Aloperine is an alkaloid found in the seeds and leaves of the medicinal plant Sophora alopecuroides L. It has been used as herbal medicine in China for centuries due to its potent anti-inflammatory, antioxidant, antibacterial, and antiviral properties. Recently, aloperine has been widely investigated for its therapeutic activities. Aloperine is proven to be an effective therapeutic agent against many human pathological conditions, including cancer, viral diseases, and cardiovascular and inflammatory disorders. Aloperine is reported to exert therapeutic effects through triggering various biological processes, including cell cycle arrest, apoptosis, autophagy, suppressing cell migration, and invasion. It has also been found to be associated with the modulation of various signaling pathways in different diseases.
- apoptosis
- cell cycle
- autophagy
- PI3K/Akt
- NF-κB
- Nrf2
- Ras
1. Introduction
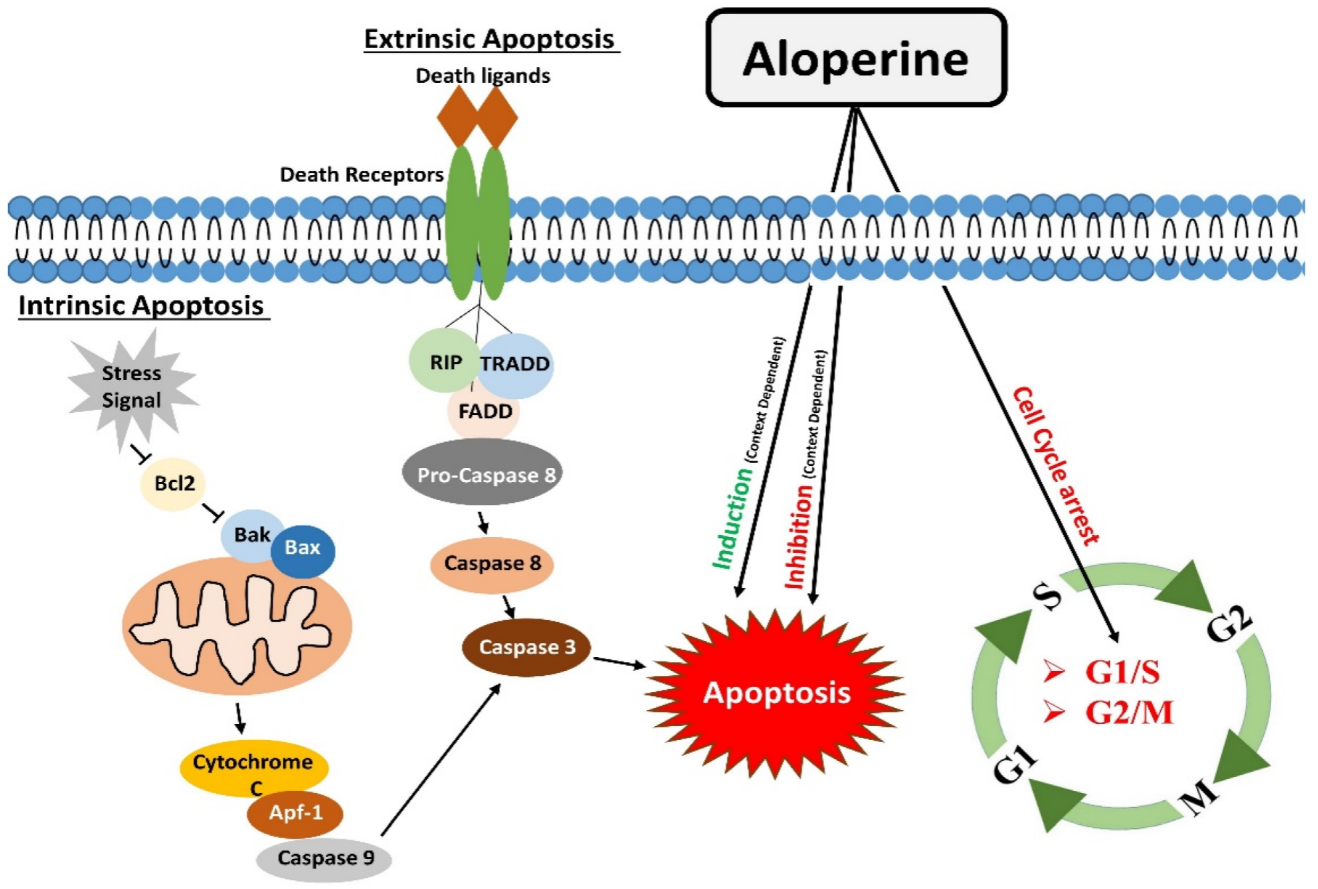
2. Regulation of Apoptosis
Apoptosis is one of the significant types of cell death [34], mainly directed by caspases (cysteine proteases). Apoptosis occurs by two main pathways: the extrinsic and intrinsic pathways. Apoptosis is complex, energy-dependent process, and it is crucial in removing dying or unwanted cells in normal conditions. Apoptosis is one of many therapeutic agents’ common mechanisms of action [10,35][10][35]. The extrinsic apoptosis or death receptors pathway works by binding death receptors with specific ligands. This binding enables the recruitment of Fas-associated death domain (FADD), which could bind to Fas, TRAIL-R1/2, or TNFR1. This interaction causes the activation of downstream events, which ultimately leads to the activation of caspase 8. Activated caspase 8 brings about apoptosis either by directly activating caspases cascade (Type I) or indirectly by cytochrome c mediated activation of a caspase cascade (Type II) [36]. The intrinsic apoptotic pathway or mitochondrial apoptotic pathway is activated in response to context-dependent stimuli. It causes the release of cytochrome c to the cytosol. Cytochrome c undergoes ATP-dependent binding with protease activating factor-1 (Apaf-1), which results in apoptosome formation. The apoptosome activates Caspase-9, which activates caspases 3,6,7 to carry out apoptosis [12]. Aloperine proved to be a potent inducer of apoptosis. One study reported that aloperine treatment caused apoptosis in U266 and MM.1S myeloma cells by activating the extrinsic apoptosis pathway. Activation of caspases 8/9/3 through aloperine therapy executed apoptosis. In thise study, aloperine was found to activate the caspase by inhibiting the anti-apoptotic cFLIP [22]. The apoptotic role of aloperine is also investigated in prostate cancer cells, which showed that aloperine induced apoptosis by changing the Bax/Bcl-2 ratio. It causes an increase in Bax (pro-apoptotic) and a decrease in Bcl-2 (anti-apoptotic). The change in the concentration of these apoptosis-related proteins activated caspase 3, which ultimately induced apoptosis in PC3, DU45, and LNCaP prostate cancer cells. These findings indicate that aloperine brought about apoptosis through the extrinsic apoptosis pathway [23]. Aloperine executed apoptosis in hepatocellular carcinoma cells. Aloperine treatment augmented cytochrome c level in the cytoplasm of hepatocellular carcinoma cells. Moreover, it caused the cleavage of caspase-9, caspase-3, and PARP and raised the levels of cleaved-caspase-9, cleaved-caspase-3, and cleaved-PARP (poly ADP ribose polymerase). This series of events lead to the apoptosis of liver cancer cells. The outcomes of this sentudry indicate that aloperine promoted apoptosis in HCC cells through the intrinsic apoptotic pathway [11]. The apoptosis induction effects of aloperine in osteosarcoma, colon cancer, breast cancer, glioma, and leukemia cells were determined. In these studies, the outcomes of western blotting and PCR experiments showed that aloperine treatment caused an increase and decrease in the levels of Bax and Bcl-2, respectively, and it also elevated cleaved caspase 3 level [7,11,14,26,37][7][11][14][26][37]. Similarly, aloperine inhibited Bcl-2 activity in bladder and NSCLC cells and caused apoptosis [24,33][24][33]. Since Bcl-2 protein and cleaved caspase-3 are the main components of the intrinsic apoptotic pathway [38[38][39],39], modulations in their levels showed that aloperine brought about apoptosis in OS cells through the intrinsic apoptotic pathway. Aloperine also triggered apoptosis in human thyroid carcinoma. IHH-4 and KMH-2 cells were found more susceptible to aloperine-induced programmed cell death. Aloperine treatment activated caspase-3 and PARP in a dose- and time-dependent manner. It also increased the levels of cleaved caspase-9 in IHH-4 and KMH-2 cells. Additionally, aloperine-treatment activated caspase-8 in KMH-2 cells. These outcomes indicate that aloperine activated intrinsic and extrinsic apoptosis pathways in human thyroid carcinoma cells [30]. The circNSUN2 RNA could promote cancer progression by binding to various RNA binding proteins. Regulation of the formation of circNSUN2 RNA-Protein complex could prevent cancer progression. Aloperine could inhibit the activity of circNSUN2 and counteract the tumor-promoting effects of circNSUN2. These findings suggest that aloperine treatment attenuated cell proliferation and increased the apoptosis in colorectal cancer cells via regulating the circNSUN2/miR-296-5p/STAT3 pathway [40]. Acute kidney disease resulting from renal ischemia and reperfusion (IR) damage is associated with high morbidity and mortality [41]. Tubular cell death frequently occurs in acute renal injury caused by IR [42]. The IR insult could raise caspase-3 levels and induce apoptosis in tubular cells. Interestingly, Hu et al. reported that aloperine treatment reduced tubular cells apoptosis in IR mice models. Protein expression analysis revealed a 1.3-fold reduction in caspase 3 levels in aloperine treated IR mice models compared to untreated mice models. These findings indicate that the treatment of aloperine could reduce apoptosis in tubular cells in IR mice [15]. This conclusion contradicts research in tumor cells where aloperine mainly promotes apoptosis in cancer cells. This variation in the outcome of aloperine treatment might be due to the differing aloperine doses utilized in cancer therapy. Hydrogen peroxide (H2O2) exposure can trigger apoptosis in N2a/Swe.D9 neuronal cells by activating the mitochondrial apoptotic pathway. Zhao et al. reported that aloperine inhibited the H2O2 mediated apoptosis in N2a/Swe.D9 cells. Hydrogen peroxide treatment promoted the release of cytochrome C from mitochondria to cytosol. Additionally, it decreased the Bcl-2 levels and activated caspase 3, but aloperine treatment reversed this apoptosis triggering effects and prevented N2a/Swe.D9 cells death [43]. Moreover, Ren et al. reported the inhibition of H2O2-mediated apoptosis in nucleus pulposus cells by aloperine. Hydrogen peroxide exposure induced apoptosis by increasing the caspase-9 activity in nucleus pulposus cells, but aloperine treatment inhibited the apoptosis of nucleus pulposus cells by attenuating the activity of caspase-9 [44]. Similarly, Zhang et al. also reported the anti-apoptotic effects of aloperine in H2O2 treated ARPE-19 cells. Hydrogen peroxide facilitated a decrease in Bcl-2 levels, and increased caspase 3 activity was mitigated by aloperine [19]. Furthermore, Li et al. evaluated the effects of aloperine in middle cerebral artery occlusion (MCAO)/reperfusion injury rat models. Brain sections of Rats models with cerebral IR injury showed a significant population of apoptotic cells and decreased Bcl-2 protein levels. Interestingly, aloperine treatment inhibited the apoptosis effects in rat models under investigation [16]. This finding shows that aloperine could regulate apoptotic pathways in a context and disease-dependent manner (Figure 1).3. Modulatory Effects on the Cell Cycle
During the cell growth and division, it undergoes a series of events known as the “cell cycle”. G1, S, G2, and M are the four main cell cycle phases. In the G1 phase, the cellular machinery makes preparation to divide. In cell division, the cell enters the S phase, during which it duplicates all of its genetic material. Hence, the suffix “S” stands for DNA synthesis. During the G2 stage, the arrangement and packaging of already duplicated genetic material are completed. The cell cycle moves to the next phase of the cell cycle. M phase is the next step in which cells physically divide into two daughter cells, and the copies of genetic material are distributed to newly formed daughter cells. At the end of the M phase, the cell cycle completes [45]. Specific serine/threonine-protein kinase regulates each cell cycle phase, known as cyclin-dependent protein kinases (CDKs). Cell cycle phase-specific CDKs make complexes with cyclin regulatory subunits and facilitate the cell cycle progression from one phase to the next [46]. Many drugs achieve their therapeutic effects by targeting the cell cycle. Blocking the cell cycle at different phases results in cell growth inhibition. A review of the literature exhibited that aloperine can effectively block the transition of the cell cycle at different stages. Cell cycle analysis of aloperine treated prostate cancer (PC) cells showed a high proportion of cells at the G1 phase. Further, western blotting analysis revealed increased p53 and p21 proteins, which confirmed that aloperine caused G1 phase cell cycle arrest in PC cells [22]. Previously, ouresearchers' research group conducted a study in NSCLC cells. WeResearchers also found that aloperine could cause G1 phase cell cycle arrest in NSCLC cells. OurResearchers' study showed that aloperine treatment upregulated the p53 and p21 proteins and downregulated the levels of Cyclin E, CDK2, pRb, and E2F1 proteins. By modifying the levels of G1 phase controlling proteins, aloperine achieved G1 phase cell cycle arrest in NSCLC cells [24]. Liu et al. reported that aloperine stopped the G2/M phase transition of the hepatocellular carcinoma cell cycle. Flow cytometry analysis of aloperine treated cells showed a high number of cells at the G2/M phase. Expression analysis exhibited low cdc25C, cdc2, and cyclin B1 proteins in aloperine treated Hep3B and Huh7 cells [23]. Moreover, G2/M phase arrest has also been observed in aloperine treated human colon cancer HCT116 cells. Cell cycle histograms showed elevated peaks at the G2/M phase of the cells cycle. The expression pattern of G2/M phase associated proteins p53, p21, cyclin D1, and B1 confirmed G2/M phase cells cycle arrest in HCT116 cells [14]. Furthermore, a study reported that aloperine executed G2/M phase cell cycle arrest in SNU-182 cancer cells. Propidium Iodide (PI) staining showed a high population of cells at the G2/M phase of the cell cycle. Interestingly, this study reported that overexpression of GRO1 oncogene reversed the cell cycle arresting effects of aloperine in SU-182 liver cancer cells. This finding indicates that aloperine may cause cell cycle arrest in SU-182 cells via downregulating GRO1 oncogene [21]. However, further investigations are needed to affirm this inference. On the contrary, aloperine treatment could not cause cell cycle arrest in IHH-4, 8505c, and KMH-2 thyroid cancer cells. There were no apparent changes in cell cycle histogram patterns [30]. This finding is inconsistent with the findings of studies conducted in other cell types, and this inconsistency might be due to differences in the genetic makeup of different cell types (Figure 1).4. Modulation of Autophagy
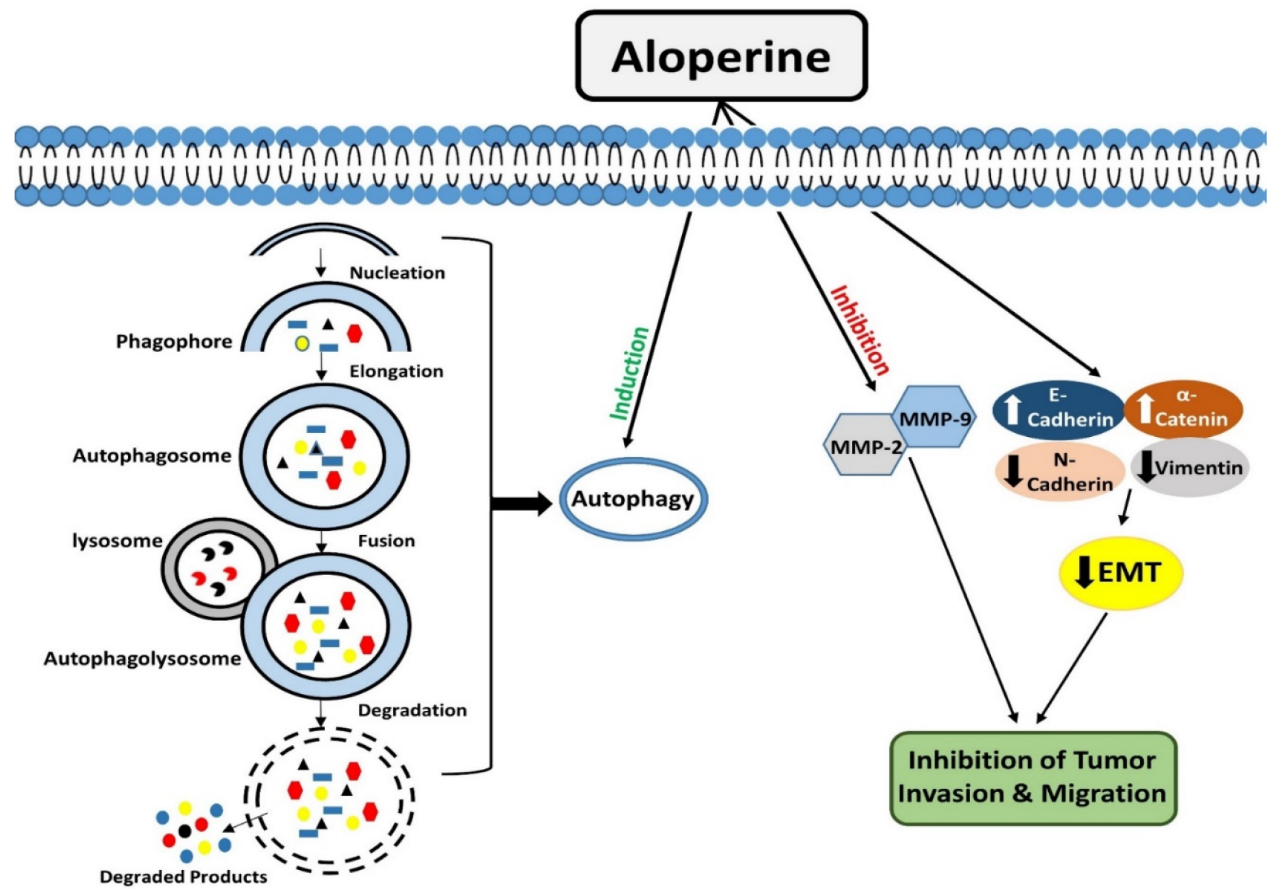
Autophagy is an evolutionarily conserved catabolic process that operates to degrade/remove undesirable cellular components, such as truncated or long-lasting proteins and unnecessary organelles [47,48][47][48]. Macro-autophagy, micro-autophagy, and chaperone-mediated autophagy are the three kinds of autophagy that have been described so far. Among all types, macro-autophagy is perhaps the most well investigated. The first step in autophagy is the formation of phagophores, which encloses truncated proteins/defective organelles. Phagophores undergo elongation and form a double membranous vesicle known as an autophagosome. These double membranous vesicles move towards and fuse with lysosomes to form autolysosomes. Finally, by the action of lysosomal enzymes, unwanted material is degraded, and recycled products are used to form new structures or used as energy sources [49]. Autophagy is a vital degradation process that maintains cellular homeostasis [50,51][50][51]. Many drugs, synthetic or natural, target autophagy to exert their therapeutic effects. Lin et al. conducted a study in HL-60 leukemia cells and evaluated the effects of aloperine treatment on autophagy. They showed that aloperine treatment for 18 h triggered the development of autophagic vacuoles. Acridine orange staining showed that the formation of autophagic vacuoles improved with the increase in the aloperine dosage. These findings demonstrated that aloperine could promote autophagy in HL-60 cells [7]. Moreover, aloperine exerted modulatory effects on autophagy were evaluated in thyroid cancer cells. Three types of thyroid cancer cells, KMH-2, IHH-4, and 8505c cells, were employed in this study. Interestingly, it was observed that aloperine treatment enhanced autophagosome formation and autophagic activity in KMH-2 and IHH-4 cells, but it did not produce such outcomes in 8505c cells. The expression analysis of LC3-II and p62 markers showed that aloperine blocked autophagic flux in 8505c cells [27]. The underlying molecular mechanism for aloperine to exhibit this dual role needs further elucidation (Figure 2).| Apoptosis | ||||||
|---|---|---|---|---|---|---|
| Pathological Conditions | Cell Lines | Animal Model | Dosage | Regulatory Effects of Aloperine | Ref. | |
| In Vitro (µM) | In Vivo | |||||
| Multiple Myeloma | U266 and MM.1S | SCID NOD mice | 50/100/250/500 | 20 mg/kg | Induced Caspase-dependent apoptosis | [12] |
| Prostate cancer | PC3, DU145 and LNCaP | BALB/C mice | 100/200 | 30 mg/kg | Induced Caspase dependent apoptosis | [22] |
| Hepatocellular carcinoma | Hep3B and Huh7 | Zebrafish embryo | 200/350/500 | 100 µM, 150 µM | Induced Mitochondria-dependent apoptosis | [23] |
| Osteosarcoma | MG-63 and U2OS | --------- | ||||
| Migration and Invasion | ||||||
| Breast cancer | ||||||
| MCF-7 and MDA-MB-231 | ||||||
| --------- | ||||||
| 100/200/400 | ||||||
| --------- | ||||||
| Inhibition of Migration and Invasion | ||||||
| [ | ||||||
| 26 | ||||||
| ] | ||||||
| Liver cancer | ||||||
| SNU-182 | ||||||
| --------- | ||||||
| 5 | ||||||
| --------- | Inhibition of Migration and Invasion | [ | 21 | ] | ||
| PI3K/Akt and Other Downstream Molecules Signaling | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathological Conditions | Cell Lines | Animal Model | Dosage | Regulatory Effects of Aloperine | Ref. | ||||||||
| In Vitro (µM) | In Vivo | ||||||||||||
| Prostate cancer | PC3, DU145 and LNCaP | BALB/C mice | 100/200 | 30 mg/kg | Inhibition of Akt/ERK signaling | [22] | |||||||
| Hepatocellular carcinoma | Hep3B and Huh7 | Zebrafish embryo | 200/350/500 | 100 µM, 150 µM | Inhibition of PI3K/Akt signaling | [23] | |||||||
| Osteosarcoma | MG-63 and U2OS | --------- | 100/200 | --------- | Inhibition of PI3K/Akt signaling | [11] | 100/200 | --------- | Induced Mitochondria-dependent apoptosis | [11] | |||
| [ | 14 | ] | Colon cancer | ||||||||||
| Colon cancer | HCT116 | ||||||||||||
| I/R-Induced Renal Injury | --------- | 250/500 | -------- | Induced Mitochondria-dependent apoptosis | [ | RAW264.7 and HK214] | |||||||
| HCT116 | C57BL/6 mice | 500 | 50 mg/kg | Inhibition of PI3K/Akt/mTOR signaling | [15] | Breast cancer | MCF-7 and MDA-MB-231 | --------- | 100/200/400 | ||||
| Thyroid Cancer | KMH-2 and IHH-4 | --------- | Induced Mitochondria-dependent apoptosis | [ | ---------26] | ||||||||
| --------- | 250/500 | --------- | Inhibition of PI3K/Akt signaling | 200 | --------- | Inhibition of Akt/mTOR signaling | [27] | I/R-Induced Renal Injury | RAW264.7 and HK2 | C57BL/6 mice | 500 | 50 mg/kg | Inhibition of Apoptosis |
| Thyroid Cancer | IHH-4,8505c and KMH-2 | [ | --------15] | ||||||||||
| 100/200 | ------- | Inhibition of Akt signaling | [30] | Thyroid Cancer | IHH-4,8505c and KMH-2 | --------- | 100/200 | --------- | Induced Caspase-dependent apoptosis | [30] | |||
| DSS-Induced Colitis | Jurkat Cells | C57BL/6 mice | 250/500 | 40 mg/kg | Inhibition of PI3K/Akt/mTOR signaling | [29] | Leukemia | HL-60 | --------- | 50/100 | --------- | Induced Mitochondria-dependent apoptosis | |
| Microembolisation-Induced cardiac Injury | --------- | Sprague-Dawley rats | ---------[ | 200 mg/kg7] | |||||||||
| Activation of the PI3K/Akt signaling | [ | 54 | ] | Alzheimer’s disease | N2a/Swe.D9 | --------- | |||||||
| I/R-Induced Cerebral injury | 100 | --------- | Induced Mitochondria-dependent apoptosis | --------- | Sprague-Dawley rats | ---------[43] | |||||||
| 2/25/50 mg/kg | Activation of the PI3K/Akt signaling | [ | 16] | Non-small cell lung cancer | H1944 and NCI-H1869 | BALB/C nude mice | 250 | 30 mg/kg | Induced Mitochondria-dependent apoptosis | [24] | |||
| Intervertebral disc degeneration | Nucleus Pulposus cells | Sprague-Dawley rats | 100 | --------- | Inhibition of Apoptosis | [44] | |||||||
| Bladder Cancer | EJ cells | --------- | 25/50/100 | --------- | Induced Mitochondria-dependent apoptosis | [59][52] | |||||||
| OGD/RP neuronal injury | Hippocampal Neuronal cells | Sprague-Dawley rats | 100/200/400 | --------- | Inhibition of Apoptosis | [60][53] | |||||||
| Colorectal Cancer | SW480 and HT29 | --------- | 200/400/800/1000 | --------- | |||||||||
| NF-κB Signaling | |||||||||||||
| Allergic airway inflammation | --------- | BALB/c mice | --------- | 100/200 mg/kg | Inhibition of NF-κB signaling | [18] | Induced Mitochondria-dependent apoptosis | [ | |||||
| Neuropathic pain | --------- | ICR mice | --------- | 80 mg/kg | Inhibition of NF-κB signaling | [40] | |||||||
| 31 | ] | Early brain injury | --------- | ||||||||||
| Intervertebral disc degeneration | Nucleus Pulposus cells | Sprague-Dawley rats | 100 | ------- | Inhibition of NF-κB signaling | [44] | |||||||
| Pulmonary arterial hypertension | --------- | Sprague-Dawley rats | --------- | 25/50/100 mg/kg | Inhibition of NF-κB signaling | [55] | Sprague-Dawley rats | --------- | 75/150 mg/kg | Inhibition of Apoptosis | [17] | ||
| Osteoporosis | RAW264.7 | C57BL/6 mice | 20 | 30 mg/Kg | Inhibition of NF-κB signaling | [56] | I/R-Induced Cerebral injury | --------- | |||||
| LPS-induced macrophage activation | Sprague-Dawley rats | --------- | 2/25/50 mg/kg | Inhibition of Apoptosis | [16] | ||||||||
| RAW264.7 | --------- | 50/100 | --------- | Inhibition of NF-κB signaling | [57] | Retinal pigment epithelial cells injury | ARPE-19 | --------- | 6.25/12.5/25 | --------- | Inhibition of Apoptosis | [19] | |
| Nrf2/HO-1 Signaling | DSS-Induced Colitis | Jurkat Cells | C57BL/6 mice | ||||||||||
| Allergic airway inflammation | --------- | BALB/c mice250/500 | 40 mg/kg | Inhibition of Apoptosis | [29] | ||||||||
| --------- | 100/200 mg/kg | Activation of Nrf2/HO-1 Signaling | [18] | Microembolisation-Induced cardiac Injury | --------- | Sprague-Dawley rats | --------- | 200 mg/kg | Inhibition of Apoptosis | [61][54] | |||
| Retinal pigment epithelial cells injury | ARPE-19 | --------- | 6.25/12.5/25 | --------- | Activation of Nrf2/HO-1 Signaling | [19] | Cell Cycle | ||||||
| High Glucose induced Schwann cells injury | RSC96 cells | --------- | 1/10/50 | --------- | Activation of Nrf2/HO-1 Signaling | [21] | Prostate cancer | PC3, DU145 and LNCaP | BALB/C mice | 100/200 | 30 mg/kg | G1 phase arrest | [22] |
| CCl4 induced mouse hepatic injury | --------- | C57BL/6 mice | --------- | 50/100 mg/kg | Activation of Nrf2/HO-1 Signaling | [58] | Hepatocellular carcinoma | Hep3B and Huh7 | Zebrafish embryo | 200/350/500 | 100 µM, 150 µM | G2 phase arrest | [23] |
| Ras Signaling | Colon cancer | HCT116 | --------- | 250/500 | --------- | G2 phase arrest | [14] | ||||||
| Thyroid Cancer | IHH-4,8505c and KMH-2 | --------- | 100/200 | --------- | No impact on Cell Cycle | [30] | |||||||
| Non-small cell lung cancer | H1944 and NCI-H1869 | BALB/C nude mice | 250 | 30 mg/kg | G1 phase arrest | [24] | |||||||
| Liver cancer | SNU-182 | --------- | 5 | --------- | G2 phase arrest | [21] | |||||||
| Autophagy | |||||||||||||
| Breast cancer | MCF-7 and MDA-MB-231 | --------- | 100/200/400 | --------- | Inhibition of Ras signaling | [26] | |||||||
| Bladder Cancer | EJ cells | ---------- | 25/50/100 | --------- | Inhibition of Ras signaling | [52] | Thyroid Cancer | KMH-2 and IHH-4 |
--------- | 200 | --------- | Autophagy induction | [27] |
| Thyroid Cancer | 8505c | --------- | 200 | --------- | Autophagy inhibition | [27] | |||||||
| Leukaemia | HL-60 | --------- | 50/100 | --------- | Autophagy induction | [7] | |||||||
References
- Chen, X.; Yi, C.; Yang, X.; Wang, X. Liquid chromatography of active principles in Sophora fla-vescens root. J. Chromatogr. B 2004, 812, 149–163.
- Wang, H.; Guo, S.; Qian, D.; Qian, Y.; Duan, J.-A. Comparative analysis of quinolizidine alkaloids from different parts of Sophora alopecuroides seeds by UPLC–MS/MS. J. Pharm. Biomed. Anal. 2012, 67–68, 16–21.
- Kangli, M.; Jianzhong, Z.; Ying, D.; Yufei, X. Research progress on the chemical compounds and pharmacology of Sophora flavescens. Nat. Prod. Res. Dev. 2001, 13, 69–73.
- Tolkachev, O.; Monakhova, T.; Sheichenko, V.; Kabanov, V.; Fesenko, O.; Proskurnina, N. Alka-loids of a new type from Sophora alopecuroides L. Chem. Nat. Compd. 1975, 11, 29–34.
- Brosius, A.; Ziller, J.; Zhang, Q. Relative and absolute configuration of aloperine. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997, 53, 1510–1512.
- Yang, L.; Chen, J.; Lin, Y. Clinical observation on the efficacy of mateling injection combined with radiotherapy in treating nasopharyngeal tumors. Strait Pharm. J. 1996, 8, 41–43.
- Lin, Z.; Huang, C.-F.; Liu, X.-S.; Jiang, J. In Vitro Anti-Tumour Activities of Quinolizidine Alkaloids Derived from Sophora Flavescens Ait. Basic Clin. Pharmacol. Toxicol. 2010, 108, 304–309.
- Zhou, C.C.; Gao, H.B.; Sun, X.B.; Shi, H.B.; Liu, W.; Yuan, H.N.; Wang, Z.X. Anti-inflammatory and anti-allergic action of aloperine. Zhongguo Yao Li Xue Bao Acta Pharmacol. Sin. 1989, 10, 360–365.
- Li Fan, S.; Zhang, S. Antiviral effect of aloperine. J. Zhong Cao Yao 1998, 29, 253–254.
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516.
- Chen, S.; Jin, Z.; Dai, L.; Wu, H.; Wang, J.; Wang, L.; Zhou, Z.; Yang, L.; Gao, W. Aloperine in-duces apoptosis and inhibits invasion in MG-63 and U2OS human osteosarcoma cells. Biomed. Pharmacother. 2018, 97, 45–52.
- Wang, H.; Yang, S.; Zhou, H.; Sun, M.; Du, L.; Wei, M.; Luo, M.; Huang, J.; Deng, H.; Feng, Y. Aloperine executes antitumor effects against multiple myeloma through dual apoptotic mecha-nisms. J. Hematol. Oncol. 2015, 8, 1–13.
- Xu, Z.; Yan, Y.; Zeng, S.; Qian, L.; Dai, S.; Xiao, L.; Wang, L.; Yang, X.; Xiao, Y.; Gong, Z. Re-ducing autophagy and inducing G1 phase arrest by aloperine enhances radio-sensitivity in lung cancer cells. Oncol. Rep. 2017.
- Zhang, L.; Zheng, Y.; Deng, H.; Liang, L.; Peng, J. Aloperine induces G2/M phase cell cycle arrest and apoptosis in HCT116 human colon cancer cells. Int. J. Mol. Med. 2014, 33, 1613–1620.
- Hu, S.; Zhang, Y.; Zhang, M.; Guo, Y.; Yang, P.; Zhang, S.; Simsekyilmaz, S.; Xu, J.-F.; Li, J.; Xiang, X. Aloperine protects mice against ischemia-reperfusion (IR)-induced renal injury by regu-lating PI3K/AKT/mTOR signaling and AP-1 activity. Mol. Med. 2015, 21, 912–923.
- Li, Z.; Cao, X.; Xiao, L.; Zhou, R. Aloperine protects against cerebral ischemia/reperfusion injury via activating the PI3K/AKT signaling pathway in rats. Exp. Ther. Med. 2021, 22, 1–8.
- Song, S.; Chen, Y.; Han, F.; Dong, M.; Xiang, X.; Sui, J.; Li, Y.; Yang, H.; Liu, J. Aloperine acti-vates the Nrf2-ARE pathway when ameliorating early brain injury in a subarachnoid hemorrhage model. Exp. Ther. Med. 2018, 15, 3847–3855.
- Wang, C.; Choi, Y.H.; Xian, Z.; Zheng, M.; Piao, H.; Yan, G. Aloperine suppresses allergic airway inflammation through NF-κB, MAPK, and Nrf2/HO-1 signaling pathways in mice. Int. Immunopharmacol. 2018, 65, 571–579.
- Zhang, J.; Zhou, H.; Chen, J.; Lv, X.; Liu, H. Aloperine protects human retinal pigment epithelial cells against hydrogen peroxide–induced oxidative stress and apoptosis through activation of Nrf2/HO-1 pathway. J. Recept. Signal Transduct. 2022, 42, 88–94.
- Casimiro, M.C.; Crosariol, M.; Loro, E.; Li, Z.; Pestell, R.G. Cyclins and cell cycle control in cancer and disease. Genes Cancer 2012, 3, 649–657.
- Huang, H.; Cao, Y.; Huang, L.; Lu, R.; Wang, J.; Zhou, Y. Aloperine suppresses the proliferation, migration and invasion of human liver cancer cells via induction of G2/M cell cycle arrest and inhibition of GROα expression. All Life 2021, 14, 392–400.
- Ling, Z.; Guan, H.; You, Z.; Wang, C.; Hu, L.; Zhang, L.; Wang, Y.; Chen, S.; Xu, B.; Chen, M. Aloperine executes antitumor effects through the induction of apoptosis and cell cycle arrest in prostate cancer n vitro and in vivo. OncoTargets Ther. 2018, 11, 2735.
- Liu, J.-S.; Huo, C.-Y.; Cao, H.-H.; Fan, C.-L.; Hu, J.-Y.; Deng, L.-J.; Lu, Z.-B.; Yang, H.-Y.; Yu, L.-Z.; Mo, Z.-X. Aloperine induces apoptosis and G2/M cell cycle arrest in hepatocellular carcinoma cells through the PI3K/Akt signaling pathway. Phytomedicine 2019, 61, 152843.
- Muhammad, T.; Sakhawat, A.; Khan, A.A.; Huang, H.; Khan, H.R.; Huang, Y.; Wang, J. Aloperine in combination with therapeutic adenoviral vector synergistically suppressed the growth of non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 861–874.
- Lv, W.; Liu, Q.; An, J.; Song, X. Aloperine prevents hypoxia-induced epithelial-mesenchymal transition in bladder cancer cells through regulating the mTOR/p70S6K/4E-BP1 pathway. Preprint 2020.
- Tian, D.; Li, Y.; Li, X.; Tian, Z. Aloperine inhibits proliferation, migration and invasion and induces apoptosis by blocking the Ras signaling pathway in human breast cancer cells. Mol. Med. Rep. 2018, 18, 3699–3710.
- Yu, H.-I.; Shen, H.-C.; Chen, S.-H.; Lim, Y.-P.; Chuang, H.-H.; Tai, T.-S.; Kung, F.-P.; Lu, C.-H.; Hou, C.-Y.; Lee, Y.-R. Autophagy Modulation in Human Thyroid Cancer Cells following Aloperine Treatment. Int. J. Mol. Sci. 2019, 20, 5315.
- Holz, R.W.; Fisher, S.K. Synaptic transmission and cellular signaling: An overview. Basic Neurochem. 2012, 235–257.
- Fu, X.; Sun, F.; Wang, F.; Zhang, J.; Zheng, B.; Zhong, J.; Yue, T.; Zheng, X.; Xu, J.-F.; Wang, C.-Y. Aloperine protects mice against DSS-induced colitis by PP2A-mediated PI3K/Akt/mTOR signaling suppression. Mediat. Inflamm. 2017, 2017, 5706152.
- Lee, Y.-R.; Chen, S.-H.; Lin, C.-Y.; Chao, W.-Y.; Lim, Y.-P.; Yu, H.-I.; Lu, C.-H. In Vitro Antitumor Activity of Aloperine on Human Thyroid Cancer Cells through Caspase-Dependent Apoptosis. Int. J. Mol. Sci. 2018, 19, 312.
- Xu, Y.-Q.; Jin, S.-J.; Liu, N.; Li, Y.-X.; Zheng, J.; Ma, L.; Du, J.; Zhou, R.; Zhao, C.-J.; Niu, Y. Aloperine attenuated neuropathic pain induced by chronic constriction injury via anti-oxidation activity and suppression of the nuclear factor kappa B pathway. Biochem. Biophys. Res. Commun. 2014, 451, 568–573.
- Chen, Y.; Ma, T.; Wang, Z.; Jia, L.; Zhang, X.; He, Q.; Liu, S. Aloperine attenuates high glucose-induced oxidative injury in Schwann cells via activation of NRF2/HO-1 pathway. Trop. J. Pharm. Res. 2020, 19, 1147–1152.
- Shin, S.-S.; Park, Y.-J.; Hwang, B.; Park, S.L.; Han, S.-W.; Park, S.-S.; Choi, Y.H.; Kim, W.-J.; Moon, S.-K. Triacanthine exerts antitumor effects on bladder cancer in vitro and in vivo. Phytomedicine 2019, 64, 153069.
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592.
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121.
- Jan, R.; Chaudhry, G.-E.-S. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218.
- Xu, Z.; Wang, X.; Chen, X.; Zeng, S.; Qian, L.; Wei, J.; Gong, Z.; Yan, Y. Identification of Aloperine as an anti-apoptotic Bcl2 protein inhibitor in glioma cells. PeerJ 2019, 7, e7652.
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104.
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707.
- Han, W.; Kong, D.; Lu, Q.; Zhang, W.; Fan, Z. Aloperine inhibits proliferation and promotes apoptosis in colorectal cancer cells by regulating the circNSUN2/miR-296-5p/STAT3 pathway. Drug Des. Dev. Ther. 2021, 15, 857.
- Schrier, R.W.; Wang, W. Acute Renal Failure and Sepsis. N. Engl. J. Med. 2004, 351, 159–169.
- Liang, H.L.; Arsenault, J.; Mortensen, J.; Park, F.; Johnson, C.P.; Nilakantan, V. Partial attenuation of cytotoxicity and apoptosis by SOD1 in ischemic renal epithelial cells. Apoptosis 2009, 14, 1176–1189.
- Zhao, J.; Zhang, G.; Li, M.; Luo, Q.; Leng, Y.; Liu, X. Neuro-protective effects of aloperine in an Alzheimer’s disease cellular model. Biomed. Pharmacother. 2018, 108, 137–143.
- Ren, D.; Ma, W.; Guo, B.; Wang, S. Aloperine attenuates hydrogen peroxide-induced injury via anti-apoptotic activity and suppression of the nuclear factor-κB signaling pathway. Exp. Ther. Med. 2017, 13, 315–320.
- Hartwell, L.H.; Kastan, M.B. Cell cycle control and cancer. Science 1994, 266, 1821–1828.
- Boward, B.; Wu, T.; Dalton, S. Concise review: Control of cell fate through cell cycle and pluripo-tency networks. Stem Cell. 2016, 34, 1427–1436.
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873.
- Suzuki, K.; Ohsumi, Y. Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett. 2007, 581, 2156–2161.
- Badadani, M. Autophagy Mechanism, Regulation, Functions, and Disorders. ISRN Cell Biol. 2012, 2012, 927064.
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12.
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2020, 17, 1–382.
- Zhang, L.; Liang, J.; Liu, X.; Wu, J.; Tan, D.; Hu, W. Aloperine Exerts Antitumor Effects on Bladder Cancer in vitro. OncoTargets Ther. 2020, 13, 10351–10360.
- Ma, N.-T.; Zhou, R.; Chang, R.-Y.; Hao, Y.-J.; Ma, L.; Jin, S.-J.; Du, J.; Zheng, J.; Zhao, C.-J.; Niu, Y.; et al. Protective effects of aloperine on neonatal rat primary cultured hippocampal neurons injured by oxygen–glucose deprivation and reperfusion. J. Nat. Med. 2015, 69, 575–583.
- Mao, Q.; Guo, F.; Liang, X.; Wu, Y.; Lu, Y. Aloperine activates the PI3K/Akt pathway and protects against coronary micro-embolisation-induced myocardial injury in rats. Pharmacology 2019, 104, 90–97.
- Li, S.; Zhou, F.; Dong, J.; Dong, Q.; Luan, H.; Li, L.; Hao, Y. Therapeutic effects of aloperine on the pulmonary arterial hyper-tension. Farmacia 2019, 67, 691–701.
- Hu, R.; Chen, L.; Chen, X.; Xie, Z.; Xia, C.; Chen, Y. Aloperine improves osteoporosis in ovariectomized mice by inhibiting RANKL-induced NF-κB, ERK and JNK approaches. Int. Immunopharmacol. 2021, 97, 107720.
- Ye, Y.; Wang, Y.; Yang, Y.; Tao, L. Aloperine suppresses LPS-induced macrophage activation through inhibiting the TLR4/NF-κB pathway. Inflamm. Res. 2020, 69, 375–383.
- Xiong, R.; Shan, S.; Wang, X.; Zhang, X.; Yu, H.; Shi, H.; Wang, X. Aloperine attenuates carbon tetrachloride-induced mouse hepatic injury via Nrf2/HO-1 pathway. Trop. J. Pharm. Res. 2020, 19, 983–988.
