Sleep-disordered breathing is associated with sleep deprivation. This sleep disruption interferes with the normal restorative functions of NREM and REM sleep, resulting in disruptions of breathing and cardiovascular function, changes in emotional reactivity, and cognitive decline in attention, memory, and decision making. As the human body goes through the different stages of sleep, physiological changes in the breathing mechanism are present. Sleep disorders, such as obstructive sleep apnea-hypopnea syndrome, are often associated with sleep-disordered breathing and sleep deprivation. Hypoxia and hypercapnia coexist with lack of sleep and undermine multiple functions of the body (e.g., cardiovascular system, cognition, immunity). Among the general population, athletes suffer from these consequences more during their performance. This concept supports the beneficial restorative effects of a good sleeping pattern.
- sleep deprivation
- sleep-disordered breathing
1. Sleep-Disordered Breathing Physiology
1.1. Respiratory Aspect
1.1. Respiratory Aspect
1.2. Neural Aspect
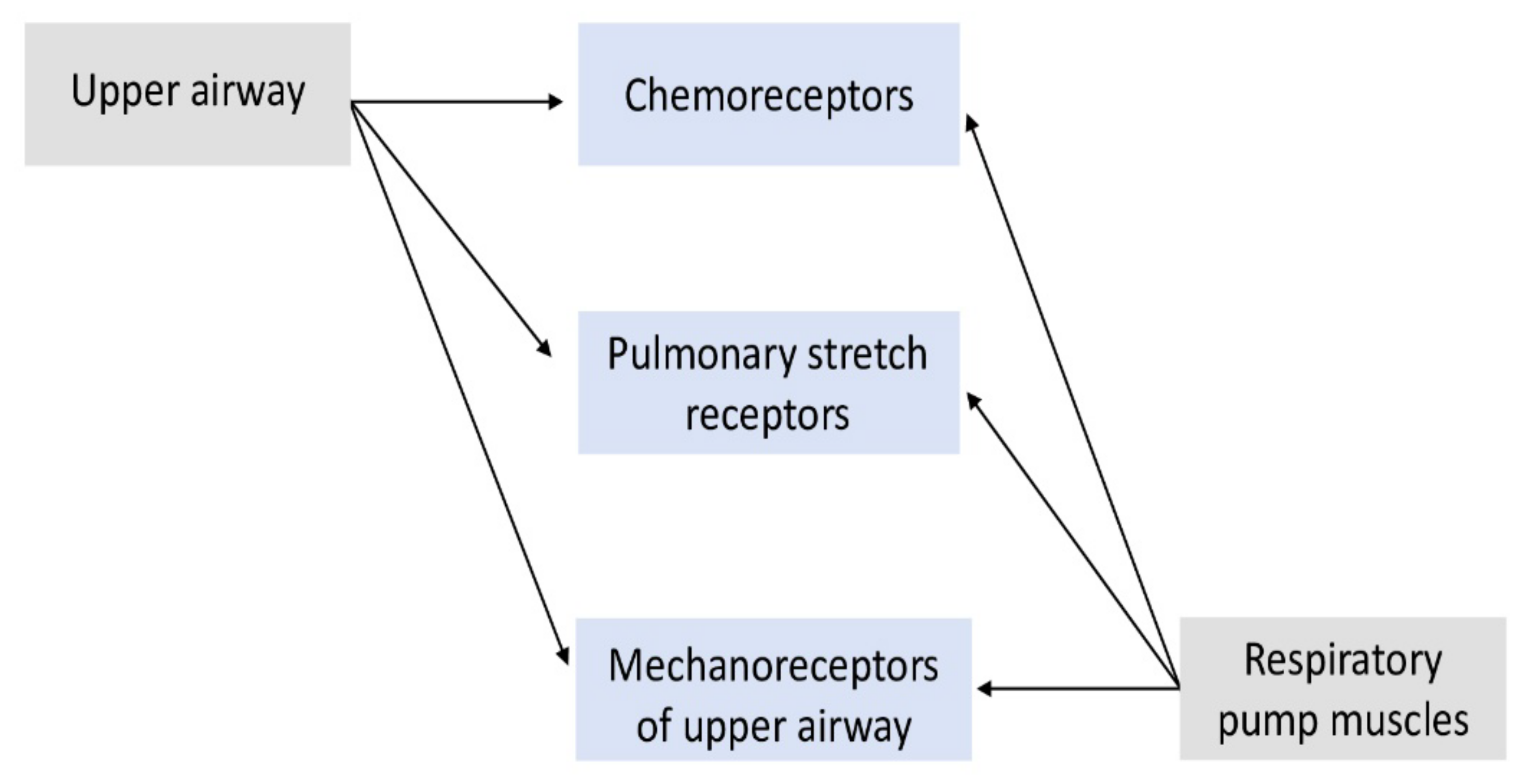
1.3. Input Sensors
1.4. Output Mediators
2. Sleep Deprivation
2.1. Sleep Deprivation and CO2 Retention
2.2. Sleep Deprivation and Exercise: Cognitive Implications
References
- Patwa, A.; Shah, A. Anatomy and physiology of respiratory system relevant to anaesthesia. Indian J. Anaesth. 2015, 59, 533–541.
- Newton, K.; Malik, V.; Lee-Chiong, T. Sleep and breathing. Clin. Chest Med. 2014, 35, 451–456.
- Sowho, M.; Amatoury, J.; Kirkness, J.P.; Patil, S.P. Sleep and respiratory physiology in adults. Clin. Chest Med. 2014, 35, 469–481.
- Dutschmann, M.; Dick, T.E. Pontine mechanisms of respiratory control. Comprehensive Physiol. 2012, 2, 2443–2469.
- Stavrou, V.T.; Astara, K.; Tourlakopoulos, K.N.; Papayianni, E.; Boutlas, S.; Vavougios, G.D.; Daniil, Z.; Gourgoulianis, K.I. Obstructive Sleep Apnea Syndrome: The Effect of Acute and Chronic Responses of Exercise. Front. Med. 2021, 8, 806924.
- Stavrou, V.T.; Astara, K.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. Respiratory Muscle Strength as an Indicator of the Severity of the Apnea-Hypopnea Index: Stepping Towards the Distinction between Sleep Apnea and Breath Holding. Cureus 2021, 13, e14015.
- Stavrou, V.; Bardaka, F.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. Brief Review: Ergospirometry in Patients with Obstructive Sleep Apnea Syndrome. J. Clin. Med. 2018, 31, 191.
- Iturriaga, R. Translating carotid body function into clinical medicine. J. Physiol. 2018, 596, 3067–3077.
- Yumino, D.; Bradley, T.D. Central sleep apnea and Cheyne-Stokes respiration. Proc. Am. Thorac. Soc. 2008, 5, 226–236.
- Halliwill, J.R.; Morgan, B.J.; Charkoudian, N. Peripheral chemoreflex and baroreflex interactions in cardiovascular regulation in humans. J. Physiol. 2003, 552, 295–302.
- Marcus, N.J.; Li, Y.L.; Bird, C.E.; Schultz, H.D.; Morgan, B.J. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: Role of the angiotensin II type 1 receptor. Respir. Physiol. Neurobiol. 2010, 171, 36–45.
- Krimsky, W.R.; Leiter, J.C. Physiology of breathing and respiratory control during sleep. Semin. Respir. Crit. Care Med. 2005, 26, 5–12.
- Harris, C.D. Neurophysiology of sleep and wakefulness. Respir. Care Clin. N. Am. 2005, 11, 567–586.
- Penzel, T.; Kantelhardt, J.W.; Lo, C.C.; Voigt, K.; Vogelmeier, C. Dynamics of heart rate and sleep stages in normals and patients with sleep apnea. Neuropsychopharmacology 2003, 28 (Suppl. 1), S48–S53.
- Appelberg, J.; Pavlenko, T.; Bergman, H.; Rothen, H.U.; Hedenstierna, G. Lung aeration during sleep. Chest 2007, 131, 122–129.
- Krieger, J. Breathing during sleep in normal subjects. Clin. Chest Med. 1985, 6, 577–594.
- Benarroch, E.E. Control of the cardiovascular and respiratory systems during sleep. Auton. Neurosci. 2019, 218, 54–63.
- Giglio, P.; Lane, J.T.; Barkoukis, T.J.; Dumitru, I. CHAPTER 3-Sleep Physiology. In Review of Sleep Medicine, 2nd ed.; Barkoukis, T.J., Avidan, A.Y., Eds.; Butterworth-Heinemann: Philadelphia, PA, USA, 2007; pp. 29–41.
- Han, F.; Chen, E.; Wei, H.; Ding, D.; He, Q. Influence of different sleep stages on respiratory regulation in normal humans. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2004, 26, 237–240.
- Wirth, K.J.; Steinmeyer, K.; Ruetten, H. Sensitization of upper airway mechanoreceptors as a new pharmacologic principle to treat obstructive sleep apnea: Investigations with AVE0118 in anesthetized pigs. Sleep 2013, 36, 699–708.
- Cummins, E.P.; Keogh, C.E. Respiratory gases and the regulation of transcription. Exp. Physiol. 2016, 101, 986–1002.
- Plataki, M.; Sands, S.A.; Malhotra, A. Clinical consequences of altered chemoreflex control. Respir. Physiol. Neurobiol. 2013, 189, 354–363.
- Brown, M.K.; Naidoo, N. The UPR and the anti-oxidant response: Relevance to sleep and sleep loss. Mol. Neurobiol. 2010, 42, 103–113.
- Yun, A.J.; Lee, P.Y.; Bazar, K.A. Autonomic dysregulation as a basis of cardiovascular, endocrine, and inflammatory disturbances associated with obstructive sleep apnea and other conditions of chronic hypoxia, hypercapnia, and acidosis. Med. Hypotheses 2004, 62, 852–856.
- Leproult, R.; Van Cauter, E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. 2010, 17, 11–21.
- Leproult, R.; Deliens, G.; Gilson, M.; Peigneux, P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep 2015, 38, 707–715.
- Wisor, J.P.; Clegern, W.C.; Schmidt, M.A. Toll-like receptor 4 is a regulator of monocyte and electroencephalographic responses to sleep loss. Sleep 2011, 34, 1335–1345.
- Shearer, W.T.; Reuben, J.M.; Mullington, J.M.; Price, N.J.; Lee, B.N.; Smith, E.O.; Szuba, M.P.; Van Dongen, H.P.; Dinges, D.F. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J. Allergy Clin. Immunol. 2001, 107, 165–170.
- Astara, K.; Siachpazidou, D.; Vavougios, G.D.; Ragias, D.; Vatzia, K.; Rapti, G.; Alexopoulos, E.; Gourgoulianis, K.I.; Xiromerisiou, G. Sleep disordered breathing from preschool to early adult age and its neurocognitive complications: A preliminary report. Sleep Sci. 2021, 14, 140–149.
- Alhola, P.; Polo-Kantola, P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr. Dis. Treat. 2007, 3, 553–567.
- Martella, D.; Plaza, V.; Estévez, A.F.; Castillo, A.; Fuentes, L.J. Minimizing sleep deprivation effects in healthy adults by differential outcomes. Acta Psychol. 2012, 139, 391–396.
- Pollicina, I.; Maniaci, A.; Lechien, J.R.; Iannella, G.; Vicini, C.; Cammaroto, G.; Cannavicci, A.; Magliulo, G.; Pace, A.; Cocuzza, S.; et al. Neurocognitive Performance Improvement after Obstructive Sleep Apnea Treatment: State of the Art. Behav. Sci. 2021, 11, 180.
- Roth, T. Effects of excessive daytime sleepiness and fatigue on overall health and cognitive function. J. Clin. Psychiatry 2015, 76, e1145.
- Stavrou, V.T.; Astara, K.; Tourlakopoulos, K.N.; Daniil, Z.; Gourgoulianis, K.I.; Kalabakas, K.; Karagiannis, D.; Basdekis, G. Sleep Quality’s Effect on Vigilance and Perceptual Ability in Adolescent and Adult Athletes. J. Sports Med. 2021, 2021, 5585573.
- Vitale, K.C.; Owens, R.; Hopkins, S.R.; Malhotra, A. Sleep Hygiene for Optimizing Recovery in Athletes: Review and Recommendations. Int. J. Sports Med. 2019, 40, 535–543.
- Axelsson, J.; Ingre, M.; Kecklund, G.; Lekander, M.; Wright, K.P.; Sundelin, T. Sleepiness as motivation: A potential mechanism for how sleep deprivation affects behavior. Sleep 2020, 43, zsz291.
- VanHelder, T.; Radomski, M.W. Sleep deprivation and the effect on exercise performance. Sports Med. 1989, 7, 235–247.
- Fullagar, H.H.K.; Skorski, S.; Duffield, R.; Hammes, D.; Coutts, A.J.; Meyer, T. Sleep and athletic performance: The effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2015, 45, 161–186.
- Dijk, D.-J.; Landolt, H.-P. Sleep Physiology, Circadian Rhythms, Waking Performance and the Development of Sleep-Wake Therapeutics. Handb. Exp. Pharmacol. 2019, 253, 441–481.
