Isatin, chemically an indole-1H-2,3-dione, is recognised as one of the most attractive therapeutic fragments in drug design and development. The template has turned out to be exceptionally useful for developing new anticancer scaffolds, as evidenced by the increasing number of isatin-based molecules which are either in clinical use or in trials. Apart from its promising antiproliferative properties, isatin has shown potential in treating Neglected Tropical Diseases (NTDs) not only as a parent core, but also by attenuating the activities of various pharmacophores.
- isatin
- antiproliferatives
- antiplasmodials
- antimycobacterials
- antimicrobials
1. Introduction
Isatin, also known as 1H-indol-2,3-dione, is a natural heterocyclic compound extracted as red-orange powder from a variety of plants worldwide, including Isatis tinctoria, Couroupita guianensis Aubl, Melochia tomentosa, and Boronia koniamboensis [1]. Moreover, it is also found in the secretion of the parotid gland of Bufo frogs as well as in the Australian mollusc Dicathais orbita [2] [2]. In humans, it is identified as a metabolite of tryptophan or epinephrine and is largely distributed in the central nervous system (CNS), peripheral tissues, as well as body fluids. Substituted isatins can also be found in plants such as melosatin alkaloids (methoxy phenyl isatins) isolated from the Caribbean tumourigenic plant Melochia tomentosa, as well as in fungi, such as 6-(3-Methylbuten-2’-yl) isatin and 5-(3’-Methylbuten-2’-yl) isatin isolated from Streptomyces albus [3] [3]. Among the most important biological activities of isatin derivatives are anticancer, antitubercular, antimalarial, antifungal, antibacterial, anticonvulsant, and antiviral properties. Despite the dominance of biological applications, other areas such as catalysis, dye compounds, nanocomposites, and polymers have merited special attention. Isatin derivatives possess a wide range of applications due to their inherent versatility in structure, which allows for the construction of diverse frameworks suitable for a specific biological or chemical property of interest. A variety of substituents, particularly at N-1, C-3 (C=O) and C5/C6/C7 (aromatic), can be introduced, modulating both the biological and chemical properties of the parent core [4].
2. Anticancer Activities of Functionalized Isatins
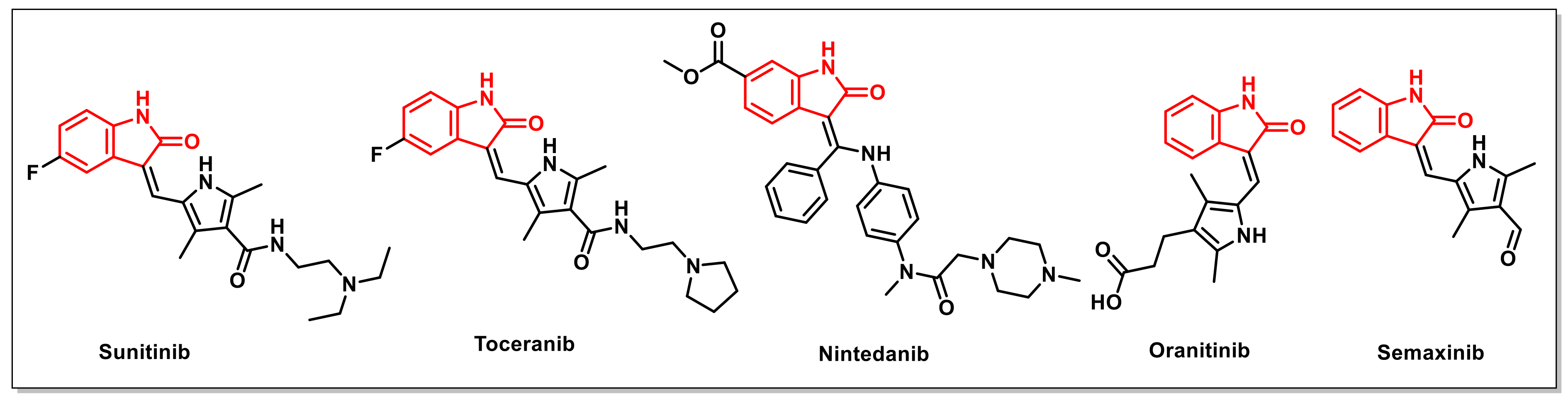
3. Antimycobacterial/Tubercular Activities of Isatin-Based Scaffolds
4. Antiplasmodial/Malarial Activities of Isatin-Based Scaffolds
P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi are members of the Plasmodium family of protozoan parasites that cause malaria. P. falciparum and P. vivax are the most virulent and are primarily responsible for the disease’s morbidity and mortality [ [13]]]. An estimated 241 million malaria cases were reported in 85 malaria-endemic countries in 2020, an increase from 227 million in 2019, with the WHO African Region accounting for the majority of the increase. The WHO African Region accounted for nearly 95 percent of all cases in 2020, with an estimated 228 million cases. India accounted for 83 percent of the cases in the Southeast Asian region. Malaria deaths increased by 11% in 2020 compared to 2019 to an estimated 627,000; of the additional 69,000 deaths, an estimated 47,000 (68%) were caused by service disruptions during the COVID-19 pandemic [14] [14]. The continuous evolving drug resistance to both the conventional (quinolines) as well as contemporary (Artemisinin combination therapy) drugs has exacerbated the need for identifying new entities with promising antiplasmodial activities. A number of reports have shown the potential of isatin-based compounds as promising antiplasmodials [15,16][15][16].5. Antimicrobial Activities of Isatin-Based Scaffolds
Antibiotics are unquestionably a blessing to human civilization, having saved millions of lives by combating infections or microbes. Various antibiotics have been used for therapeutic purposes over the years. In the mid-twentieth century, antibiotics were regarded as the “wonder drug”. There was an idealistic belief at the time that communicable disease was on its way out. Antibiotics were thought to be a magic bullet that selectively targeted microbes responsible for disease eradication. Antibiotic resistance progresses rapidly and is therefore a major source of concern. A growing number of infections, such as pneumonia and gonorrhoea, are becoming more difficult and, in some cases, impossible to treat, while antibiotics have become less effective [17].6. Conclusions
Isatin is a highly promising scaffold in drug discovery due to its ubiquitous presence in biological systems; it is a molecular architecture that can be easily modulated in addition to a plethora of biological activities. Of course, isatin’s anticancer potential is overwhelming, as evidenced by the number of isatin-based compounds in use as therapeutics or in various stages of clinical trials. Isatin derivatives have demonstrated promising antiproliferative attributes against various cancer cells, targeting specific biomolecules or organelles as free ligands or those coordinated to metal ions. Adherence to metal ions frequently enhances its bioactivities, indicating a synergistic mechanism comprising the metal and the ligand. They also disclose a variety of modes of action, including the ability to bind DNA, generate reactive species that induce oxidative damage, and suppress specific proteins. Isatin-pharmacophore hybrids have the potential to overcome drug resistance and provide new functional entities with multiple mechanisms of action and good safety profiles. In the case of antimycobacterials, the inclusion of isatin with isoniazid has not only improved the lipophilicity as well as the activity of the hybrids, but also minimized the frequency of the development of resistance. The inclusion of isatin core with quinoline core afforded hybrids better antiplasmodial profiles than CQ itself against the CQ-resistant species of P. falciparum. The current evidence therefore suggests that further exploiting this promising moiety can provide efficient clinical candidates with a reduced incidence of drug resistance. WThe researchers anticipate that introducing isatin into various drugs/organic moieties will significantly impact the treatment of various diseases in the near future, given the multitude of biological activities and mechanistic pathways provided by isatin-based compounds.References
- Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Taib, K. A Review on Antibiotic Resistance: Alarm Bells are Ringing Origin of antibiotic resistance. Cureus 2017, 9.World Health Organization. Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2010; Volume 1.
- Esmaeelian, B.; Abbott, C.A.; Le Leu, R.K.; Benkendorff, K. 6-Bromoisatin found in muricid mollusc extracts inhibits colon cancer cell proliferation and induces apoptosis, preventing early stage tumor formation in a colorectal cancer rodent model. Mar. Drugs. 2014, 12, 17–35.Esmaeelian, B.; Abbott, C.A.; Le Leu, R.K.; Benkendorff, K. 6-Bromoisatin found in muricid mollusc extracts inhibits colon cancer cell proliferation and induces apoptosis, preventing early-stage tumor formation in a colorectal cancer rodent model. Mar. Drugs. 2014, 12, 17–35.
- Varuna, S.R.K. Isatin and its derivatives: A survey of recent syntheses, reactions, and applications. Med. Chem. Commun. 2019, 10, 351–368.Varuna, S.R.K. Isatin and its derivatives: A survey of recent syntheses, reactions, and applications. Med. Chem. Commun. 2019, 10, 351–368.
- Kumar, G., Singh, N.P. and Kumar, K. Recent Advancement of Synthesis of Isatins as a Versatile Pharmacophore: A review. Drug Res. 2021, 71, 115–121.Kumar, G.; Singh, N.P.; Kumar, K. Recent Advancement of Synthesis of Isatins as a Versatile Pharmacophore: A review. Drug Res. 2021, 71, 115–121.
- International Agency for Research on, All Cancers Factsheet. World Heal. Organ. 2020, 419, 199–200. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 24 February 2022).International Agency for Research on, All Cancers Factsheet. World Health Organ. 2020, 419, 199–200. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 24 February 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLO-BOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, CA. Cancer J. Clin. 2021, 71, 209–249.Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, CA. Cancer J. Clin. 2021, 71, 209–249.
- Enoque, R.; De Paiva, F.; Vieira, E.G.; Rodrigues, D. Anticancer Compounds Based on Isatin-Derivatives: Strategies to Ame-liorate Selectivity and Efficiency. Front. Mol. Biosci. 2021, 7, 1–24.Enoque, R.; De Paiva, F.; Vieira, E.G.; Rodrigues, D. Anticancer Compounds Based on Isatin-Derivatives: Strategies to Ameliorate Selectivity and Efficiency. Front. Mol. Biosci. 2021, 7, 627272.
- Schwartz, N.G.; Sandy, F.P.; Robert, H.P.; Adam, J.L. Tuberculosis—United States, 2019. Morb. Mortal. Wkly Rep. 2020, 69, 286.Schwartz, N.G.; Sandy, F.P.; Robert, H.P.; Adam, J.L. Tuberculosis—United States, 2019. Morb. Mortal. Wkly Rep. 2020, 69, 286.
- Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.D.M.C.; et al. Global Tuberculosis Report 2020–Reflections on the Global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. 2021, 113, S7-S12.Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.D.M.C.; et al. Global Tuberculosis Report 2020–Reflections on the Global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. 2021, 113, S7–S12.
- Huszár, S.; Chibale, K.; Singh, V. The quest for the holy grail: New antitubercular chemical entities , targets and strategies. Drug Discov. Today 2020, 25, 772–780.Huszár, S.; Chibale, K.; Singh, V. The quest for the holy grail: New antitubercular chemical entities, targets and strategies. Drug Discov. Today 2020, 25, 772–780.
- Yang, L.; Hu, X.; Chai, X.; Ye, Q.; Pang, J.; Li, D. Opportunities for overcoming tuberculosis: Emerging targets and their in-hibitors. Drug Discov. Today 2022, 27, 326–336.Yang, L.; Hu, X.; Chai, X.; Ye, Q.; Pang, J.; Li, D. Opportunities for overcoming tuberculosis: Emerging targets and their inhibitors. Drug Discov. Today 2022, 27, 326–336.
- Perveen, S.; Kumari, D.; Singh, K.; Sharma, R. Tuberculosis drug discovery: Progression and future interventions in the wake of emerging resistance. Eur. J. Med. Chem. 2022, 229, 114066.Perveen, S.; Kumari, D.; Singh, K.; Sharma, R. Tuberculosis drug discovery: Progression and future interventions in the wake of emerging resistance. Eur. J. Med. Chem. 2022, 229, 114066.
- Kumar, N.; Singh, R.; Rawat, D.S. Tetraoxanes: Synthetic and Medicinal Chemistry Perspective. Med. Res. Rev. 2012, 32, 581–610.Kumar, N.; Singh, R.; Rawat, D.S. Tetraoxanes: Synthetic and Medicinal Chemistry Perspective. Med. Res. Rev. 2012, 32, 581–610.
- World Malaria Report, 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 24 February 2022).World Malaria Report. 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 24 February 2022).
- Chauhan, M.; Saxena, A.; Saha, B. An insight in anti-malarial potential of indole scaffold: A review. Eur. J. Med. Chem. 2021, 218, 113400.Chauhan, M.; Saxena, A.; Saha, B. An insight in anti-malarial potential of indole scaffold: A review. Eur. J. Med. Chem. 2021, 218, 113400.
- Zhou, W.; Wang, H.; Yang, Y.; Chen, Z.; Zou, C.; Zhang, J. Chloroquine against malaria , cancers and viral diseases. Drug Discov. Today 2020, 25, 2012–2022.Zhou, W.; Wang, H.; Yang, Y.; Chen, Z.; Zou, C.; Zhang, J. Chloroquine against malaria, cancers and viral diseases. Drug Discov. Today 2020, 25, 2012–2022.
- Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Taib, K. A Review on Antibiotic Resistance: Alarm Bells are Ringing Origin of antibiotic resistance. Cureus 2017, 9.Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Taib, K. A Review on Antibiotic Resistance: Alarm Bells are Ringing Origin of antibiotic resistance. Cureus 2017, 9.
