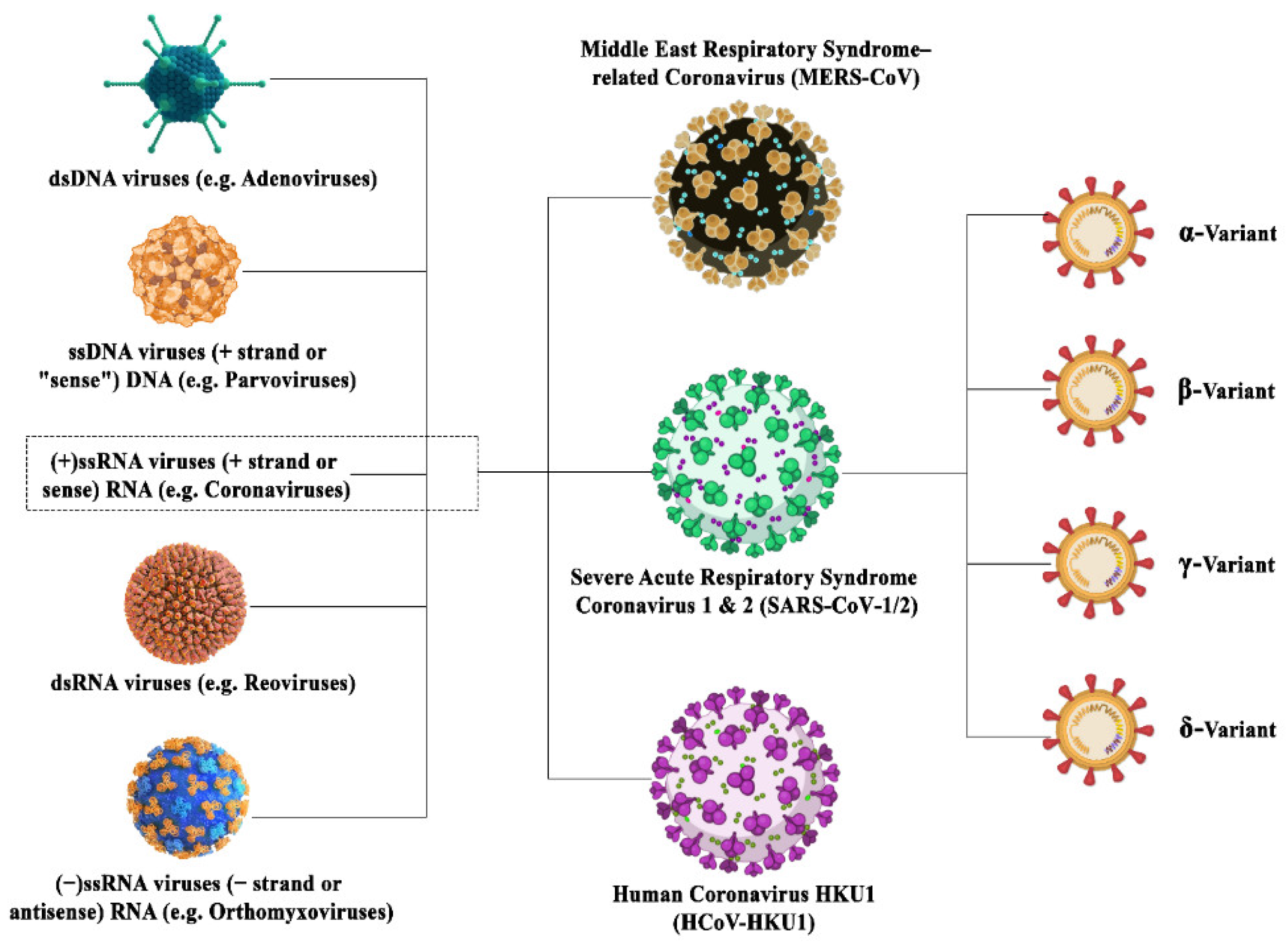
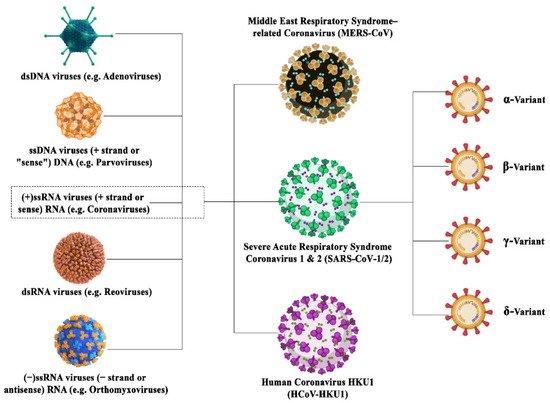
Figure 1. Illustration depicting the family inclusions of different coronavirus variants surged in this century with their subtypes.
Illustration depicting the family inclusions of different coronavirus variants surged in this century with their subtypes.
2. Viral Mutations
Replication of the virus leads to ample mutations in the SARS-CoV-2 genome. A sampling of the genomes of the SARS-CoV-2 virion particles mostly yielded mutations that are neutral or mildly deleterious. However, viral mutations that might confer fitness to the virus occur and may affect the pathogenicity, infectivity, transmissibility and/or antigenicity of the virus [15]. The virus mutated at a rate of approximately two mutations/month from December 2019 to October 2020 [16][17][16,17]. Even though the viral outbreak has been catastrophic, the virus has adopted a milder phenotype from the end of May 2020 [18]. Mutation in the ORF-8 has been shown to have resulted in a less severe viral strain, this region is involved in immune evasion and deletion of this region led to a robust immune response against the variant. This deletion might have been selected as a result of the pressure of the host immune system. This deletion coincides with the one found in the ORF-8 of the SARS-CoV-1 virus [19]. According to the WHO, mutations in the viral genome that affect the efficiency of the host immune system have occurred in several countries worldwide by the end of 2020. The mutations may enhance their ability to evade immune surveillance [20] by antibodies, thereby evading the humoral immune response of the body. These include the B.1.1.7 (α-variant), B.1.351 (β-variant) and B1.1.28 (E484K variant) from the UK, the B.1.351/501.YV2 variant from South Africa, the B.1.1.248/B1.1.28/P1 variant from Brazil and the Cluster 5 variant found in Denmark and Netherlands. Another variant, omicron (BA. 1), was first identified in Botswana and South Africa in late November 2021 [21]. The B.1.351 variant has a mutated receptor-binding domain (RBD), protecting the virus against neutralizing antibodies. This mutation leads to higher transmission rates due to the improved affinity of the RBD towards the ACE-2 receptor. Although no variant has yet been found to confer vaccine resistance, the virus will continue to accumulate more variations under the pressure of vaccination. In addition to the variants already discussed, a few more variants are being thoroughly examined, namely the P.2 variant in Brazil and the CAL.20C variant in California [22][23][24][22,23,24]. At the end of November 2021, the omicron variant of coronavirus was detected with more than 32 mutations in the spike protein [25]. Most recently, another recombinant strain has been identified in the UK which is a recombinant of the omicron/BA.1 and its subvariant BA.2. According to the WHO, it has been named the most transmissible variant so far [26]. The regions of the mutations found in the different variants of SARS-CoV-2 are listed in the Table 1 below.
Table 1.
Regions of mutations in the different SARS-CoV-2 variants.
| Type of Variant | Region of Viral Mutations | Types of Mutations | References |
|---|
- [39,40]. The most popular laboratory-based NAAT is the quantitative reverse transcription-polymerase chain reaction [41][42][41,42]. The samples that are used for diagnosis include sputum, saliva, nasal, pharyngeal and tracheal swabs, broncho-alveolar lavage, pleural effusion fluid, blood, faeces and sometimes urine and semen [6].
| Detected Component |
|---|
- According to the World Health Organization (WHO), the detection of a single RNA sequence of coronavirus by RT-PCR is enough for the confirmation of the disease. This test is used for the real-time qualitative detection of nucleic acids from suspected viral pathogens[43]. In case of detection of SARC-CoV-2, the nucleic acid (RNA) isolated from the specific sample is first reverse transcribed into cDNA and then amplified. During the amplification process, the probe anneals to a specific target sequence located between the forward and reverse primers. During the extension phase of the PCR cycle, the 5′ nuclease activity of Taq polymerase degrades the bound probe, causing the reporter dye to separate from the quencher dye, generating a fluorescent signal. Fluorescence intensity is monitored at each PCR cycle [43]. According to the WHO, based on the first sequences of SARS-CoV-2 made available on the GISAID database on 11 January 2020, primers and probes (nCoV_IP2 and nCoV_IP4) were designed to target the RdRp gene spanning nt 12,621–12,727 and 14,010–14,116 [44]. The current test kits may lead to false-negative results because the detection of COVID-19 in the early stages of the infection is challenging due to the improper isolation of RNA or inadequate methods for detection.
| α-variant | Spike protein | 60–70 del, 145 del, N501Y, A570D, D614G, P681H, T7161, S982A, D1118H | [27] |
| β-variant |
- Chest computerized tomography (CT): CT scans are used for the diagnosis and imaging of viral pneumonia. It has been extensively used for the timely diagnosis and treatment of other coronavirus outbreaks caused by SARS-CoV and MERS-CoV. It is a confirmatory scan that is used to detect any false negatives as robust detection of COVID-19 is imperative for avoiding these false negatives because a CT scan can detect the infection even before the manifestation of symptoms resulting in timely treatment [39].
Table 2.
Diagnostic tests for SARS-CoV-2 detection.
4.1. Molecular Targets for Diagnostic Kits
The disease-specific biomarkers may serve as effective diagnostic tools. As a result, in vitro diagnostic kits are being designed for the early detection of SARS-CoV-2. The most common receptor through which SARS-CoV-2 enters the host system is the angiotensin-converting enzyme receptor (ACE-2) which is abundantly found in the lower respiratory tract [48][49][50][48,49,50]. SP glycoprotein is a viral ligand that binds to the ACE-2 receptor through its receptor-binding domain which is found in one of the two subunits (S1) of the SP protein (S1 and S2). S2, on the other hand, aids in membrane fusion [60][61][60,61]. This SP protein serves as a target of various neutralizing antibodies and vaccines. NP, a phosphor protein, is another important viral ligand that is produced and divided into copious amounts during viral infection [62]. However, both the viral ligands, the SP and NP glycoproteins, possess high immunogenicity. The highest amount of NP is detected after 10 days of viral infection which can be easily detected using a sandwich immunoassay [63]. The most abundant membrane protein (MP) and the smallest structural envelope protein (EP), which are involved in important viral functions such as assembly, release and pathogenesis, may also serve as potential targets for viral detection kits [37][64][65][66][37,64,65,66].
D-Dimer-Based Detection
D-dimer detection is used for fast diagnosis of COVID-19 infection, as abnormal coagulation is closely associated with its progression. The severity of the infection can be determined by D-dimer levels, which are protein fragments formed when there is clotting of blood. Higher levels of D-dimer showed a higher probability of pulmonary embolism in patients with COVID-19 infection [67].
The advantages and disadvantages of the different tests are described in the Table 3 below:
Table 3.
Advantages and disadvantages of various diagnostic tests used for SARS-CoV-2 detection.
| Diagnostic Technique | Advantages | Disadvantages | References | |||||
|---|---|---|---|---|---|---|---|---|
| NAAT |
|
| [68][69] | [68,69][6,46,47] | ||||
| Spike protein | K417N, E484K and N501Y | [ | 28] | |||||
| Epsilon variant | Spike protein | S13I, W152C and L452R | [29] | |||||
| γ-variant | Spike protein | K417T, E484K, N501Y | [30] | |||||
| Eta-variant | Spike protein | E484K ΔH69/ΔV70 deletion | [31] | |||||
| Cluster 5 | Spike protein | ΔH69/ΔV70 deletion | [32] |
3. Coronavirus Adaptations
The killer viral strain SARS-CoV-2 has emerged as a powerful pathogen due to its robust adaptations enabling it to survive for longer periods. The novel coronavirus has been categorized as one of the most contagious diseases. Since the inception of COVID-19, it has been an incredibly difficult journey to come up with a vaccine against the virus. Unlike other viruses with high mutation rates, it is known to possess a competent proofreading system allowing it to attack the host cells at multiple sites and amongst them, the respiratory system is the primary target [33]. It is a widely known fact that most viruses lack a proofreading mechanism which ultimately limits their ability to protect their genome against mutations. However, the genome of the coronavirus is extremely stable due to the presence of a sophisticated proofreading system [33]. The virus adopts a unique process that may protect against vaccines. They evolve through the process of recombination by swapping sequences of RNA with another coronavirus. When two distant coronavirus types end up in the same cell, they undergo recombination. This recombination generates a mutated viral strain that possesses the ability to infect new cell types and transverse to other species [34][35][34,35]. One of the favourable organisms that house as many as 61 coronavirus species are the bats and, therefore, these viruses undergo recombination inside the bats without infecting them [36][37][36,37].
4. Diagnosis of Coronavirus
Patients infected with the coronavirus suffer from pneumonia-like symptoms, including fever, shortness of breath, sputum production and myalgia or fatigue. The virus majorly infects the upper respiratory tract (URTIs), along with infecting other tissues such as the digestive tract (diarrhoea, poor appetite, nausea and vomiting), nervous system (confusion and headache) and cardiovascular system (palm’s, chest distress and cardiac injury) in the body [38]. The different techniques used in the diagnosis of SARS-CoV-2 are described below:
| 2. | PCR with fluorescently labelled probes | ||||||||||||||||||
| CT scan |
| Polymerase chain reaction | RNA | 6–8 h |
| Viral RNA |
| [41] | |||||||||||
| [70][71] | [70,71] | 3. | ||||||||||||||||
| Serological immunoassays | Chest computerized tomography (CT) | X-ray |
|
| Chest X-ray | 30–60 min | Small nodules in the chest | [48] | 48 | [49 | [72 | ] | ,49 | [50] | [,50] | ||||
| ] | 4. | Rapid lateral Flow Immunoassay (LFIA) | A liquid sample consisting of analyte transports without the help of capillary action through 3 zones of polymeric strips, upon which molecules that can interact with the analyte are attached | Throat swab or sputum | IgG | [51] | |||||||||||||
| 5. | Automated chemiluminescence immunoassay (CLIA) | Chemiluminescent methods utilizing luminophore markers | serum | IgM and IgG antibodies | [52] | ||||||||||||||
| 6. | Manual ELISA | Specific antibody-antigen interactions | Saliva, serum, plasma | 5–6 h | IgG antibodies | [53][54] | [53,54] | ||||||||||||
| 7. | Rapid antigen test | Rapid membrane-based lateral flow immunoassay | Saliva | 15–30 min | nucleocapsid protein antigen of the coronavirus SARS-CoV-2 | [55] | |||||||||||||
| 8. | Detection of D-dimer levels | Coagulation | Blood | 1–2 days | D-dimer concentrations | [56] | |||||||||||||
| 9. | Microbial culture test | NAAT (nucleic acid amplification test) | Nucleic acids | 2–3 days | Viral growth | [57] | |||||||||||||
| 10. | Lateral flow antigen test | Immunochromatographic assay | Throat swab or sputum | 30–60 min | Nucleocapsid protein antigen | [58] | |||||||||||||
| 11. | Neutralizing antibody test | Rapid membrane-based lateral flow immunoassay | Serum, throat swab or sputum | 30–60 min | Neutralizing antibodies | [59] |
- Serological immunoassays: A plethora of serological immunoassays exists which detect SARS-CoV-2 viral proteins and antibodies against those proteins in the plasma or serum. The most popular biomolecules detected by commercial immunological tests such as rapid lateral flow immunoassay (LFIA) tests, automated chemiluminescence immunoassay (CLIA) and manual ELISA and other formats are IgM and IgG antibodies. The antibodies are released into the bloodstream in the second week of viral infection. IgM and IgG may be detected within 10–30 days and 20 days post-infection, respectively
- [40]. The IgM antibody unveils a drop in concentration, whereas IgG persists in the systemic circulation for prolonged periods and may play a role in adaptive immunity against SARS-CoV-2 in a possible second encounter. ELISA kits against nucleocapsid (NP) and spike (SP) viral proteins exist in the market but they are mainly used for rsearch and development purposes only [45]. The details of all the diagnostic techniques used for SARS-CoV-2 detection are summarized in the Table 2 below:
4.2. Effect of Viral Mutations on the Accuracy of Diagnostic Tests
Since the virus accumulates mutations over time, the analysis of the sequenced data from time to time is critical to evaluate their effect on the diagnostic tests [73]. If tests continuously give false-negative results, the viral genome must be immediately sequenced to fish out any mutations that may be responsible for the same. In addition, the popular NAAT is designed in a way that enables binding to multiple targets. Therefore, even if a mutation occurs concerning one target site, the test will continue to work [74]. For example, the widely known S-gene drop out/S-gene target failure of the Thermo Fisher TaqPath test may lead to false-negative test results as a consequence of the Δ69/70 mutation in the Spike-gene. However, the test continues to deliver accurate test results because of the presence of primers specific to two other target genes. Even though viral evolution is inevitable, the provision of multiple target binding in most diagnostic tests works as a boon in the fight against the pandemic. In addition, the S-gene failure occurred in the detection of the B.1.1.7 variant, and a majority of the PCR-based diagnostic tests do not target the S-gene, and even if they do, they work against multiple targets. S-gene failure is widely being used to screen variants from positive PCR results, if the S-gene failure occurs, the genome is sequenced to identify the potential mutations in the S-gene. However, the S-gene is not the only mutation that affects the diagnostic tests, and instances of target failure have been reported as a result of mutations in other genes [75]. Therefore, sequencing is an important tool to curb the problem of diagnostic test failure.
5. Transmission of Coronavirus
The transmission of this deadly virus occurs through the air when an infected person sneezes or coughs, or even talks. The microdroplets produced in the respiratory tract travel through the air and come in contact with the other person(s), which have a shelf life of approximately half an hour. The droplets are laced with virions and may enter the host, ultimately eliciting an infection [76]. Even though the virus can persist in aerosols for an only half-hour, it still settles onto surfaces later and can persist for longer time durations, up to 72 h depending upon the type of surface it comes in contact with, such as steel, cardboard, plastic, etc. [76]. About 80% of COVID-19 cases are asymptomatic (mild–moderate symptoms), 15% of cases progress to pneumonia and only about 5% of cases result in acute respiratory distress syndrome (ARDS), septic shock and/or multiple organ failure. The major cell surface receptor for the coronavirus through which it enters the host cell is the ACE-2 receptor. The ACE-2 receptor is scattered throughout the human body but is present abundantly in the epithelia of the lung and small intestine, serving as routes for viral entry [77].
