Rheumatoid arthritis (RA) is an autoimmune disorder affecting a vast variety of the population. The onset of RA as well as the development of systematic immunization is affected by both genetic and environmental risk factors. TIn this review aims to point out the role antioxidant setting of natural products, RA patients may find the use of natural products in the management of RA, focusing on the reports of basic research (in vitro and animal studies) emphasizing the antioxidant andbeneficial. Although there is conflicting evidence of the role of antioxidants in RA, as this field remains poorly explored, the value of antioxidants in fighting inflammation is well-documented, which explains the fact that the antioxidant properties of natural products are commonly evaluated and usually represent the first step of in vitro evaluation before that of the anti-inflammatory properties considered in the field of RA.
- rheumatoid arthritis
- antioxidant
- natural products
- anti-inflammatory
- animal studies
- cell models
1. Introduction
2. Natural Products
2.1. Date (
Phoenix dactylifera
L.)
Date (Phoenix dactylifera L.) seeds are a known traditional Moroccan remedy against pathological conditions involving inflammation, such as RA [33]. Date seeds are rich in dietary fiber, phenolics, and antioxidants while their protein contains the majority of essential amino acids [34]. An interesting study on the methanol extracts of different date seed varieties (namely: Boufgous, Bousthammi, Jihl, and Majhoul) aimed to evaluate their anti-inflammatory effects, as reflected via membrane stabilization activity, suppression of protein denaturation, and nitric oxide (NO) radical scavenging activity (IC50 is reported in all cases). In all cases, 30 g of pulverized date seeds were used for the extraction. The results of this research showed that Boufgous seeds had higher rates of membrane stabilization activity (241.65 ± 6.69 mg/mL) and relatively high outcomes in terms of the inhibition of protein denaturation and NO radical scavenging activity (167.32 ± 5.82 mg/mL and 144.45 ± 7.63 mg/mL, respectively) compared to Trolox, which was used as a reference standard. Majhoul seeds had the highest outcomes in terms of the inhibition of protein denaturation and NO radical scavenging activity (193.71 ± 7.25 mg/mL and 163.63 ± 6.39 mg/mL, respectively), although their results for membrane stabilization were slightly lower than those of the Boufgous seeds (209.38 ± 9.01 mg/mL). Interestingly, these outcomes were related in the article to the phenolic and flavonoid contents of each product as an indicative factor of their antioxidant and anti-inflammatory activity [33]. The research was further carried in a collagen-induced arthritis (CIA) animal model in which, in relation to the previously reported outcomes, date seeds (at a dose of 30 mg/kg body weight (b.wt.)) exhibited significant ability to reduce carrageenan and croton oil-induced paw and ear edema, respectively, as compared to indomethacin (10 mg/kg b.wt.). More specifically, regarding the carrageenan-induced paw edema, Bousthammi and Jihl varieties showed 76.27% and 87.28% reductions, respectively, while the Boufgous and Majhoul varieties resulted in reductions of over 40%. Similarly, croton oil-induced ear edema had in all cases over 50% reduction, reaching up to 77.17% for the Bousthammi seeds, also compared to indomethacin (95.11%) [33].2.2. Wild Pomegranate (
Punica granatum
Linn.)
Punica granatum has been used as a traditional medicine for the treatment of various conditions including pain and inflammation [35,36][35][36]. As described in a recently conducted study in the setting of lipopolysaccharide (LPS)-induced RAW 264.7 macrophages, pomegranate has shown potential NO inhibition, as well as decreased paw edema in carrageenan-induced mice after administration of 100 mg/kg [36]. An interesting study was conducted to evaluate the antiarthritic activity of butanol fraction (administered at doses of 50 and 75 mg/kg body weight) of Punica granatum Linn. rind methanolic extract versus dexamethasone (5 mg/kg) against Freund’s complete adjuvant (FCA)-induced arthritis in rats. The results of this study s showed dose-dependent effects on biophysical (body weight, paw volume, arthritic score, and joint diameter) and hematological parameters (such as red and white blood cell counts, Erythrocyte Sedimentation Rate (ESR) and Hemoglobin (Hb) concentration), while active phytochemicals such as phenolic compounds, iridoid glycosides, and flavonoids were underlined as the source of the antiarthritic potential of the butanol fraction of Punica granatum Linn. rind extract [37]. It is worth noting that NO inhibitory effects of Punicalagin, Punicalin, Strictinin A, and Granatin B (hydrolysable tannins found in this natural product) were measured in LPS-induced RAW 264.7 macrophage cells showing inducible NO synthetase (iNOS) activities of 15.7 ± 0.4, 46.7 ± 7.0, 25.4 ± 2.4, and 41.7 ± 0.7, respectively (results presented as NO inhibition % for each compound). Although the IC50 values (mM) for each of these compounds regarding NO inhibition was relatively high (69.8, 78.6, 63.1, and 33.6 mM, respectively), this could also explain the reported levels of cytotoxicity (%) (35.5 ± 0.7, 67.6 ± 1.4, 45.8 ± 1.1, and 41.7 ± 0.7, respectively). However, from further investigation using lower concentrations (12.5–100 μΜ) and although cytotoxicity was still exhibited at 100 μM, dose-dependent inhibitory effects of the iNOS expression were found, which were also related to the time of the reaction showing significant iNOS inhibition at 8 h as well as 18 h [37]. Pomegranate juice is one of the natural products which have also led to promising outcomes in the setting of clinical trials for the management of RA symptoms, which can also be related to the polyphenolic compounds that exert antioxidant and anti-inflammatory activities. Pomegranate extract (250 mg/capsule) has been investigated and compared to cellulose (250 mg/capsule) in 55 RA patients in an 8-week trial. The results demonstrated reductions in major targets of RA management such as swelling and tenderness as well as morning stiffness and pain compared with the placebo group. Additionally, markers such as ESR levels, which have also been investigated in animal studies, have also shown a reduction in the clinical setting, although others such as mean matrix metalloproteinase—3 (MMP3) or C-Reactive protein (CRP) presented no substantial differences between the studied groups [38].2.3. Ribes (
Ribes orientale
,
Ribes alpestre
Decne)
Ribes is a genus comprised of about 200 known species and the only genus in the family Grossulariaceae, which originates from many parts of the Mediterranean area as well as most of Asia [39,40][39][40]. The roots of Ribes orientale aqueous ethanolic extract (30:70), aqueous and n-butanol fractions at the effective dose of 200 mg/kg (initial screening included doses of 50, 100, and 200 mg/kg b.wt.) were evaluated in treating RA in Sprague–Dawley rats (FCA model) and compared to piroxicam (10 mg/kg b.wt.). The interventions were orally administered 30 min before adjuvant injection from the beginning of the study and until 28 days after the injection. Paw volume/diameter was dose-dependently decreased by the intervention, which also suppressed paw swelling. Additionally, the interventions successfully downregulated gene expression levels of TNF-α, Cyclooxygenase type 2 (COX-2), IL-6, NF-κB, IL-1β, and Prostaglandin E2 (PGE2) and upregulated those of IL-4 and IL-10 compared with FCA control rats [41]. Similar results were obtained in the study of Ribes alpestre Decne, which has been also commonly used in the treatment of joint complaints. In the study of 28-day supplementation with aqueous ethanol extract or n-butanol and aqueous fractions at a 200 mg/kg oral dose in Sprague–Dawley rats (FCA model), paw volume and thickness and arthritic score were scientifically reduced by the intervention. Notably, on day 28, paw volume was decreased at approximately 81%, 61%, and 76% for the aqueous ethanol extract or n-butanol and aqueous fractions, respectively, with similar rates reported for paw diameter. Additionally, downregulation of proinflammatory cytokines IL-1β, TNF-α, IL-6, COX-2, PGE2, and NF-κB was also observed in thise study. It is worth mentioning that NF-κB, TNF-α, and IL-1β fold change rates were similar, if not the same, in the cases of piroxicam, aqueous ethanol extract, and aqueous fraction compared to the control group [42]. It should be noted that previous research has indicated the beneficial properties of anthocyanins and proanthocyanidins (the better-known phytochemicals found in Ribes) in conditions involving inflammation such as RA among others [43,44][43][44]. These effects will be further discussed later in this entreviewy. Additionally, regarding the antioxidant properties of plant extract and its fractions, free radical scavenging activity was measured against the 1,1-diphenyl-1-picryl-hydrazyl (DPPH) radical using solutions of various concentrations (50–6400 μg/mL) and ascorbic acid as a reference standard. This sItudy showed that the ethanolic (70%) plant extract exhibited 84.53% inhibition of oxidation at 6400 μg/mL, while butanol and aqueous fraction showed 74.07% and 85.74% DPPH radical scavenging potentials at maximum concentration. Similar results were obtained via the ferric reducing antioxidant power (FRAP) protocol, showing a significant and concentration-dependent (50–6400 μg/mL) increase in reducing power of aqueous ethanol extract and its fractions, with higher results observed in the plant extract at 6400 μg/mL, followed by the aqueous and butanol fractions at the same concentration [43,44][43][44].2.4. Southern Dewdrop Grass (
Circaea mollis
Sieb. and Zucc)
Circaea mollis Sieb. and Zucc. is the scientific name of Southern Dewdrop Grass originally written as “南方露珠草”, a natural product known in traditional Chinese medicine. This plant has been investigated for the treatment of joint swelling and pain in RA in the form of its ethanol extract in an animal study. The effects of the supplementation with various doses of the ethanolic extract of the plant were evaluated using dimethyl benzene (induction of inflammatory swelling in the ear), hot-plate (induction of pain), and FCA models. The selected doses of 170, 680, and 1350 mg/kg (delivered daily for a week) successfully inhibited the induced swelling (at least 3 mg reduction in swelling) and the high dose resulted in effects similar to the positive control (aspirin 35 mg/kg) (p < 0.01). Similar results were observed in the evaluation of time of reaction to pain induced by the hot-plate. In this case, the reaction time was successfully prolonged after treatment, mostly in the groups of 680 and 1350 mg/kg. A dose-dependent effect on both paw swelling and arthritis index was observed after FCA-induced arthritis for both selected doses (470 and 940 mg/kg). However, it is highlighted that the timing of the effects differed 15 days between the low and the high doses. Additionally, the treatment downregulated serum TNF-α and IL-1β and increased the production of serum IL-10 in FCA-induced rats [45].3. Natural Compounds
3.1. Polyphenols
Resveratrol (Stilbenes)
Resveratrol (Figure 1) is a well-known and thoroughly studied stilbene derived from stilbenoid biosynthesis. This phytoalexin polyphenolic compound, mostly recognized for its antioxidant profile, is commonly abundant in various natural products, such as grapes, berries, and peanuts [85][46]. As previously reported, there is compelling evidence on the beneficial effects of this plant-derived constituent on autoimmune diseases, while a correlation has been described between the disease activity of RA and the presence of oxidative stress, especially oxidative DNA damage. In the case of RA, the benefits of resveratrol are established, inter alia, on the basis of proinflammatory cytokine (IFN-γ, TNF-α, IL-6, IL-1, and IL-4) modulation, as well as MMPs and RANKL inhibition [86][47]. A study demonstrated that the upregulation of Sirt1 by resveratrol suppressed the Bradykinin-induced COX-2/PGE2 production through inhibiting the interactions of AP-1 and NF-κB with COX-2 promoter in RA synovial fibroblasts, while resveratrol also inhibited the phosphorylation and acetylation of p65, and reduced the binding to the COX-2 promoter, thereby attenuating COX-2 expression [87][48]. Similar results were observed in a study employing an acute model of antigen-induced arthritis in rats. As reported, pretreatment with resveratrol (12.5 mg/kg daily for 2 months before antigen-induced arthritis (AIA) induction) led to a significant reduction in knee swelling, which was documented after the continuous oral administration of resveratrol 2 days after the injection [88][49].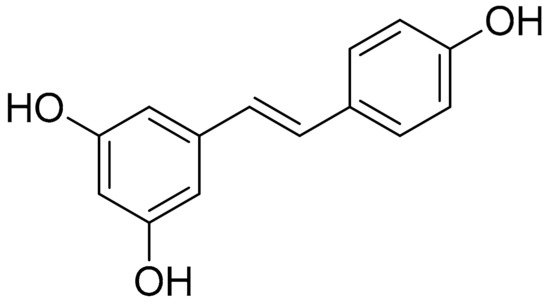
Silibinin (Flavonolignans)
Silibinin (Figure 2) is a natural polyphenolic flavonoid, with well-documented antioxidant, anti-inflammation, and anticancer properties [91][52]. A study showed that silibinin suppressed cell viability, suppressed the NF-κB pathway, decreased Sirtuin1, and increased the percentage of apoptotic RA-FLS, while RA-FLS transfection with a short hairpin RNA (shRNA) of SIRT1 enhanced silibinin-induced apoptosis. Notably, the selected concentrations of 0, 50, 100, and 200 μM of silibinin (cells treated for 48 h) dose-dependently induced the apoptosis of RA-FLS cells (apoptosis rates were approximately increased by 5% from 50 to 100 μΜ and from 100 to 200 μΜ), while in a similar way the same selected doses inhibited TNF-α-induced IL-6 and IL-1β production. It is noteworthy that further Western plot analysis toward these findings indicated that TNF-α-induced phosphorylation of NF-κB p65 and IκBα was also suppressed by silibinin in a concentration-dependent manner. Furthermore, as has also been investigated for RA-FLS cells, the combined effect of SIRT1-shRNA the treatment seemed to have higher outcomes (almost 30%) regarding the apoptosis rate compared to each treatment alone (apoptosis rate for silibinin 100 μΜ or SIRT1-shRNA were approximately 10%–15%). Similar results were observed in the setting of a CIA rat model, where 50, 100, and 150 mg/kg of silibinin were the selected doses of treatment. In these conditions, all treatments improved the arthritis score (especially after 8 days of treatment) with small variations reported regarding dose-dependency. Finally, TNF-α, IL-1β, and IL-6 levels in the treated group were also significantly decreased, as compared to the untreated group, in a dose-dependent manner [92][53].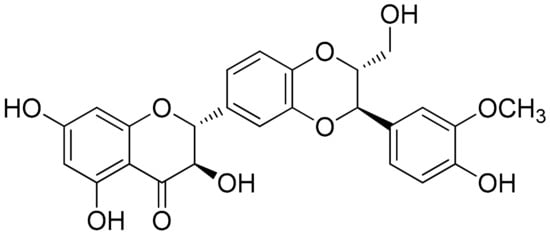
Curcumin (Flavonoids)
Curcumin (Figure 3) is a well-studied polyphenol and the predominant active compound found in turmeric (Curcumina longa); although it is described as a compound with low bioavailability due to its fast metabolism in the liver (an event that has been previously described to be partially modulated via the coadministration of piperine), it was found to inhibit the expression of proinflammatory cytokines and chemokines, through suppression of the NF-κB signaling pathway [93][54]. It should be noted that a previous study investigating Curcuma longa (among other plants) has demonstrated that Curcumin Monoglucoside and Curcumin Diglucoside, exhibited strong molecular interactions at the potential ligand binding sites of TNF-α and IL-1, thus supporting the inhibitory effects against the proinflammatory cytokines TNF-α and IL-1 [60][55]. A recent study investigated cell viability (CCK-8 assay) and macrophage apoptotic effect of curcumin (flow cytometry, TUNEL assay), showing that curcumin inhibited the degradation of IκBα and reduced the production of COX-2 in LPS-induced inflammatory RAW264.7 cells. This research concludes that curcumin significantly induced macrophage apoptosis, presumably via the inhibition of the NF-κB signaling pathway [94][56]. The same study also investigated the therapeutic effects of orally administered curcumin versus MTX (0.3 mg/kg) on CIA rats. The intervention (200 and 100 mg/kg) provided daily (for approximately 1 week after CIA induction) was able to attenuate the degree of joint swelling, while the higher dose, as well as MTX, seemed to have better results regarding the evaluation of arthritis score, synovial hyperplasia score, and pannus formation score as compared to the higher dose of treatment. Finally, the increased levels of NF-α, IL-17, IL-1β, and TGF-β in CIA rat synovium were significantly inhibited by the treatment with 200 mg/kg curcumin or MTX (p < 0.05). Interestingly, although the high-dose treatment and MTX were closer together, regarding proinflammatory cytokines modulation, the effects of the lower dose were not far behind compared to those of the high dose, especially in terms of TNF-α and IL-17 levels [94][56].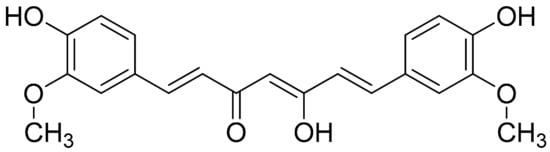
3.2. Alkaloids
Sinomenine (Morphine Alkaloid)
Sinomenine or cocculine (Figure 5Figure 4) is a well-known morphinane alkaloid (morphinan-derived alkaloid) commonly found in the root of Sinomenium acutum (native to Japan and China). This plant is traditionally known in herbal medicine for over 2000 years and has been utilized in the treatment of RA in China [100][57]. Although Sinomenine is a prescription drug for RA in China, a recent study was conducted to further evaluate and analyze its effect in the setting of LPS-stimulated RAW264.7 cells via measuring the expression of cytokines and chemokines related to inflammatory progression. In this research, concentrations of 0–1000 μg/mL Sinomenine were tested and those of 10 and 50 µg/mL of Sinomenine was selected based on the cytotoxicity screening previously conducted via the cell counting kit-8 (CCK-8) assay. In particular, the 24 h treatment with Sinomenine 0.1–50 μg/mL resulted in almost 100% cell viability. LPS treatment was tested in various concentrations as well (0.01–20 μg/mL), which showed a gradual reduction in cell viability after the concentration of 1 μg/mL in the 24 h treatment (2 h preincubation time). In a more targeted approach, dose-dependent reductions in the secretion of IL-6, GMC-SF, IL-1a, IL-1b, TNF-α, and Eotaxin-2, were also observed in LPS-induced RAW264.7 model (1 μg/mL) cells. This sItudy was carried further in CIA DBA/1 mice (groups: placebo, 50 or 100 mg/kg/day treatments). Lower inflammatory cell infiltration and synovial hyperplasia were demonstrated in addition to ameliorated clinical arthritis scores, while paw swelling score, inflammation score, and cartilage damage score were also decreased in a dose-dependent manner [101][58].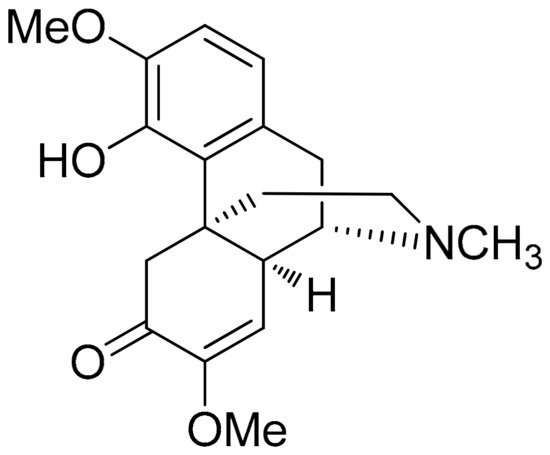
3.3. Terpenes
Taraxasterol (Pentacyclic Triterpene)
Taraxacum officinale has been previously investigated in various settings regarding its bioactive properties including those against inflammation [102,103,104][59][60][61]. A recent study investigated taraxasterol (Figure 6Figure 5), a pentacyclic-triterpene derived from taraxastane (mevalonate pathway), found in Taraxacum officinale of Chinese origin, in IL-1β-stimulated (10 ng/mL) human RA-FLS in vitro as well in a CIA model in mice (CIA was induced by two immunizations in mice (secondary immunization was given 12 days after the primary immunization)). Results of this study as related to the in vitro model report that cell viability was not affected by the treatment while the selected doses (0.3 to 30 μM) showed no inhibition on IL-1β-induced proliferation of human RA-FLS. Additionally, also regarding the in vitro model, the anti-inflammatory activity of the treatment was detected based on significant suppressions of TNF-α, IL-6, and IL-8 (mostly observed in the doses of 3, 10, and 30 μM) and production of matrix metalloproteinases MMP-1 and MMP-3. Notably, the supplementation at a high dose (30 μM) significantly blocked the IL-1β-mediated NF-κB p65 nuclear translocation. The same study, investigating CIA mice, reported that the treatment (10 mg/kg, intragastrical administration every other day from day 0 to day 48), significantly decreased the expression of TNF-α, IL-6, and IL-8 in joint tissues (p < 0.01, compared with the vehicle-treated group) in addition to the modulation of CIA-induced levels of IKKα/β and IκBα phosphorylation and IκBα degradation [105][62].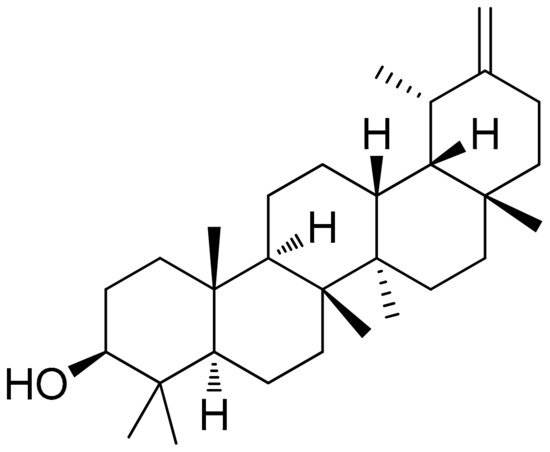
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038.
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400.
- Muravyev, Y.V. Extra-articular manifestations of rheumatoid arthritis. Nauchno Prakt. Revmatol. 2018, 56, 356–362.
- Littlejohn, E.A.; Monrad, S.U. Early Diagnosis and Treatment of Rheumatoid Arthritis. Prim. Care Clin. Off. Pract. 2018, 45, 237–255.
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880.
- Gulati, M.; Farah, Z.; Mouyis, M. Clinical features of rheumatoid arthritis. Medicine 2018, 46, 211–215.
- Gavrilă, B.I.; Ciofu, C.; Stoica, V. Biomarkers in Rheumatoid Arthritis, what is new? J. Med. Life 2016, 9, 144–148.
- Atzeni, F.; Talotta, R.; Masala, I.F.; Bongiovanni, S.; Boccassini, L.; Sarzi-Puttini, P. Biomarkers in rheumatoid arthritis. Isr. Med. Assoc. J. IMAJ 2017, 19, 512–516.
- Lindstrom, T.M.; Robinson, W.H. Biomarkers for rheumatoid arthritis: Making it personal. Scand. J. Clin. Lab. Invest. 2010, 70, 79–84.
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348.
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15, 1–10.
- Schett, G.; Emery, P.; Tanaka, Y.; Burmester, G.; Pisetsky, D.S.; Naredo, E.; Fautrel, B.; Van Vollenhoven, R. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: Current evidence and future directions. Ann. Rheum. Dis. 2016, 75, 1428–1437.
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF therapy: Past, present and future. Int. Immunol. 2015, 27, 55–62.
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; Van Vollenhoven, R.F.; De Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020.
- Rennie, K.L.; Hughes, J.; Lang, R.; Jebb, S.A. Nutritional management of rheumatoid arthritis: A review of the evidence. J. Hum. Nutr. Diet. 2003, 16, 97–109.
- Hänninen, O.; Kaartinen, K.; Rauma, A.L.; Nenonen, M.; Törrönen, R.; Häkkinen, S.; Adlercreutz, H.; Laakso, J. Antioxidants in vegan diet and rheumatic disorders. Toxicology 2000, 155, 45–53.
- Quinonez-Flores, C.M.; Gonzalez-Chavez, S.A.; Del Rio Najera, D.; Pacheco-Tena, C. Oxidative Stress Relevance in the Pathogenesis of the Rheumatoid Arthritis: A Systematic Review. Biomed Res. Int. 2016, 2016, 1–14.
- Fernández-Llanio Comella, N.; Fernández Matilla, M.; Castellano Cuesta, J.A. Have complementary therapies demonstrated effectiveness in rheumatoid arthritis? Reumatol. Clin. 2016, 12, 151–157.
- Efthimiou, P.; Kukar, M.; MacKenzie, C.R. Complementary and alternative medicine in rheumatoid arthritis: No longer the last resort! HSS J. 2010, 6, 108–111.
- Mbizo, J.; Okafor, A.; Sutton, M.A.; Burkhart, E.N.; Stone, L.M. Complementary and Alternative Medicine Use by Normal Weight, Overweight, and Obese Patients with Arthritis or Other Musculoskeletal Diseases. J. Altern. Complement. Med. 2016, 22, 227–236.
- Van de Laar, M. Pain Treatment in Arthritis-Related Pain: Beyond NSAIDs. Open Rheumatol. J. 2012, 6, 320–330.
- Proudman, S.M.; James, M.J.; Spargo, L.D.; Metcalf, R.G.; Sullivan, T.R.; Rischmueller, M.; Flabouris, K.; Wechalekar, M.D.; Lee, A.T.; Cleland, L.G. Fish oil in recent onset rheumatoid arthritis: A randomised, double-blind controlled trial within algorithm-based drug use. Ann. Rheum. Dis. 2013, 74, 89–95.
- Dudics, S.; Langan, D.; Meka, R.R.; Venkatesha, S.H.; Berman, B.M.; Che, C.T.; Moudgil, K.D. Natural products for the treatment of autoimmune arthritis: Their mechanisms of action, targeted delivery, and interplay with the host microbiome. Int. J. Mol. Sci. 2018, 19, 2508.
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130.
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Inflammation. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128137925.
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618.
- Astry, B.; Venkatesha, S.H.; Laurence, A.; Christensen-Quick, A.; Garzino-demo, A.; Frieman, M.B.; O’Shea, J.J.; Moudgil, K.D. Celastrol, a Chinese herbal compound, controls autoimmune inflammation by altering the balance of pathogenic and regulatory T cells in the target organ. Clin. Immunol. 2015, 157, 228–238.
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 30, 1343–1352.
- Maitra, R.; Porter, M.A.; Huang, S.; Gilmour, B.P. Inhibition of NFB by the natural product withaferin a in cellular models of cystic fibrosis inflammation. J. Inflamm. 2009, 6, 15.
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10.
- Nanjundaiah, S.M.; Lee, D.Y.W.; Berman, B.M.; Moudgil, K.D. Chinese herbal formula Huo-luo-xiao-ling dan protects against bone damage in adjuvant arthritis by modulating the mediators of bone remodeling. Evid. Based Complement. Altern. Med. 2013, 2013, 1–10.
- Che, C.T.; Wong, M.S.; Lam, C.W.K.; McPhee, D.J. Natural products from Chinese medicines with potential benefits to bone health. Molecules 2016, 21, 239.
- Bouhlali, E.d.T.; Hmidani, A.; Bourkhis, B.; Khouya, T.; Ramchoun, M.; Filali-Zegzouti, Y.; Alem, C. Phenolic profile and anti-inflammatory activity of four Moroccan date (Phoenix dactylifera L.) seed varieties. Heliyon 2020, 6, e03436.
- Al-Farsi, M.A.; Lee, C.Y. Usage of date (Phoenix Dactylifera L.) seeds in human health and animal feed. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123756886.
- Zahin, M.; Ahmad, I.; Gupta, R.C.; Aqil, F. Punicalagin and Ellagic Acid Demonstrate Antimutagenic Activity and Inhibition of Benzopyrene Induced DNA Adducts. Biomed Res. Int. 2014, 2014, 1–10.
- Lee, C.J.; Chen, L.G.; Liang, W.L.; Wang, C.C. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010, 118, 315–322.
- Gautam, R.K.; Sharma, S.; Sharma, K.; Gupta, G. Evaluation of antiarthritic activity of butanol fraction of Punica granatum linn. Rind extract against freund’s complete adjuvant-induced arthritis in rats. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 53–62.
- Ghavipour, M.; Sotoudeh, G.; Tavakoli, E.; Mowla, K.; Hasanzadeh, J.; Mazloom, Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur. J. Clin. Nutr. 2017, 71, 92–96.
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007, 102, 777–783.
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856.
- Uttra, A.M.; Alamgeer; Shahzad, M.; Shabbir, A.; Jahan, S.; Bukhari, I.A.; Assiri, A.M. Ribes orientale: A novel therapeutic approach targeting rheumatoid arthritis with reference to pro-inflammatory cytokines, inflammatory enzymes and anti-inflammatory cytokines. J. Ethnopharmacol. 2019, 237, 92–107.
- Hassan, U.H.; Alamgeer; Shahzad, M.; Shabbir, A.; Jahan, S.; Saleem, M.; Bukhari, I.A.; Assiri, A.M. Amelioration of adjuvant induced arthritis in Sprague Dawley rats through modulation of inflammatory mediators by Ribes alpestre Decne. J. Ethnopharmacol. 2019, 235, 460–471.
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 1–25.
- He, Y.H.; Zhou, J.; Wang, Y.S.; Xiao, C.; Tong, Y.; Tang, J.C.O.; Chan, A.S.C.; Lu, A.P. Anti-inflammatory and anti-oxidative effects of cherries on Freund’s adjuvant-induced arthritis in rats. Scand. J. Rheumatol. 2006, 35, 356–358.
- Zhang, Q.; Yu, Y.; Li, J.; Guan, Y.; Huang, J.; Wang, Z.; Zhang, Z.; Zhang, W.; Guo, J.; Li, J.; et al. Anti-arthritic activities of ethanol extracts of Circaea mollis Sieb. & Zucc. (whole plant) in rodents. J. Ethnopharmacol. 2018, 225, 359–366.
- Csaki, C.; Keshishzadeh, N.; Fischer, K.; Shakibaei, M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 2008, 75, 677–687.
- De Brito Oliveira, A.L.; Monteiro, V.V.S.; Navegantes-Lima, K.C.; Reis, J.F.; de Souza Gomes, R.; Rodrigues, D.V.S.; de França Gaspar, S.L.; Monteiro, M.C. Resveratrol role in autoimmune disease—A mini-review. Nutrients 2017, 9, 1306.
- Yang, C.M.; Chen, Y.W.; Chi, P.L.; Hsiao, L. Der Resveratrol inhibits BK-induced COX-2 transcription by suppressing acetylation of AP-1 and NF-κB in human rheumatoid arthritis synovial fibroblasts. Biochem. Pharmacol. 2017, 132, 77–91.
- Riveiro-Naveira, R.R.; Valcárcel-Ares, M.N.; Almonte-Becerril, M.; Vaamonde-García, C.; Loureiro, J.; Hermida-Carballo, L.; López-Peláez, E.; Blanco, F.J.; López-Armada, M.J. Resveratrol lowers synovial hyperplasia, inflammatory markers and oxidative damage in an acute antigen-induced arthritis model. Rheumatology 2016, 55, 1889–1900.
- Almonte-Becerril, M.; Fernandez-Rodriguez, J.; Fernandez-Rodriguez, J.; Ramil-Gomez, O.; Riveiro-Naveira, R.; Hermida-Carballo, L.; Concha, A.; Vela-Anero, A.; Viñas, S.; Blanco, F.; et al. FRI0063 Resveratrol attenuates synovial hyperplasia in an acute antigen-induced arthritis model by augmenting autophagy and decreasing angiogenesis. Ann. Rheum. Dis. 2017, 76, 502.
- Fernández-Rodríguez, J.A.; Almonte-Becerril, M.; Ramil-Gómez, O.; Viñas-Diz, S.; Hermida-Carballo, L.; Vela-Anero, Á.; Concha, Á.; Camacho-Encina, M.; Blanco, F.J.; Lopez-Armada, M.J. AB0121 Resveratrol-enhanced autophagic flux reduces severity of experimental rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 1254.
- Anestopoulos, I.; Sfakianos, A.P.; Franco, R.; Chlichlia, K.; Panayiotidis, M.I.; Kroll, D.J.; Pappa, A. A novel role of silibinin as a putative epigenetic modulator in human prostate carcinoma. Molecules 2017, 22, 62.
- Tong, W.W.; Zhang, C.; Hong, T.; Liu, D.H.; Wang, C.; Li, J.; He, X.K.; Xu, W.D. Silibinin alleviates inflammation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes and has a therapeutic effect on arthritis in rats. Sci. Rep. 2018, 8, 1–12.
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S.S.R. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356.
- Xu, S.; Peng, H.; Wang, N.; Zhao, M. Inhibition of TNF-α and IL-1 by compounds from selected plants for rheumatoid arthritis therapy: In vivo and in silico studies. Trop. J. Pharm. Res. 2018, 17, 277.
- Wang, Q.; Ye, C.; Sun, S.; Li, R.; Shi, X.; Wang, S.; Zeng, X.; Kuang, N.; Liu, Y.; Shi, Q.; et al. Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects. Int. Immunopharmacol. 2019, 72, 292–300.
- Kok, T.W.; Yue, P.Y.K.; Mak, N.K.; Fan, T.P.D.; Liu, L.; Wong, R.N.S. The anti-angiogenic effect of sinomenine. Angiogenesis 2005, 8, 3–12.
- Liu, W.; Zhang, Y.; Zhu, W.; Ma, C.; Ruan, J.; Long, H.; Wang, Y. Sinomenine Inhibits the Progression of Rheumatoid Arthritis by Regulating the Secretion of Inflammatory Cytokines and Monocyte/Macrophage Subsets. Front. Immunol. 2018, 9.
- Ma, C.; Zhu, L.; Wang, J.; He, H.; Chang, X.; Gao, J.; Shumin, W.; Yan, T. Anti-inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 2015, 168, 349–355.
- Jeon, D.; Kim, S.J.; Kim, H.S. Anti-inflammatory evaluation of the methanolic extract of Taraxacum officinale in LPS-stimulated human umbilical vein endothelial cells. BMC Complement. Altern. Med. 2017, 17, 1–10.
- Jeon, H.J.; Kang, H.J.; Jung, H.J.; Kang, Y.S.; Lim, C.J.; Kim, Y.M.; Park, E.H. Anti-inflammatory activity of Taraxacum officinale. J. Ethnopharmacol. 2008, 115, 82–88.
- Chen, J.; Wu, W.; Zhang, M.; Chen, C. Taraxasterol suppresses inflammation in IL-1β-induced rheumatoid arthritis fibroblast-like synoviocytes and rheumatoid arthritis progression in mice. Int. Immunopharmacol. 2019, 70, 274–283.
- Lee, A.Y.; Lee, S.; Kim, H.Y.; Lee, S.; Cho, E.J. Anti-inflammatory effects of luteolin and luteoloside from Taraxacum coreanum in RAW264.7 macrophage cells. Appl. Biol. Chem. 2016, 59, 747–754.
