Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Bettina Toth.
As chemotherapy—in contrast to radiation—is mainly applied to all cancer patients, a myriad of studies already exists, which indicate that commonly used agents cause premature ovarian insufficiency (POI) by inducing the death or accelerated loss of primordial follicles as well as damage to blood vessels, stromal cells, and immune components.
- low-dose radiation
- ovarian damage
1. Introduction
The overall five-year relative survival rate of all childhood cancers has significantly improved over the last thirty years from 58% to 83%, giving rise to a special population: adult survivors of childhood cancer after cytotoxic chemo- and radiotherapy (RT) with the desire to have children regardless of their medical history [1,2][1][2]. Fertile women have lower chances to become a mother after surviving cancer treatments [3].
As chemotherapy—in contrast to radiation—is mainly applied to all cancer patients, a myriad of studies already exists, which indicate that commonly used agents cause premature ovarian insufficiency (POI) by inducing the death or accelerated loss of primordial follicles as well as damage to blood vessels, stromal cells, and immune components [4,5,6,7,8,9,10,11,12][4][5][6][7][8][9][10][11][12]. However, data on (low-dose) radiation in fertile women are urgently needed.
Radiotherapy accelerates oocyte depletion by different mechanisms such as apoptosis or oxidative stress, leading to acute ovarian failure (AOF), POI, and menopause. Radiotherapy is a cornerstone of state-of-the-art cancer therapy in children, women, and men. At present, around 50–60% of all patients with long-term cancer (45–50% curative success for all cancer types) receive radiation therapy alone or in combined treatment schedules [13]. The direct and undisputed beneficial effects of targeted radiation on tumor cells result from preventing further cell proliferation or inducing cell death. Irrespective of this, the surrounding organs can be directly damaged by scattered radiation [13,14][13][14].
The human ovary contains a finite pool of primordial follicles (PMF), which comprise the ovarian reserve. Its maximum is already reached as a fetus at five months of gestation, which then declines with increasing age and culminates in the menopause at an average age of 50–52 years [14,15][14][15]. This fixed number of oocytes is non-renewable and must provide for the entire reproduction cycle throughout adult life. The maximum reserve during fetal life is followed by atresia and the loss of more than half of the originally developed germ cell pool at the time of birth. Only oocytes that are enclosed by a sufficient number of epithelial and stromal cells are able to survive. Therefore, the interaction between oocytes and stromal cells is of utmost importance even during prenatal development [16,17,18][16][17][18]. Different cell populations of the ovarian cortex can be defined by gene expression analyses: stroma (83%); oocytes (0.2%); perivascular (10%); endothelial (5%); granulosa (1.2%); and theca and immune cells (0.4%) [19,20][19][20].
In the last decade, maintaining the gonadal function and preserving fertility after successful cancer treatment have become critical and have increased the concerns of young fertile patients. The options depend upon the age of the patient, their physical state, the administered agent, and the start of the cancer treatment. Regarding radiation of the pelvis, ovaries can be transposed outside the radiation field (ovarian transposition). This surgical technique has been performed since 1952 with low success rates and a high risk of POI (33–100%) also due to scattered radiation [21,22,23][21][22][23]. Patients undergoing brachytherapy seem to benefit most with ovarian survival rates from 77.8–100% [24]. However, an increased risk of ovarian cysts after ovarian transposition has been reported, ranging between 0 and 34% [25,26][25][26]. Together with other complications such as abdominal pain, hematomas, tubal ligation, ischemia, or small bowel obstructions, reoperation was necessary in 34.7% of patients with complications [24]. Therefore, ovarian transposition may be offered to women with planned pelvic radiation without chemotherapy as being recommended by international guidelines [27,28,29,30][27][28][29][30]. Of note, supporting evidence is weak; therefore, ovarian transposition may not act as a safe procedure for fertility preservation with special regard to the above-mentioned complications. Another technique—which can only be offered after puberty—is the cryopreservation of oocytes before the legal age and the cryopreservation of (non) fertilized oocytes thereafter. In order to cryopreserve, women need to undergo controlled hormonal stimulation before the oocytes are transvaginally retrieved [31]. Therefore, this technique can only be offered if the cancer treatment can be postponed for at least 2–3 weeks.
The cryopreservation of ovarian tissue was first performed by Prof. Dr. Donnez in 1997. After successful cancer treatments, the tissue can be thawed and transplanted onto the remaining ovary or into a peritoneal tissue pouch near the ovaries. As the first successful ovarian tissue transplantation was performed in 2004, this technique is now routinely performed. The resumption of cyclic hormone production can be achieved in up to 63% of cases, and live birth rates (LBR) are described as 23% per transplantation [32,33][32][33]. To date, more than 170 live births after the transplantation of frozen–thawed ovarian cortical pieces have been reported, and the transplanted ovarian tissue remains active from six months to three years [32]. However, the cryopreservation of ovarian tissue is not established in children and only parts of the ovary (40–50%) can be cryopreserved whereas the major part stays within the pelvis and is exposed to radio-chemotherapy.
2. The Human Ovary and Folliculogenesis
Folliculogenesis
Human folliculogenesis has been described in detail in the literature [45,46][34][35]. Meiosis of the oogonia starts in early fetal life and the final PMF pool is formed around 24 gestational weeks in humans and around birth in rodents (Figure 21). Once meiosis is initiated, mitosis ends; therefore, the individual PMF pool is already fixed before birth. Of note, POI occurs in 1% of women under the age of 45 years without the influence of gonadotoxic treatment due to a low PMF pool [47][36]. After menarche, the follicles either start to grow or become atretic. The activation of PMF includes different pathways such as PI3K/PTEN7Akt and Hippo [48,49,50,51][37][38][39][40]. However, the detailed mechanisms of the regulation of the PMF pool over the reproductive female lifespan remain unknown. PMFs are mainly located in the ovarian cortex, which represents the poorest vascularized zone in the human ovary. Both primordial and early growing follicles are dependent on stromal vessels as they do not rely on an independent vascular network [52][41]. PMFs consist of an oocyte surrounded by granulosa cells [53][42]. The high proliferation rate of granulosa cells explains their sensitivity to chemotherapy and radiation. The secondary follicle is characterized by the formation of a zona pellucida, stromal cells, and an undifferentiated theca layer. After differentiation into a theca interna and a theca externa layer, the stage of a pre-antral follicle is reached followed by an early antral follicle with fluid-filled cavities and a large antral follicle with a visible large antrum and a marginal oocyte [46,53][35][42].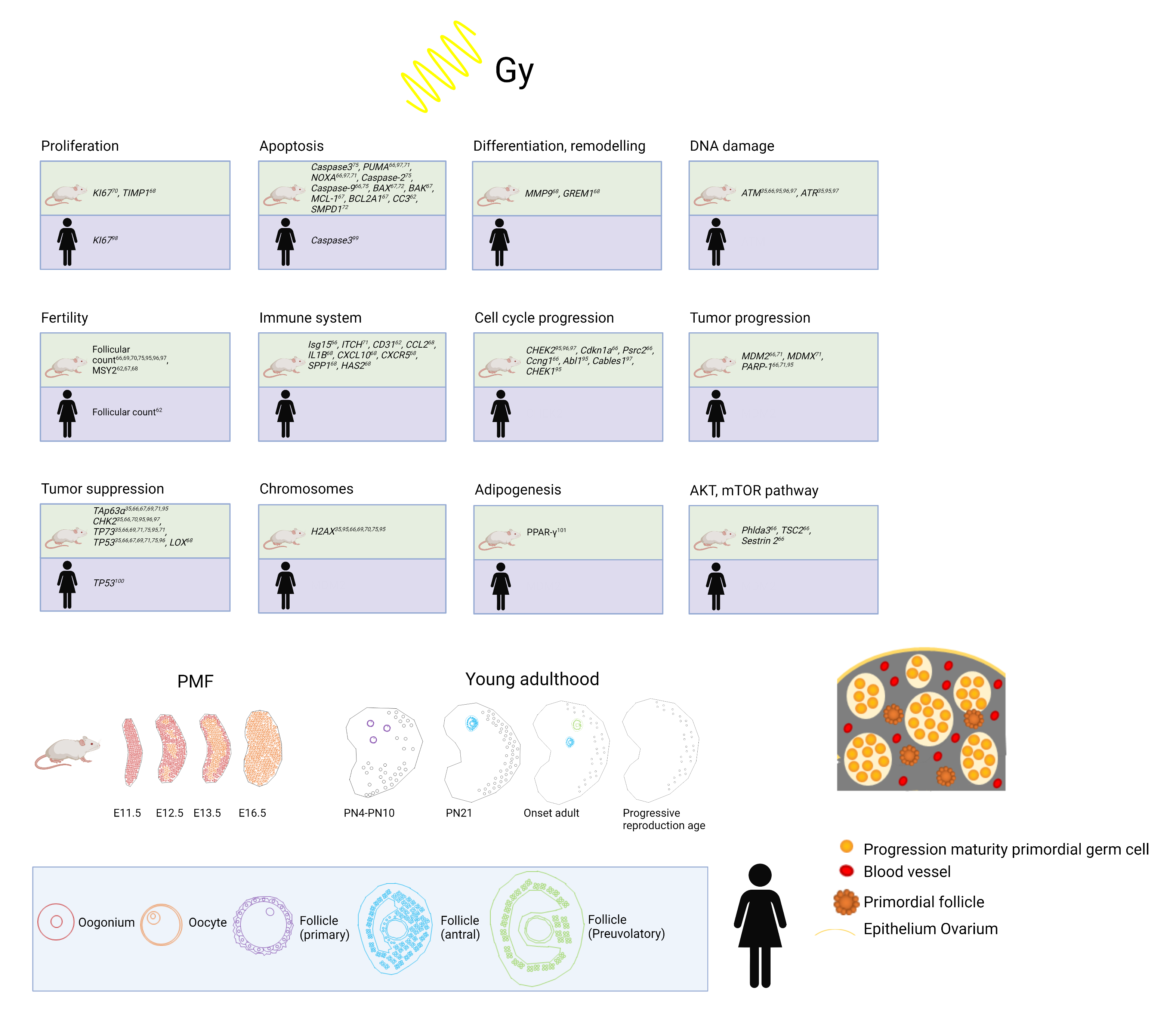
Figure 21. Top: Overview of already established markers concerning ovarian damage including proliferation, apoptosis, differentiation and remodeling, DNA damage, fertility, immune system, cell cycle progression, tumor progression, tumor suppression, chromosomes, adipogenesis, and AKT/mTOR pathway after radiation in human and mouse models. Bottom: Development from PMF pool to young adulthood in mice and humans and human folliculogenesis and detail in PMF pool in humans at birth. Adapted from [54,55,56,57,58,59,60,61,62].
3. Application of Low-Dose Radiation in Clinical Practice
Cancer treatment can cause female infertility in different ways. Fertility impairment as well as an elevated risk of pregnancy complications primarily depend on the applied radiation dose and on the composition of a systemic co-treatment. Thereby, an additive effect is likely for combined chemoradiation schedules. In addition, the age at treatment and the location of the treatment target are decisive factors [63][43]. Pediatric cancer survivors are known to exhibit a significant increase in the risk of infertility at doses ranging from 1 to 5 Gray (Gy) to the uterine region (RR = 1.33), which is further elevated at >20 Gy (RR = 2.50) [64][44]. The increasing use of intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) techniques can cause higher co-radiation doses (radiation leakage) deposited in the uterine and ovarian region compared with standard conformal RT [65][45]. Modern radiation planning techniques with ovary-sparing protocols can also be applied to significantly reduce ovarian radiation exposure [66][46]. However, the proof of functional outcome advantages using this method is still lacking. Moreover, position variability as well as ovarian movements complicate ovarian protection using IMRT. The effect of co-radiation ranging from the lowest mGy doses up to 0.5–1.0 Gy as a consequence of more distant target sites or optimized organs at risk-sparing during treatment is still speculative or a matter of linear extrapolation (linear non-threshold (LNT) hypothesis) [67][47]. In addition, individual and organ-specific differences in the response to LDR as well as cell–biological mechanisms such as the bystander effect further complicate a generally valuable risk prediction [68][48]. Nevertheless, for a few very low doses, the overall risk of a certain health effect may be clinically identical with the absence of a radiation-induced excess risk. This might also be valuable for uncertain risk estimations of infertility or germ cell mutations following curative radiotherapy with a low-dose involvement of the reproductive tract. In order to prove the presence, extent, or absence of such risks for female cancer patients at a certain assumptive threshold, the only available data are provided by epidemiological studies, which principally employ an observational, non-experimental approach. However, data from epidemiological studies are generated by the uncontrolled conditions of everyday life and randomized controlled trials are unacceptable for the investigation of actual or potential hazardous exposures [69,70,71][49][50][51]. Thus, radiobiological clues to investigate the low-dose effects of high-energy radiation first have to be obtained from dose-relationship studies using in vitro and in vivo animal models [72][52]. The dose–response relationship describing the excess risk of stochastic health effects (radiation-induced cancer and germ cell mutations) following low-dose levels of exposure to ionizing radiation is more and more controversially discussed. The standard approach for the purposes of radiological protection is based on the hypothesis that radiation-induced risks are directly proportional to the administered dose, as described by the LNT hypothesis. Nevertheless, several radiobiologists have argued that this approach underestimates the current risks (i.e., the relationship is properly described by dose–response curves of a supralinear shape) or that there is a threshold dose below which either no effect or even a beneficial (hormetic) effect is likely to exist [73][53]. In the past, the International Commission on Radiological Protection (ICRP) released several reports concluding that a low dose threshold seems to exist for radiation-induced malignancies of certain tissues as well as for infertility or germ cell mutations [74,75,76][54][55][56]. In their opinion, this evidence does not favor the existence of a universal threshold. The LNT hypothesis, combined with an uncertain dose and dose-rate effectiveness factor (DDREF) for extrapolation from high doses, still represents the propagated basis for radiation protection at low doses and low-dose rates by the ICRP, World Health Organization (WHO), and other official authorities. However, the almost dogmatic LNT approach is being increasingly questioned within the expert community due to the continuously increasing amount of reports that prove hormesis, an adaptive response, and individually varying susceptibility to high-energy low-dose and/or low-dose-rate radiation [77][57]. With regard to the risk of infertility after chemo-co-radiation, it is of utmost relevance to gain further experimental insight from suitable translational research and bias-revised epidemiological studies in order to investigate the potential existence of lower and upper threshold doses in gonadotoxicity. Both the evidenced presence of threshold doses and a finally confirmed linear dose relationship from below 0.5 to 1 Gy would decisively improve the process of therapy decision making for fertile female cancer patients with a good survival prognosis.References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA A Cancer J. Clin. 2016, 66, 271–289.
- Von der Weid, N.X. Adult life after surviving lymphoma in childhood. Support. Care Cancer 2008, 16, 339–345.
- Anderson, R.A.; Brewster, D.H.; Wood, R.; Nowell, S.; Fischbacher, C.; Kelsey, T.W.; Wallace, W.H.B. The impact of cancer on subsequent chance of pregnancy: A population-based analysis. Hum. Reprod. 2018, 33, 1281–1290.
- Bedoschi, G.M.; Navarro, P.A.; Oktay, K.H. Novel insights into the pathophysiology of chemotherapy-induced damage to the ovary. Panminerva Med. 2019, 61, 68–75.
- Ben-Aharon, I.; Bar-Joseph, H.; Tzarfaty, G.; Kuchinsky, L.; Rizel, S.; Stemmer, S.M.; Shalgi, R. Doxorubicin-induced ovarian toxicity. Reprod. Biol. Endocrinol. 2010, 8, 20.
- Bildik, G.; Akin, N.; Senbabaoglu, F.; Sahin, G.N.; Karahuseyinoglu, S.; Ince, U.; Taskiran, C.; Selek, U.; Yakin, K.; Guzel, Y.; et al. GnRH agonist leuprolide acetate does not confer any protection against ovarian damage induced by chemotherapy and radiation in vitro. Hum. Reprod. 2015, 30, 2912–2925.
- Yuksel, A.; Bildik, G.; Senbabaoglu, F.; Akin, N.; Arvas, M.; Unal, F.; Kilic, Y.; Karanfil, I.; Eryılmaz, B.; Yilmaz, P.; et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum. Reprod. 2015, 30, 2926–2935.
- Chang, E.M.; Lim, E.; Yoon, S.; Jeong, K.; Bae, S.; Lee, D.R.; Yoon, T.K.; Choi, Y.; Lee, W.S. Cisplatin Induces Overactivation of the Dormant Primordial Follicle through PTEN/AKT/FOXO3a Pathway which Leads to Loss of Ovarian Reserve in Mice. PLoS ONE 2015, 10, e0144245.
- Chow, E.J.; Stratton, K.L.; Leisenring, W.M.; Oeffinger, K.C.; Sklar, C.A.; Donaldson, S.S.; Ginsberg, J.P.; Kenney, L.B.; Levine, J.M.; Robison, L.L.; et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2016, 17, 567–576.
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Morgan, F.H.; Strasser, A.; Scott, C.L.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018, 9, 1–12.
- Özcan, P.; Fıçıcıoğlu, C.; Yıldırım, Ö.K.; Özkan, F.; Akkaya, H.; Aslan, İ. Protective effect of resveratrol against oxidative damage to ovarian reserve in female Sprague-Dawley rats. Reprod. Biomed. Online 2015, 31, 404–410.
- Liu, Z.-Q.; Shen, M.; Wu, W.-J.; Li, B.-J.; Weng, Q.-N.; Li, M.; Liu, H.-L. Expression of PUMA in Follicular Granulosa Cells Regulated by FoxO1 Activation During Oxidative Stress. Reprod. Sci. 2015, 22, 696–705.
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800.
- Motta, P.M.; Makabe, S.; Nottola, S.A. The ultrastructure of human reproduction. I. The natural history of the female germ cell: Origin, migration and differentiation inside the developing ovary. Hum. Reprod. Update 1997, 3, 281–297.
- Grive, K.J.; Freiman, R.N. The developmental origins of the mammalian ovarian reserve. Development 2015, 142, 2554–2563.
- Mork, L.; Maatouk, D.M.; McMahon, J.A.; Guo, J.J.; Zhang, P.; McMahon, A.P.; Capel, B. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol. Reprod. 2012, 86, 37.
- Liu, C.; Peng, J.; Matzuk, M.M.; Yao, H.H. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat. Commun. 2015, 6, 6934.
- Rotgers, E.; Jørgensen, A.; Yao, H.H. At the Crossroads of Fate-Somatic Cell Lineage Specification in the Fetal Gonad. Endocr. Rev. 2018, 39, 739–759.
- Wagner, M.; Yoshihara, M.; Douagi, I.; Damdimopoulos, A.; Panula, S.; Petropoulos, S.; Lu, H.; Pettersson, K.; Palm, K.; Katayama, S.; et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat. Commun. 2020, 11, 1147.
- Fan, X.; Bialecka, M.; Moustakas, I.; Lam, E.; Torrens-Juaneda, V.; Borggreven, N.V.; Trouw, L.; Louwe, L.A.; Pilgram, G.S.K.; Mei, H.; et al. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat. Commun. 2019, 10, 3164.
- Thomas, P.R.; Winstanly, D.; Peckham, M.J.; Austin, D.E.; Murray, M.A.; Jacobs, H.S. Reproductive and endocrine function in patients with Hodgkin’s disease: Effects of oophoropexy and irradiation. Br. J. Cancer 1976, 33, 226–231.
- Yamamoto, R.; Okamoto, K.; Yukiharu, T.; Kaneuchi, M.; Negishi, H.; Sakuragi, N.; Fujimoto, S. A study of risk factors for ovarian metastases in stage Ib-IIIb cervical carcinoma and analysis of ovarian function after a transposition. Gynecol. Oncol. 2001, 82, 312–316.
- van Beurden, M.; Schuster-Uitterhoeve, A.; Lammes, F. Feasibility of transposition of the ovaries in the surgical and radiotherapeutical treatment of cervical cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 1990, 16, 141–146.
- Hoekman, E.J.; Broeders, E.; Louwe, L.A.; Nout, R.A.; Jansen, F.W.; de Kroon, C.D. Ovarian function after ovarian transposition and additional pelvic radiotherapy: A systematic review. Eur J. Surg Oncol 2019, 45, 1328–1340.
- Pahisa, J.; MartÍNez-RomÁN, S.; MartÍNez-Zamora, M.A.; TornÉ, A.; CaparrÓS, X.; SanjuÁN, A.; LejÁRcegui, J.A. Laparoscopic ovarian transposition in patients with early cervical cancer. Int. J. Gynecol. Cancer 2008, 18, 584–589.
- Husseinzadeh, N.; Van Aken, M.L.; Aron, B. Ovarian transposition in young patients with invasive cervical cancer receiving radiation therapy. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 1994, 4, 61–65.
- Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; Maslin, C.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 2020, hoaa052.
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84.
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006, 24, 2917–2931.
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001.
- Nagy, Z.P.; Anderson, R.E.; Feinberg, E.C.; Hayward, B.; Mahony, M.C. The Human Oocyte Preservation Experience (HOPE) Registry: Evaluation of cryopreservation techniques and oocyte source on outcomes. Reprod. Biol. Endocrinol. 2017, 15, 1–10.
- Balcerek, M.; Wolff, M.; Nawroth, F. Indikation Und Durchführung Fertilitätsprotektiver Massnahmen Bei Onkologischen Und Nichtonkologischen Erkrankungen. 2017. Available online: https://www.sggg.ch/fr/formation/formation-continue/evenements/detail/1/indikation-und-durchfuehrung-fertilitaetsprospektiver-massnahmen-bei-onkologischen-und-nicht-onkologischen-erkrankungen/ (accessed on 12 December 2021).
- Winkler-Crepaz, K.; Bottcher, B.; Toth, B.; Wildt, L.; Hofer-Tollinger, S. What is new in 2017? Update on fertility preservation in cancer patients. Minerva Endocrinol. 2017, 42, 331–339.
- Hirshfield, A.N. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991, 124, 43–101.
- Gougeon, A.; Ecochard, R.; Thalabard, J.C. Age-related changes of the population of human ovarian follicles: Increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol. Reprod. 1994, 50, 653–663.
- Coulam, C.B.; Adamson, S.C.; Annegers, J.F. Incidence of premature ovarian failure. Obstet. Gynecol. 1986, 67, 604–606.
- Ernst, E.H.; Grøndahl, M.L.; Grund, S.; Hardy, K.; Heuck, A.; Sunde, L.; Franks, S.; Andersen, C.Y.; Villesen, P.; Lykke-Hartmann, K. Dormancy and activation of human oocytes from primordial and primary follicles: Molecular clues to oocyte regulation. Hum. Reprod. 2017, 32, 1684–1700.
- Demeestere, I.; Brice, P.; Peccatori, F.A.; Kentos, A.; Dupuis, J.; Zachee, P.; Casasnovas, O.; Van Den Neste, E.; Dechene, J.; De Maertelaer, V.; et al. No Evidence for the Benefit of Gonadotropin-Releasing Hormone Agonist in Preserving Ovarian Function and Fertility in Lymphoma Survivors Treated With Chemotherapy: Final Long-Term Report of a Prospective Randomized Trial. J. Clin. Oncol. 2016, 34, 2568–2574.
- Grosbois, J.; Demeestere, I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum. Reprod. 2018, 33, 1705–1714.
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C.H.; Kawamura, N.; Tamura, M.; Hashimoto, S.; et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479.
- Delgado-Rosas, F.; Gaytán, M.; Morales, C.; Gómez, R.; Gaytán, F. Superficial ovarian cortex vascularization is inversely related to the follicle reserve in normal cycling ovaries and is increased in polycystic ovary syndrome. Hum. Reprod. 2009, 24, 1142–1151.
- Peters, H.; Byskov, A.G.; Grinsted, J. Follicular growth in fetal and prepubertal ovaries of humans and other primates. Clin. Endocrinol. Metab. 1978, 7, 469–485.
- Palmer, J.D.; Tsang, D.S.; Tinkle, C.L.; Olch, A.J.; Kremer, L.C.M.; Ronckers, C.M.; Gibbs, I.C.; Constine, L.S. Late effects of radiation therapy in pediatric patients and survivorship. Pediatr. Blood Cancer 2021, 68 (Suppl. 2), e28349.
- Barton, S.E.; Najita, J.S.; Ginsburg, E.S.; Leisenring, W.M.; Stovall, M.; Weathers, R.E.; Sklar, C.A.; Robison, L.L.; Diller, L. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013, 14, 873–881.
- Oonsiri, P.; Vannavijit, C.; Wimolnoch, M.; Suriyapee, S.; Saksornchai, K. Estimated radiation doses to ovarian and uterine organs in breast cancer irradiation using radio-photoluminescent glass dosimeters (RPLDs). J. Med. Radiat. Sci. 2021, 68, 167–174.
- Kovtun, K.A.; Yeo, W.-P.; Phillips, C.H.; Viswanathan, A.; Baldini, E.H. Ovary-Sparing Radiation Planning Techniques Can Achieve Ovarian Dose Reduction for Soft Tissue Sarcoma of the Buttock and Thigh. Sarcoma 2017, 2017, 2796925.
- Doss, M. Are We Approaching the End of the Linear No-Threshold Era? J. Nucl. Med. 2018, 59, 1786–1793.
- Tang, F.R.; Loke, W.K. Molecular mechanisms of low dose ionizing radiation-induced hormesis, adaptive responses, radioresistance, bystander effects, and genomic instability. Int. J. Radiat. Biol. 2015, 91, 13–27.
- Jaworowski, Z. Observations on the Chernobyl Disaster and LNT. Dose Response 2010, 8, 148–171.
- Sutou, S. Low-dose radiation from A-bombs elongated lifespan and reduced cancer mortality relative to un-irradiated individuals. Genes Environ. 2018, 40, 26.
- Shore, R.E.; Beck, H.L.; Boice, J.D.; Caffrey, E.A.; Davis, S.; Grogan, H.A.; Mettler, F.A.; Preston, R.J.; Till, J.E.; Wakeford, R.; et al. Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J. Radiol. Prot. 2018, 38, 1217–1233.
- Rodgers, B.E.; Holmes, K.M. Radio-adaptive response to environmental exposures at Chernobyl. Dose Response 2008, 6, 209–221.
- Shore, R.; Walsh, L.; Azizova, T.; Rühm, W. Risk of solid cancer in low dose-rate radiation epidemiological studies and the dose-rate effectiveness factor. Int. J. Radiat. Biol. 2017, 93, 1064–1078.
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. Ann. ICRP 2007, 37, 1–332.
- Stewart, F.A.; Akleyev, A.V.; Hauer-Jensen, M.; Hendry, J.H.; Kleiman, N.J.; Macvittie, T.J.; Aleman, B.M.; Edgar, A.B.; Mabuchi, K.; Muirhead, C.R.; et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs--threshold doses for tissue reactions in a radiation protection context. Ann. ICRP 2012, 41, 1–322.
- Harrison, J.D.; Balonov, M.; Bochud, F.; Martin, C.; Menzel, H.G.; Ortiz-Lopez, P.; Smith-Bindman, R.; Simmonds, J.R.; Wakeford, R. ICRP Publication 147: Use of Dose Quantities in Radiological Protection. Ann. ICRP 2021, 50, 9–82.
- Barnett, G.C.; West, C.M.; Dunning, A.M.; Elliott, R.M.; Coles, C.E.; Pharoah, P.D.; Burnet, N.G. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat. Rev. Cancer 2009, 9, 134–142.
More
