Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Camila Xu and Version 2 by Camila Xu.
Unlike natural polymers, the chemical composition, functional group type and extent of functionalization, molecular weight, charge density and distribution, degradation and stability of synthetic polymers can be engineered to maximize antiviral activity against a specific virus type.
- dendrimers
- synthetic polymers
- sialyl-based polymers
1. Introduction
Polymers are chain-like molecules formed by polymerization of small, reactive organic molecules. Polymers due to their long chain length or high molecular weight are used extensively in surface coating applications because each polymer chain can make many physical bonds to irreversibly coat the surface [1][2]. As a result, polymers are very attractive as an antiviral material to bond irreversibly to viral glycoproteins to cover and conceal the viral surface, thus blocking the interaction of viral particles with the host cell [3][4][5][6][7][8][9][10][11][12][13][14][15][16][17]. Unlike natural polymers, the chemical composition, functional group type and extent of functionalization, molecular weight, charge density and distribution, degradation and stability of synthetic polymers can be engineered to maximize antiviral activity against a specific virus type [14][18][19]. Among synthetic polymers, dendrimers and sialyl-based polymers have been extensively studied as antiviral agents to fight against infections.
2. Dendrimers
Dendrimers due to their nanoscale size and structural uniformity are attractive as a carrier for delivery of antiviral agents [20]. The branched structure of dendrimers results in spherical and symmetric nanostructures with a monodisperse size in the 2–10 nm range [21]. The dendrimer generation which is the number of branches or the number of repeated synthetic cycles, controls the size of dendrimers [21]. The chemical groups on the dendrimer surface can be functionalized to produce polycationic or polyanionic dendrimers [21][22]. Peptides and drug molecules can be conjugated to dendrimers via cleavable linkages to form functional dendrimers with controlled biological activity [23]. Antiviral activity of dendrimers is attributed to their multivalency, symmetrical and monodisperse molecular structure, and the presence of multiple functional groups on the dendrimer surface. The dendrimer-based antiviral agents act mechanistically by interfering with the interaction of virus with the host cell [24]. As a result, antiviral activity depends on dendrimer molecular weight and type of functional groups present on the dendrimer surface [24]. Among different types, peptide-conjugated dendrimers show excellent antiviral activity against many virus types. A sulfonated derivative of polylysine dendrimer with a benzhydrylamine core was used to prevent infection against intravaginal herpes simplex virus (HSV) and simian immunodeficiency virus/human immunodeficiency virus (SIV/HIV) chimeric viruses [25][26]. In vitro studies with monkey kidney epithelial cells (Vero) demonstrated that this dendrimer completely prevented adsorption and penetration of the virus in host cells and inhibited DNA synthesis in the infected cells [26]. Figure 1 shows the chemical structure and mechanism of action of an FDA approved VivaGel® dendrimer (SPL7013), which is used in preventing genital herpes (HSV-2) and HIV. The VivaGel® is composed of anionic G4-poly(L-lysine) dendrimer with a benzylhydramine-amide-lysine core with 32 sodium (1-naphthyleneyl-3,6-disulphonic acid)-oxyacetamide functional groups [27]. The functional groups on the dendrimer surface act to prevent infection by blocking the virus from binding to host cells [27].
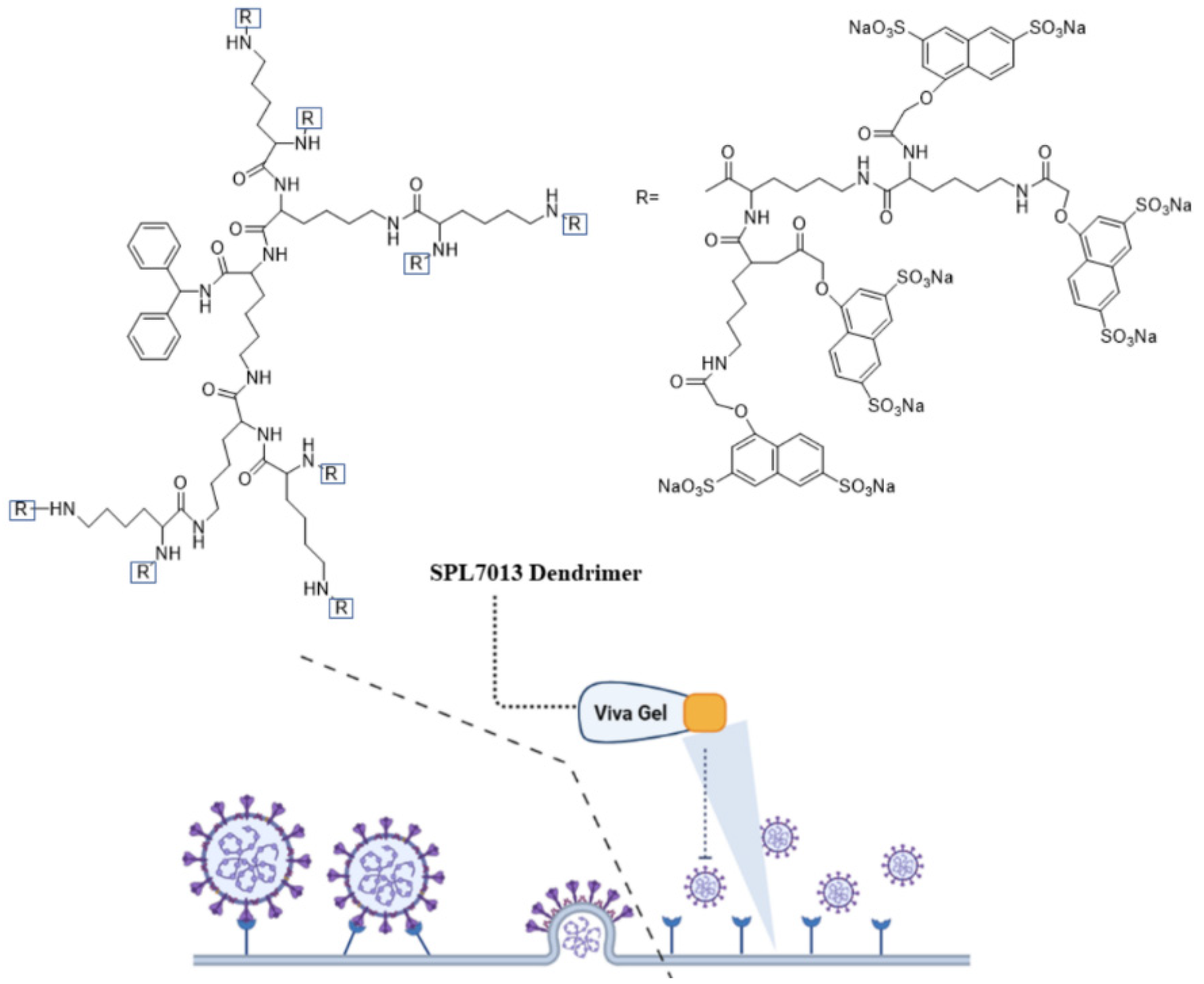 Figure 1. Chemical structure and mechanism of action of VivaGel® as an antiviral agent. The image was created by BioRender software.
Figure 1. Chemical structure and mechanism of action of VivaGel® as an antiviral agent. The image was created by BioRender software.Paull and coworkers evaluated the antiviral activity of SPL7013 dendrimer against SARS-CoV-2 [28]. Compared to polyanionic biopolymers such as io-ta-carrageenan and heparin (as linear sulphated molecules), the branched sulfonated structure of SPL7013 dendrimer inhibited SARS-CoV-2 infection in Vero E6 cells early in the replication cycle by blocking viral entry to the cells. In another study, a new class of dendrimers with tryptophan (Trp) functional groups on the surface were synthe-sized and their antiviral activity was evaluated against HIV and enterovirus-71 (EV71) [29]. Dendrimers with different surface amino acid groups including aromatic and non-aromatic, decarboxylated Trp (tryptamine), and methylated Trp were synthe-sized to study the relation between amino acid structure and antiviral activity [29]. According to the results, Trp-conjugated dendrimers act mechanistically by interacting with cell surface glycoproteins to interfere with virus-cell interaction early in the HIV replication cycle prior to viral entry in the host cell [29]. The type of peptide and its structure (aliphatic versus aromatic) affected antiviral activity of the dendrimer. Dendrimers conjugated with aromatic amino acids like tryptophan showed antiviral activity against both HIV and EV71 whereas dendrimers conjugated with aliphatic amino acids like alanine were inactive. The natural peptide LL-37 which is a member of cathelicidin family of peptides show antiviral activity against the respiratory syn-cytial virus (RSV), which is a common lower respiratory tract pathogen in children [30]. However, LL-37 peptide has limited stability in physiological medium. In a re-cent study, linear (SA-35) and hyperbranched (dendrimeric, LTP) cationic peptides from the helical region of LL-37 peptide were synthesized and their antiviral activity were compared with the natural peptide [31]. The results showed that the linear and hyperbranched peptides had higher antiviral activity as compared to the natural pep-tide which was attributed to destabilization of the viral envelope and competitive in-hibition of virus attachment/fusion with the host cell.
A range of polycationic and polyanionic dendrimers have been synthesized and evaluated against viral infections [32]. The antiviral activity of these dendrimers is due to electrostatic interaction between the viral envelope proteins and the den-drimer’s ionic functional groups [33]. In one study, polycationic dendrimers with N-alkylated 4,4′-bipyridinium units of varying charge density were synthesized and evaluated with respect to inhibition of HIV-1 [34]. Results showed that the inhibition of HIV-1 in MT-4 host T cells by the dendrimers was mediated by CXCR4 coreceptor and was independent of charge density of dendrimers. In another study, a polyanionic carbosilane dendrimer (PCD), designed to inhibit HIV-1 infection, was used to prevent viral infections causing sexually transmitted diseases [35]. The PCD dendrimer inhib-ited herpes simplex virus-2 (HSV-2) infection by reducing plaque formation in HSV-2 infected Vero cells, which was attributed to direct binding between the sulfonate groups on the dendrimer surface and glycoprotein B of the virus [36]. In another study, a PCD consisting of a polyphenolic core with 24 sulfonate groups on the den-drimer surface was shown to possess antiviral activity against hepatitis C virus (HCV) at low and high concentrations [36]. In another study, PCDs with surface sulfonate or naphthalene sulfonate functional groups prevented not only HIV-1 infection by inter-fering with cell-virus fusion but also inhibited cell-to-cell transmission [20]. List of studies on antiviral activity of dendrimers against different viruses are presented in Table 1.
Table 1. Antiviral activity of dendrimers and their mechanisms of action.
| Dendrimer Type | Name | Virus | Cell Line | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| Polyanionic | Phenyldicarboxylic acid (BRI6195), Naphthyldisulfonic acid (BRI2923) | HIV-1 | MT-4 | Inhibit viral binding and replication | [24] |
| Polyanionic | SPL-2999 | HSV-1, HSV-2 | Vero | Inhibit late stages of viral replication | [26] |
| Polyanionic | carbosilane dendrimer G2-STE16 | HIV-1 | TZM.bl | Inhibit viral entry | [33] |
| Polyanionic | G2-S16 | HIV | PBMC | Inhibit viral replication by blocking gp120/CD4/CCR5 interaction | [37] |
| Polyanionic | G3-S16 and G2-NF16 | HIV-1 | HeLa P4.R5MAGI, TZM.bl | Inhibit viral replication by blocking gp120/CD4/CCR5 interaction | [20] |
| Polycationic | Viologen | HIV-1 | MT-4 cells | Block viral entry | [34] |
3. Sialyl-Based Polymers
The sialylated glycans in the protective mucin layer of the nasal cavity disrupts the entry of influenza virus (IFV) particles to host cells by complexation with viral hemagglutinin (HA) [38]. The mucin barrier is the first line of defense in the immune system that traps and clears the viral particles upon mucin turnover, as shown in Figure 2 [39]. Although sialic acid (SA) and derivatives of SA have a strong affinity for the viral HA, the monovalent interaction between HA and SA is relatively weak [40]. Therefore, polymers modified with SA that can overcome the low interaction energy by complexation with multiple SA groups can effectively inhibit IFV infections [41]. In one study, the antiviral activity of a multivalent polymer conjugate consisting of poly (methyl vinyl ether-alt-maleic anhydride) modified with thiosialoside against PR8 influenza strain was comparable to those of Zanamivir® and Oseltamivir® drugs, as measured by growth inhibition assay [42]. The antiviral activity was attributed to multivalent binding of the modified polymer to neuraminidase on the viral particles to inhibit enzymatic activity, leading to viral particle aggregation and inhibition of virus-cell fusion. These results imply that polymers functionalized with monomeric sialoside groups which possess neuraminidase inhibitory activity can serve as an antiviral scaffold against early and late stages of influenza infection.
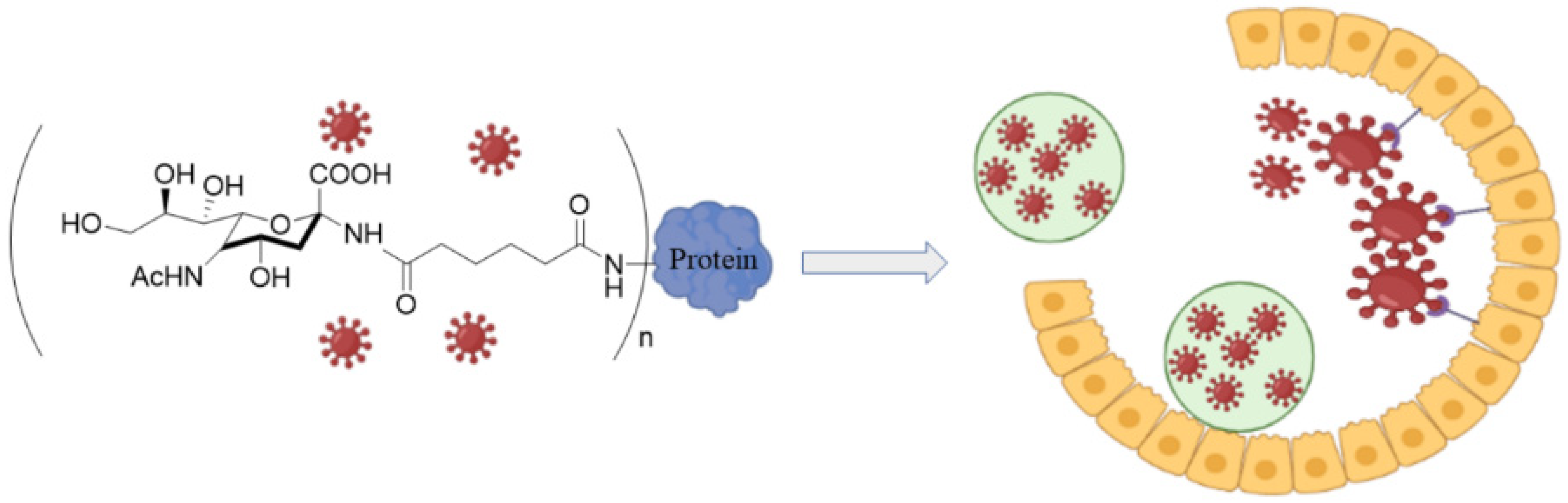 Figure 2. Schematic representation for the clearance of influenza virus by the mucin layer or mucin-mimetic glycol-conjugated proteins prior to uptake by epithelial cells. The image was created by BioRender.com software.
Figure 2. Schematic representation for the clearance of influenza virus by the mucin layer or mucin-mimetic glycol-conjugated proteins prior to uptake by epithelial cells. The image was created by BioRender.com software.Glycopolymers based on sialyl oligosaccharides with a range of chain lengths and sugar densities were synthesized by reversible addition-fragmentation chain transfer polymerization (RAFT) for interaction with hemagglutinin on the surface of influenza virus [43]. The extent of interaction and molecular specificity of the copolymers against different types of influenza virus depended on sugar units (6′- versus 3′-sialyllactose), sugar density, and polymer chain length [43]. In another study, tri-arm star glycopolymers with homogenous number of arms consisting of inert spacers and glycomonomer segments were synthesized by a core-first approach. The star glycopolymers had three arms with each arm having a degree of polymerization ranging from 30 to 100 to match the sugar-binding pockets of hemagglutinin on influenza virus [44]. The inert core segment was based on poly(N,N-dimethylacrylamide) (PDMA) whereas the arms were made of 6′-Sialyllactose glycomonomer which served as a natural ligand for HA binding in human influenza virus [44]. The use of arms with different degrees of polymerization allowed tunning the ligand-receptor interaction to the desired strength for surface HA in influenza virus. In another study, a polymer brush consisting of side chains ending in α-2,6-linked sialic acid (SA) was synthesized by protection-group-free, ring-opening metathesis polymerization (ROMP) to enhance multivalent interaction as determined by hemagglutination binding [45]. In this work, the anomeric hydroxyl group of 6′-sialyllatose in the oligosaccharide was activated and converted to an azide by nucleophilic substitution to form an azido oligosaccharide. Next, the azido oligosaccharide was reacted with α-norbornenyl, ω-acetylene terminated PEG, prepared from amine-terminated hydroxyl PEG, using a copper-catalyzed click reaction. Then, the α-norbornenyl terminated oligosaccharide PEG was polymerized by ROMP to produce a brush polymer with oligosaccharide side chains terminated with SA [45]. According to the results, brush polymers with high degree of polymerization, high SA density, and long side chains produced strongest antiviral activity as measured by hemagglutination inhibition assay [45]. These brush polymers are useful as a model system to study the antiviral properties of native mucins with the passage of influenza A virus (IAV) through the epithelial mucin barrier as measured by the strength of interaction between the viral HA and the sialyloligosaccharides of mucin (Figure 2) [46]. List of studies on antiviral activity of sialylated polymers are presented in Table 2.
Table 2. List of studies on antiviral activity of sialyl-based polymers with their mechanism of action.
| Sialyl-Based Polymers | Virus | Cell Line | Mechanism of Action | Ref. |
|---|---|---|---|---|
| Amide-sialoside protein conjugates | IAV | H1N1, H3N2, H9N2 | Binding to the virus and inhibiting replication | [39] |
| Thiosialoside-modified poly (methyl vinyl ether-alt-maleic anhydride) | IAV | MDCK | inhibiting both early attachment and late release during viral infection | [42] |
| silyl oligosaccharides modified glycopolymers | IAV | H1N1 | Binding with hemagglutinin on viral surface | [43] |
| Bioinspired brush polymers containing α-2,6-linked sialic acid | IAV | H1N1 | binding hemagglutination on viral surface | [45] |
References
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 1–19.
- Woo, J.; Seo, H.; Na, Y.; Choi, S.; Kim, S.; Choi, W.I.; Park, M.H.; Sung, D. Facile synthesis and coating of aqueous antifouling polymers for inhibiting pathogenic bacterial adhesion on medical devices. Prog. Org. Coat. 2020, 147, 10.
- Chun, H.; Yeom, M.; Kim, H.-O.; Lim, J.-W.; Na, W.; Park, G.; Park, C.; Kang, A.; Yun, D.; Kim, J. Efficient antiviral co-delivery using polymersomes by controlling the surface density of cell-targeting groups for influenza A virus treatment. Polym. Chem. 2018, 9, 2116–2123.
- Sun, Y.Z.; Gong, L.D.; Yin, Y.; Zhang, L.; Sun, Q.M.; Feng, K.; Cui, Y.M.; Zhang, Q.; Zhang, X.H.; Deng, X.L.; et al. A gradient pH-sensitive polymer-based antiviral strategy via viroporin-induced membrane acidification. Adv. Mater. 2022, 2, e2109580.
- Soares, D.C.F.; Poletto, F.; Eberhardt, M.J.; Domingues, S.C.; De Sousa, F.B.; Tebaldi, M.L. Polymer-hybrid nanosystems for antiviral applications: Current advances. Biomed. Pharmacother. 2022, 146, 112249.
- Chen, P.Y.; Lang, J.Y.; Zhou, Y.L.; Khlyustova, A.; Zhang, Z.Y.; Ma, X.J.; Liu, S.; Cheng, Y.F.; Yang, R. An imidazolium-based zwitterionic polymer for antiviral and antibacterial dual functional coatings. Sci. Adv. 2022, 8, eab18812.
- Ordon, M.; Zdanowicz, M.; Nawrotek, P.; Stachurska, X.; Mizielinska, M. Polyethylene films containing plant extracts in the polymer matrix as antibacterial and antiviral materials. Int. J. Mol. Sci. 2021, 22, 13438.
- Nasri, N.; Rusli, A.; Teramoto, N.; Jaafar, M.; Ishak, K.M.K.; Shafiq, M.D.; Hamid, Z.A.A. Past and current progress in the development of antiviral/antimicrobial polymer coating towards COVID-19 prevention: A Review. Polymers 2021, 13, 4234.
- Li, J.H.; Wang, W.Z.; Jiang, R.; Guo, C.C. Antiviral electrospun polymer composites: Recent advances and opportunities for tackling COVID-19. Front. Mater. 2021, 8, 773205.
- Leong, J.Y.; Shi, D.R.; Tan, J.P.K.; Yang, C.; Yang, S.C.; Wang, Y.M.; Ngow, Y.S.; Kng, J.; Balakrishnan, N.; Peng, S.Q.; et al. Potent antiviral and antimicrobial polymers as safe and effective disinfectants for the prevention of infections. Adv. Healthc. Mater. 2021, 2101898.
- Kuroki, A.; Tay, J.; Lee, G.H.; Yang, Y.Y. Broad-Spectrum Antiviral Peptides and Polymers. Adv. Healthc. Mater. 2021, 10, e2101898.
- Steinman, N.Y.; Hu, T.; Dombrovsky, A.; Reches, M.; Domb, A.J. Antiviral polymers based on N-halamine polyurea. Biomacromolecules 2021, 22, 4357–4364.
- Yadavalli, T.; Mallick, S.; Patel, P.; Koganti, R.; Shukla, D.; Date, A.A. Pharmaceutically acceptable carboxylic acid-terminated polymers show activity and selectivity against HSV-1 and HSV-2 and synergy with antiviral drugs. ACS Infect. Dis. 2020, 6, 2926–2937.
- Bianculli, R.H.; Mase, J.D.; Schulz, M.D. Antiviral polymers: Past approaches and future possibilities. Macromolecules 2020, 53, 9158–9186.
- Soria-Martinez, L.; Bauer, S.; Giesler, M.; Schelhaas, S.; Materlik, J.; Janus, K.; Pierzyna, P.; Becker, M.; Snyder, N.L.; Hartmann, L. Prophylactic antiviral activity of sulfated glycomimetic oligomers and polymers. J. Am. Chem. Soc. 2020, 142, 5252–5265.
- Roner, M.R.; Carraher, C.E.; Miller, L.; Mosca, F.; Slawek, P.; Haky, J.E.; Frank, J. Organotin polymers as antiviral agents including inhibition of Zika and Vaccinia viruses. J. Inorg. Organomet. Polym. Mater. 2020, 30, 684–694.
- Gunther, S.C.; Maier, J.D.; Vetter, J.; Podvalnyy, N.; Khanzhin, N.; Hennet, T.; Stertz, S. Antiviral potential of 3′-sialyllactose- and 6′-sialyllactose-conjugated dendritic polymers against human and avian influenza viruses. Sci. Rep. 2020, 10, 768.
- Kouhi, M.; Prabhakaran, M.P.; Ramakrishna, S. Edible polymers: An insight into its application in food, biomedicine and cosmetics. Trends Food Sci. Technol. 2020, 103, 248–263.
- Kenawy, E.-R.; Worley, S.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384.
- Vacas-Córdoba, E.; Maly, M.; De la Mata, F.J.; Gomez, R.; Pion, M.; Muñoz-Fernández, M.Á. Antiviral mechanism of polyanionic carbosilane dendrimers against HIV-1. Int. J. Nanomed. 2016, 11, 1281.
- Kim, Y.; Park, E.J.; Na, D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018, 41, 571–582.
- Caminade, A.M.; Turrin, C.O.; Laurent, R.; Rebout, C.; Majoral, J.P. Phosphorus dendritic architectures: Polyanionic and polycationic derivatives. Polym. Int. 2006, 55, 1155–1160.
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Ferreira, E.I.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharmaceut. 2020, 573, 118814.
- Witvrouw, M.; Fikkert, V.; Pluymers, W.; Matthews, B.; Mardel, K.; Schols, D.; Raff, J.; Debyser, Z.; De Clercq, E.; Holan, G. Polyanionic (i.e., polysulfonate) dendrimers can inhibit the replication of human immunodeficiency virus by interfering with both virus adsorption and later steps (reverse transcriptase/integrase) in the virus replicative cycle. Mol. Pharmacol. 2000, 58, 1100–1108.
- Bernstein, D.; Stanberry, L.; Sacks, S.; Ayisi, N.; Gong, Y.; Ireland, J.; Mumper, R.; Holan, G.; Matthews, B.; McCarthy, T. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob. Agents Chemother. 2003, 47, 3784–3788.
- Gong, Y.; Matthews, B.; Cheung, D.; Tam, T.; Gadawski, I.; Leung, D.; Holan, G.; Raff, J.; Sacks, S. Evidence of dual sites of action of dendrimers: SPL-2999 inhibits both virus entry and late stages of herpes simplex virus replication. Antivir. Res. 2002, 55, 319–329.
- Mumper, R.J.; Bell, M.A.; Worthen, D.R.; Cone, R.A.; Lewis, G.R.; Paull, J.R.; Moench, T.R. Formulating a sulfonated antiviral dendrimer in a vaginal microbicidal gel having dual mechanisms of action. Drug Dev. Ind. Pharm. 2009, 35, 515–524.
- Paull, J.R.A.; Heery, G.P.; Bobardt, M.D.; Castellarnau, A.; Luscombe, C.A.; Fairley, J.K.; Gallay, P.A. Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro. Antivir. Res. 2021, 191, 105089.
- Martínez-Gualda, B.; Sun, L.; Rivero-Buceta, E.; Flores, A.; Quesada, E.; Balzarini, J.; Noppen, S.; Liekens, S.; Schols, D.; Neyts, J. Structure-activity relationship studies on a Trp dendrimer with dual activities against HIV and enterovirus A71. Modifications on the amino acid. Antivir. Res. 2017, 139, 32–40.
- Anderson, L.J.; Dormitzer, P.R.; Nokes, D.J.; Rappuoli, R.; Roca, A.; Graham, B.S. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 2013, 31 (Suppl. S2), B209–B215.
- Kozhikhova, K.V.; Shilovskiy, I.P.; Shatilov, A.A.; Timofeeva, A.V.; Turetskiy, E.A.; Vishniakova, L.I.; Nikolskii, A.A.; Barvinskaya, E.D.; Karthikeyan, S.; Smirnov, V.V. Linear and dendrimeric antiviral peptides: Design, chemical synthesis and activity against human respiratory syncytial virus. J. Mater. Chem. B 2020, 8, 2607–2617.
- Ortega, M.A.; Guzman Merino, A.; Fraile-Martinez, O.; Recio-Ruiz, J.; Pekarek, L.; Guijarro, L.G.; Garcia-Honduvilla, N.; Alvarez-Mon, M.; Bujan, J.; Garcia-Gallego, S. Dendrimers and dendritic materials: From laboratory to medical practice in infectious diseases. Pharmaceutics 2020, 12, 874.
- Sepulveda-Crespo, D.; Lorente, R.; Leal, M.; Gomez, R.; Francisco, J.; Jiménez, J.L.; Muñoz-Fernández, M.Á. Synergistic activity profile of carbosilane dendrimer G2-STE16 in combination with other dendrimers and antiretrovirals as topical anti-HIV-1 microbicide. Nanomedicine 2014, 10, 609–618.
- Asaftei, S.; Huskens, D.; Schols, D. HIV-1 X4 activities of polycationic “viologen” based dendrimers by interaction with the chemokine receptor CXCR4: Study of structure–activity relationship. J. Med. Chem. 2012, 55, 10405–10413.
- Relaño-Rodríguez, I.; Muñoz-Fernández, M.Á. Emergence of nanotechnology to fight HIV sexual transmission: The trip of G2-S16 polyanionic carbosilane dendrimer to possible pre-clinical trials. Int. J. Mol. Sci. 2020, 21, 9403.
- Sepúlveda-Crespo, D.; Jiménez, J.L.; Gómez, R.; De La Mata, F.J.; Majano, P.L.; Muñoz-Fernández, M.Á.; Gastaminza, P. Polyanionic carbosilane dendrimers prevent hepatitis C virus infection in cell culture. Nanomedicine 2017, 13, 49–58.
- Chonco, L.; Pion, M.; Vacas, E.; Rasines, B.; Maly, M.; Serramia, M.; Lopez-Fernandez, L.; De la Mata, J.; Alvarez, S.; Gomez, R. Carbosilane dendrimer nanotechnology outlines of the broad HIV blocker profile. J. Contr. Rel. 2012, 161, 949–958.
- Ogata, M.; Umemura, S.; Sugiyama, N.; Kuwano, N.; Koizumi, A.; Sawada, T.; Yanase, M.; Takaha, T.; Kadokawa, J.-I.; Usui, T. Synthesis of multivalent sialyllactosamine-carrying glyco-nanoparticles with high affinity to the human influenza virus hemagglutinin. Carbohydr. Polym. 2016, 153, 96–104.
- Zhong, M.; Yu, Y.; Song, J.-Q.; Jia, T.-W.; Liu, A.-Y.; Zhao, T.-F.; He, H.-J.; Yang, M.-B.; Zhang, W.-X.; Yang, Y. Amide-sialoside protein conjugates as neomucin bioshields prevent influenza virus infection. Carbohydr. Res. 2020, 495, 108088.
- Stencel-Baerenwald, J.E.; Reiss, K.; Reiter, D.M.; Stehle, T.; Dermody, T.S. The sweet spot: Defining virus–sialic acid interactions. Nat. Rev. Microbiol. 2014, 12, 739–749.
- Gulati, S.; Smith, D.F.; Cummings, R.D.; Couch, R.B.; Griesemer, S.B.; George, K.S.; Webster, R.G.; Air, G.M. Human H3N2 influenza viruses isolated from 1968 to 2012 show varying preference for receptor substructures with no apparent consequences for disease or spread. PLoS ONE 2013, 8, e66325.
- Yu, Y.; Qin, H.-J.; Shi, X.-X.; Song, J.-Q.; Zhou, J.-P.; Yu, P.; Fan, Z.-C.; Zhong, M.; Yang, Y. Thiosialoside-decorated polymers use a two-step mechanism to inhibit both early and late stages of influenza virus infection. Eur. J. Med. Chem. 2020, 199, 112357.
- Nagao, M.; Fujiwara, Y.; Matsubara, T.; Hoshino, Y.; Sato, T.; Miura, Y. Design of glycopolymers carrying sialyl oligosaccharides for controlling the interaction with the influenza virus. Biomacromolecules 2017, 18, 4385–4392.
- Nagao, M.; Matsubara, T.; Hoshino, Y.; Sato, T.; Miura, Y. Topological design of star glycopolymers for controlling the interaction with the influenza virus. Bioconj. Chem. 2019, 30, 1192–1198.
- Tang, S.; Puryear, W.B.; Seifried, B.M.; Dong, X.; Runstadler, J.A.; Ribbeck, K.; Olsen, B.D. Antiviral agents from multivalent presentation of sialyl oligosaccharides on brush polymers. ACS Macro Lett. 2016, 5, 413–418.
- Matrosovich, M.N.; Gambaryan, A.S.; Teneberg, S.; Piskarev, V.E.; Yamnikova, S.S.; Lvov, D.K.; Robertson, J.S.; Karlsson, K.A. Avian influenza a viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology 1997, 233, 224–234.
More
