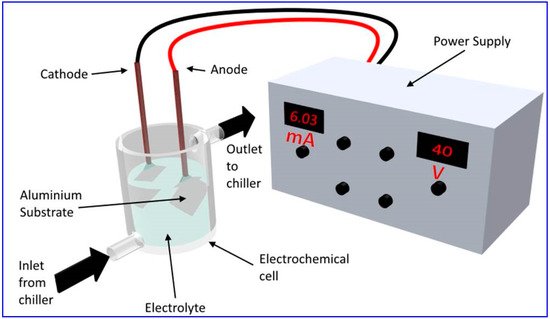
The fabrication of a thick oxide layer onto an aluminum surface via anodization has been a subject of intense research activity for more than a century, largely due to protective and decorative applications. The capability to create well-defined pores via a cost-effective electrochemical oxidation technique onto the surface has made a major renaissance in the field, as the porous surfaces exhibit remarkably different properties compared to a bulk oxide layer. Amongst the various nanoporous structures being investigated, nanoporous anodic alumina (NAA) with well-organized and highly ordered hexagonal honeycomb-like pores has emerged as the most popular nanomaterial due to its wide range of applications, ranging from corrosion resistance to bacterial repelling surfaces.
- nanoporous anodic alumina
- nanomaterials
1. Types of Anodic Alumina Films
2. Structure of NAA
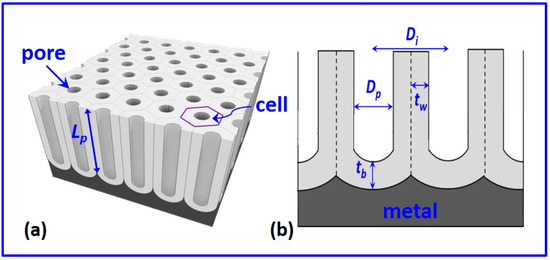
2.1. Pore Diameter
References
- Thompson, G. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Film. 1997, 297, 192–201.
- Despić, A.; Parkhutik, V.P. Electrochemistry of Aluminum in Aqueous Solutions and Physics of Its Anodic Oxide. In Modern Aspects of Electrochemistry No. 20; Bockris, J.O.M., White, R.E., Conway, B.E., Eds.; Springer: Boston, MA, USA, 1989; pp. 401–503.
- Sulka, G.; Stroobants, S.; Moshchalkov, V.; Borghs, G.; Celis, J.-P. Synthesis of well-ordered nanopores by anodizing aluminum foils in sulfuric acid. J. Electrochem. Soc. 2002, 149, D97–D103.
- Schwirn, K.; Lee, W.; Hillebrand, R.; Steinhart, M.; Nielsch, K.; Gösele, U. Self-Ordered Anodic Aluminum Oxide Formed by H2SO4 Hard Anodization. ACS Nano 2008, 2, 302–310.
- Choudhari, K.; Sudheendra, P.; Udayashankar, N. Fabrication and high-temperature structural characterization study of porous anodic alumina membranes. J. Porous Mater. 2012, 19, 1053–1062.
- Choudhari, K.S.; Kulkarni, S.D.; Santhosh, C.; George, S.D. Photoluminescence enhancement and morphological properties of nanoporous anodic alumina prepared in oxalic acid with varying time and temperature. Microporous Mesoporous Mater. 2018, 271, 138–145.
- Masuda, H.; Yada, K.; Osaka, A. Self-ordering of cell configuration of anodic porous alumina with large-size pores in phosphoric acid solution. Jpn. J. Appl. Phys. 1998, 37, L1340.
- Akiya, S.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Optimum exploration for the self-ordering of anodic porous alumina formed via selenic acid anodizing. J. Electrochem. Soc. 2015, 162, E244–E250.
- Nishinaga, O.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Rapid fabrication of self-ordered porous alumina with 10-/sub-10-nm-scale nanostructures by selenic acid anodizing. Sci. Rep. 2013, 3, 2748.
- Lee, W.; Nielsch, K.; Gösele, U. Self-ordering behavior of nanoporous anodic aluminum oxide (AAO) in malonic acid anodization. Nanotechnology 2007, 18, 475713.
- Kikuchi, T.; Yamamoto, T.; Suzuki, R.O. Growth behavior of anodic porous alumina formed in malic acid solution. Appl. Surf. Sci. 2013, 284, 907–913.
- Akiya, S.; Kikuchi, T.; Natsui, S.; Sakaguchi, N.; Suzuki, R.O. Self-ordered porous alumina fabricated via phosphonic acid anodizing. Electrochim. Acta 2016, 190, 471–479.
- Ma, Y.; Wen, Y.; Li, J.; Lu, J.; Li, Y.; Yang, Y.; Feng, C.; Hao, C.; Zhang, Z.; Hu, J. Pore Nucleation Mechanism of Self-Ordered Alumina with Large Period in Stable Anodization in Citric Acid. J. Electrochem. Soc. 2018, 165, E311–E317.
- Mínguez-Bacho, I.; Scheler, F.; Büttner, P.; Bley, K.; Vogel, N.; Bachmann, J. Ordered nanopore arrays with large interpore distances via one-step anodization. Nanoscale 2018, 10, 8385–8390.
- Sepúlveda, M.; Castaño, J.G.; Echeverría, F. Influence of temperature and time on the fabrication of self-ordering porous alumina by anodizing in etidronic acid. Appl. Surf. Sci. 2018, 454, 210–217.
- Elaish, R.; Curioni, M.; Gowers, K.; Kasuga, A.; Habazaki, H.; Hashimoto, T.; Skeldon, P. Effect of fluorozirconic acid on anodizing of aluminium and AA 2024-T3 alloy in sulphuric and tartaric-sulphuric acids. Surf. Coat. Technol. 2018, 342, 233–243.
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Fabrication of self-ordered porous alumina via etidronic acid anodizing and structural color generation from submicrometer-scale dimple array. Electrochim. Acta 2015, 156, 235–243.
- Takenaga, A.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Exploration for the self-ordering of porous alumina fabricated via anodizing in etidronic acid. Electrochim. Acta 2016, 211, 515–523.
- Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Growth behavior of anodic oxide formed by aluminum anodizing in glutaric and its derivative acid electrolytes. Appl. Surf. Sci. 2014, 321, 364–370.
- Takenaga, A.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Self-ordered aluminum anodizing in phosphonoacetic acid and its structural coloration. ECS Solid State Lett. 2015, 4, P55–P58.
- Diggle, J.W.; Downie, T.C.; Goulding, C. Anodic oxide films on aluminum. Chem. Rev. 1969, 69, 365–405.
- Lohrengel, M.M. Thin anodic oxide layers on aluminium and other valve metals: High field regime. Mater. Sci. Eng. R Rep. 1993, 11, 243–294.
- Alwitt, R.; Vijh, A. Sparking voltages observed on anodization of some valve metals. J. Electrochem. Soc. 1969, 116, 388.
- Lee, W.; Park, S.-J. Porous anodic aluminum oxide: Anodization and templated synthesis of functional nanostructures. Chem. Rev. 2014, 114, 7487–7556.
- Lee, W. The anodization of aluminum for nanotechnology applications. JOM 2010, 62, 57–63.
- Jani, A.M.M.; Losic, D.; Voelcker, N.H. Nanoporous anodic aluminium oxide: Advances in surface engineering and emerging applications. Prog. Mater. Sci. 2013, 58, 636–704.
- Vega, V.; García, J.; Montero-Moreno, J.M.; Hernando, B.; Bachmann, J.; Prida, V.M.; Nielsch, K. Unveiling the hard anodization regime of aluminum: Insight into nanopores self-organization and growth mechanism. ACS Appl. Mater. Interfaces 2015, 7, 28682–28692.
- Lee, W.; Kim, J.C.; Gösele, U. Spontaneous current oscillations during hard anodization of aluminum under potentiostatic conditions. Adv. Funct. Mater. 2010, 20, 21–27.
- Sun, C.; Luo, J.; Wu, L.; Zhang, J. Self-ordered anodic alumina with continuously tunable pore intervals from 410 to 530 nm. ACS Appl. Mater. Interfaces 2010, 2, 1299–1302.
- Li, Y.; Ling, Z.; Chen, S.; Wang, J. Fabrication of novel porous anodic alumina membranes by two-step hard anodization. Nanotechnology 2008, 19, 225604.
- Chen, X.; Yu, D.; Cao, L.; Zhu, X.; Song, Y.; Huang, H.; Lu, L.; Chen, X. Fabrication of ordered porous anodic alumina with ultra-large interpore distances using ultrahigh voltages. Mater. Res. Bull. 2014, 57, 116–120.
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747.
- Choi, J.; Wehrspohn, R.B.; Gösele, U. Mechanism of guided self-organization producing quasi-monodomain porous alumina. Electrochim. Acta 2005, 50, 2591–2595.
- Masuda, H.; Hasegwa, F.; Ono, S. Self-ordering of cell arrangement of anodic porous alumina formed in sulfuric acid solution. J. Electrochem. Soc. 1997, 144, L127.
- Schneider, J.J.; Engstler, J.; Budna, K.P.; Teichert, C.; Franzka, S. Freestanding, Highly Flexible, Large Area, Nanoporous Alumina Membranes with Complete through-Hole Pore Morphology. Eur. J. Inorg. Chem. 2005, 2005, 2352–2359.
- Vrublevsky, I.; Parkoun, V.; Schreckenbach, J. Analysis of porous oxide film growth on aluminum in phosphoric acid using re-anodizing technique. Appl. Surf. Sci. 2005, 242, 333–338.
- Choudhari, K.S.; Jidesh, P.; Sudheendra, P.; Kulkarni, S.D. Quantification and morphology studies of nanoporous alumina membranes: A new algorithm for digital image processing. Microsc. Microanal. 2013, 19, 1061–1072.
- Sulka, G.D.; Zaraska, L.; Stepniowski, W.J. Anodic porous alumina as a template for nanofabrication. In Encyclopedia of Nanoscience and Nanotechnology; Scientific Publishers: Valencia, Spain, 2011; Volume 11, pp. 261–349.
- O’sullivan, J.; Wood, G. The morphology and mechanism of formation of porous anodic films on aluminium. Proc. R. Soc. Lond. A 1970, 317, 511–543.
- Zhao, N.-Q.; Jiang, X.-X.; Shi, C.-S.; Li, J.-J.; Zhao, Z.-G.; Du, X.-W. Effects of anodizing conditions on anodic alumina structure. J. Mater. Sci. 2007, 42, 3878–3882.
- Sulka, G.; Parkoła, K. Temperature influence on well-ordered nanopore structures grown by anodization of aluminium in sulphuric acid. Electrochim. Acta 2007, 52, 1880–1888.
- Sellarajan, B.; Sharma, M.; Ghosh, S.; Nagaraja, H.; Barshilia, H.C.; Chowdhury, P. Effect of electrolyte temperature on the formation of highly ordered nanoporous alumina template. Microporous Mesoporous Mater. 2016, 224, 262–270.
- Kostaras, C.; Dellis, S.; Christoulaki, A.; Anastassopoulos, D.L.; Spiliopoulos, N.; Vradis, A.; Toprakcioglu, C.; Priftis, G.D. Flow through polydisperse pores in an anodic alumina membrane: A new method to measure the mean pore diameter. J. Appl. Phys. 2018, 124, 204307.
