The use of nanoparticles (NPs) has surely grown in recent years due to their versatility, with a spectrum of applications that range from nanomedicine to the food industry. Recent research focuses on the development of NPs for the oral administration route rather than the intravenous one, placing the interactions between NPs and the intestine at the centre of the attention. This allows the NPs functionalization to exploit the different characteristics of the digestive tract, such as the different pH, the intestinal mucus layer, or the intestinal absorption capacity. On the other hand, these same characteristics can represent a problem for their complexity, also considering the potential interactions with the food matrix or the microbiota.
- nanoparticles
- nanocarriers
- insulin delivery
- inflammatory bowel diseases
- colon cancer
- food additives
1. Introduction
1.1. The Intestinal Barrier
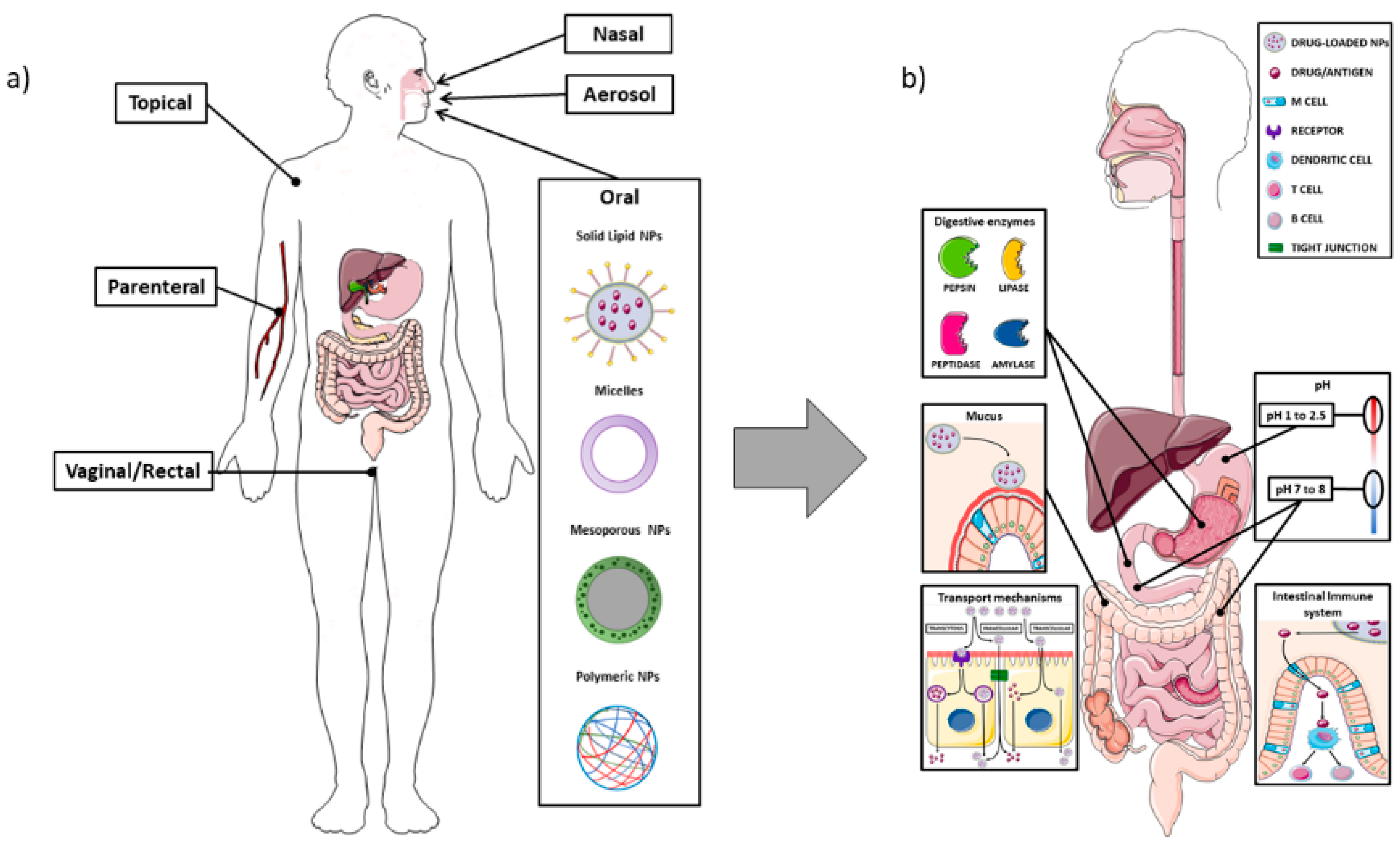
1.2. Nanoparticles
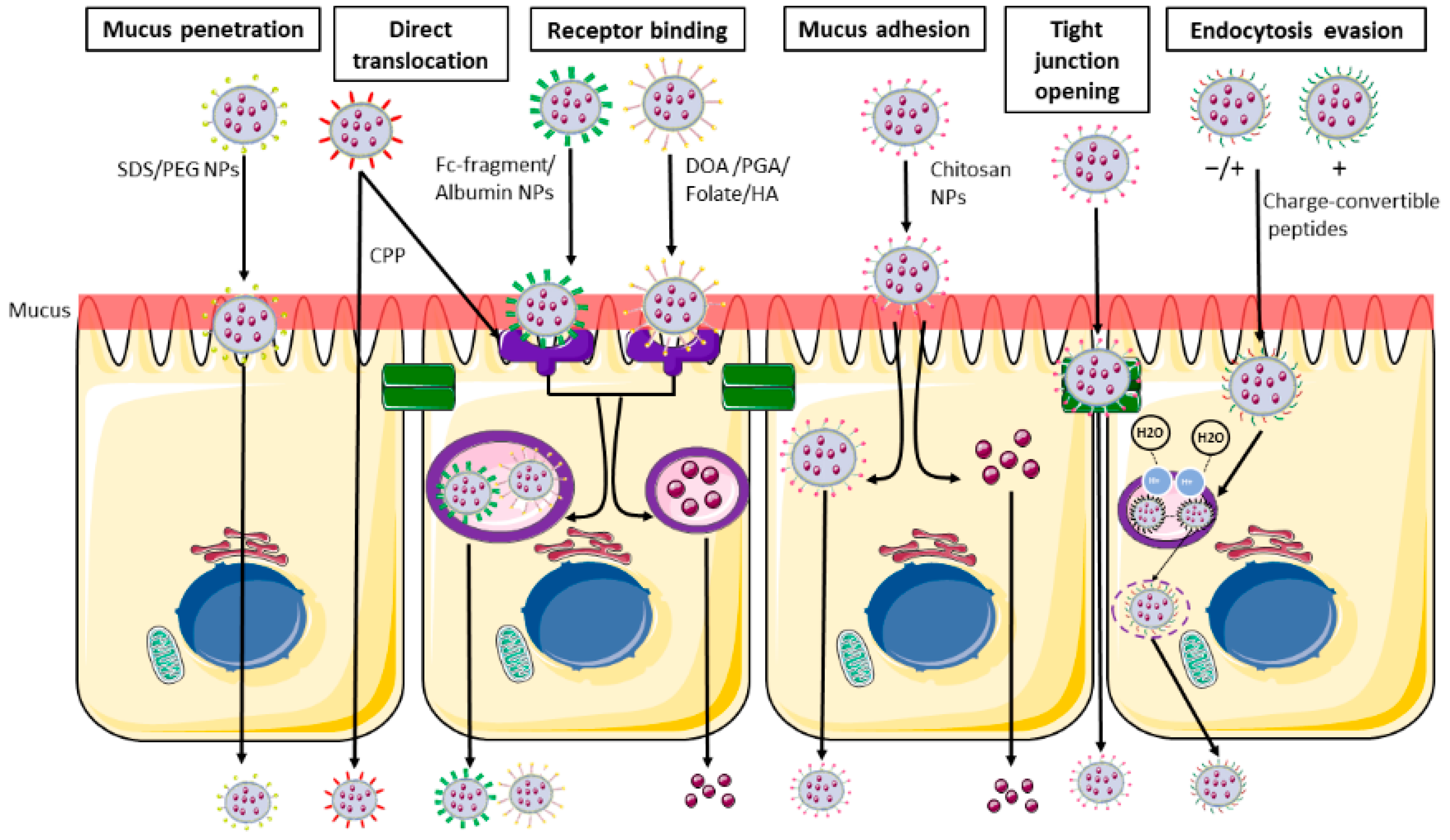
1.3. Nanoparticles—Intestine Interaction
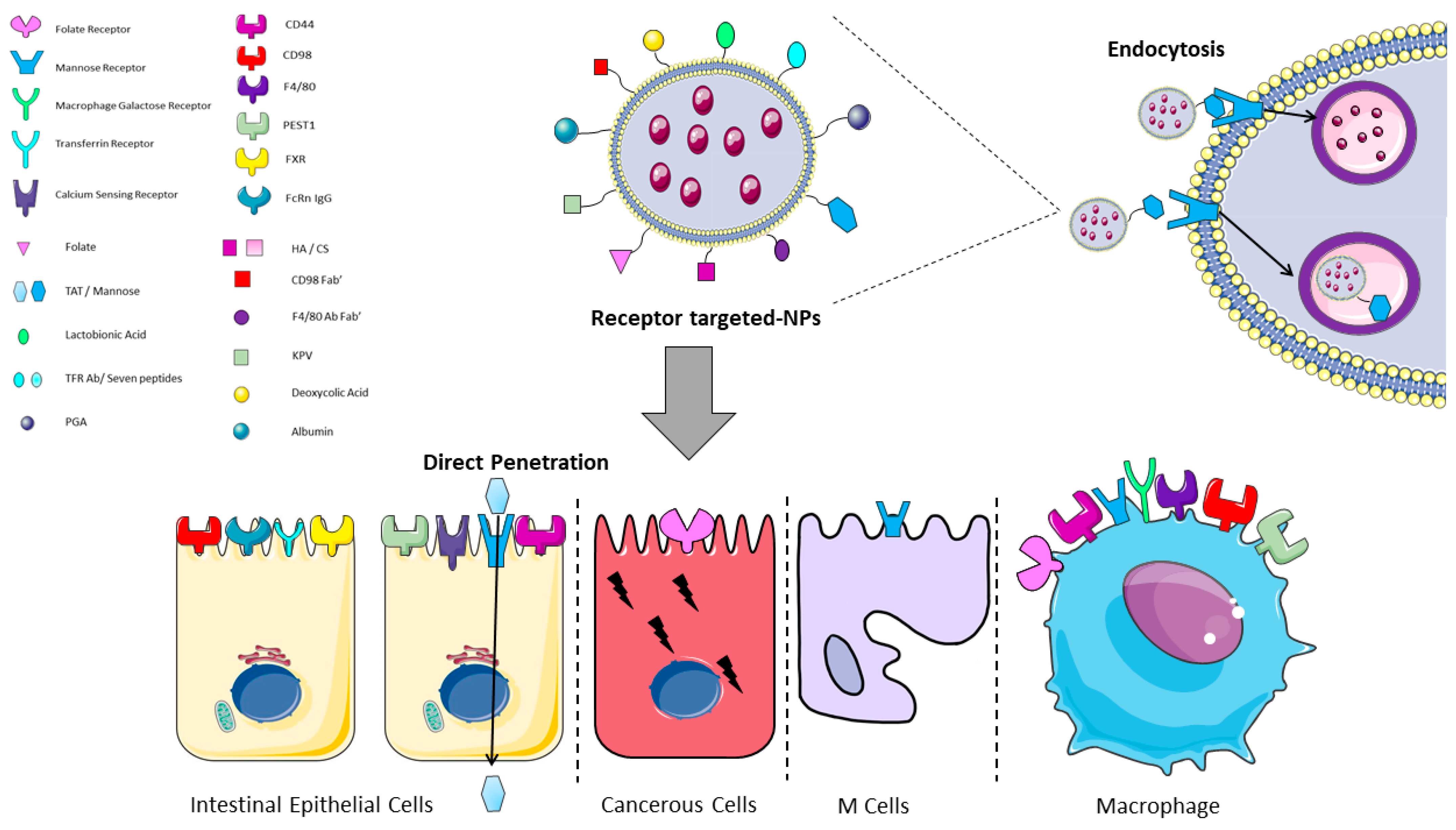
| Reference | Receptor | Ligand | Cell Type Expression |
Direct Penetration |
Endocytosis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hua S 2020 [43 | ||||||||||
| Li L 2017 | ] | [58Mannose Receptor | Mannose | Macrophages, Enterocytes, M cells |
No | Yes | ||||
| ] | Chitosan | CPP | n/a | Yes | Tian 2018 [44] |
CD44 | HA/CS | Macrophages, Intestinal Epithelial Cells |
No | Yes |
| Wu J-Z 2017 [59] | diethylene glycol dimethacrylate | n/a | phenylboronic acid | Yes | Xiao, 2018 [45] |
CD98 | CD98 Fab’/single chain CD98 Ab | |||
| Alfatama 2018 [60 | Intestinal | Epithelial Cells, Macrophages | No | ] | Alginate/Chitosan | Yes | ||||
| n/a | n/a | Yes | Peng L, 2021 [46] |
F4/80 | F4/80 Ab Fab’ | Macrophages | No | Yes | ||
| Liu W, 2018 [47] |
Macrophage Galactose Receptor | Lactobionic Acid | Macrophages | No | Yes | |||||
| Xi Z 2022, Álvarez-González, 2020 [48][49] | ||||||||||
| Czuba 2018 [61] | PLGA | SDS | n/a | Yes | Folate Receptor | Folate | ||||
| Fan 2018 [62] | Chitosan | Deoxycholic acid | Macrophages, Epithelial Cancer Cells | No | Yes | |||||
| n/a | Yes | |||||||||
| Hou 2018 [63] | Mesoporous silica nanoparticle | n/a | phenylboronic acid | Yes | Yong, 2019 | |||||
| Jamshidi 2018 [64] | [50] | Transferrin Receptor | TFR Ab/Seven peptides | ChitosanIntestinal Epithelial Cells |
No | n/a | n/a | Yes | ||
| Yes | Zhang W, 2021 [51] |
PEST1 | KPV | |||||||
| Ji N 2018 [65] | Macrophages, Intestinal | Zein + CSA | n/aEpithelial Cells | No | Yes | |||||
| n/a | n/a | Liu L, 2018 [52] |
Mannose Receptor | TAT | Intestinal Epithelial Cells, Macrophages |
Yes | No | |||
| Liu L 2018 [52] | Azevedo, 2020 [53] |
FcRn IgG | Albumin | Intestinal Epithelial Cells |
No | Yes | ||||
| Huang X, 2021 [54] |
FXR | Deoxycolic Acid | Intestinal Epithelial Cells |
No | Yes | |||||
| Urimi, 2019 [55] |
Calcium Sensing Receptor | PGA | Intestinal Epithelial Cells |
No | Yes |
2. Nanoparticles for Systemic Drug Delivery
The possibility to deliver drugs through the intestinal route rather than using other more invasive ways has been quite captivating for various pharma products, in particular anti-cancer drugs or vaccines. However, due to the large number of the employed molecules and the great differences among the NPs, wresearchers decided to focus on a single molecule tackling another disorder, i.e., insulin. Due to the high social impact of diabetes and the need to administer the drug few times during the day, several groups throughout the world have been involved in the development of NPs able to provide the oral delivery of recombinant insulin. The most used cores for NPs are polymers, either natural or synthetic ones; among the natural polymers there is chitosan, either alone or in combination with alginate; these NPs have some characteristics that make them suitable for insulin delivery, such as biodegradability, nontoxicity, muco-adhesiveness, and low immunogenicity, as previously described (see Table 2). Other employed natural polymers are HA, albumin, starch (amylose), zein, and lignin, as reported in Table 2.| Reference | Core of the NPs | Further Functionalization for Adhesion/Passage |
Release Control | Reduces Glycaemia in Animal Model |
|---|---|---|---|---|
| Chitosan + hydrogel | ||||
| n/a | ||||
| n/a | ||||
| Yes | ||||
| Song M 2018 [66] | Cyclodextrin/chitosan | n/a | n/a | Yes |
| Tian 2018 [44] | Chitosan/hyaluronic acid | n/a | n/a | Yes |
| Wang W 2018 [67] | Polyamidoamine/polyaspartic acid/phenylboronic acid/PEG | PEG | phenylboronic acid | Yes |
| Xu Y 2018 [68] | solid lipid nanoparticle + endosomal escape agent | n/a | n/a | Yes |
| Zhang Y 2018 [69] | hydroxyapatite | PEG | n/a | Yes |
| Zhang L. 2018 [70] | PLGA + chitosan + alginate | n/a | pH dependent | Yes |
| Alsulays 2019 [71] | Solid lipid nanoparticles | CPP | n/a | Yes |
| Guo 2019 [72] | Chitosan | CPP | n/a | yes |
| Hu 2019 [73] | phospholipids | n/a | n/a | Yes |
| Jamwal 2019 [74] | dextran | n/a | Glucose oxidase | n/a |
| Ji 2019 [75] | Chitosan/zein-carboxymethylated short-chain amylose | n/a | n/a | Yes |
| Mohammadpour 2019 [76] | PLGA + chitosan | n/a | Glucose oxidase | Yes |
| Muntoni 2019 [77] | Lipid nanoparticles | n/a | n/a | Yes |
| Mudassir 2019 [78] | Methyl methacrylate/itaconic acid nanogels | n/a | pH dependent | Yes |
| Tsai 2019 [79] | Chitosan + fucoidan | n/a | pH dependent | n/a |
| Urimi 2019 [55] | Chitosan | Polyglutamic acid | n/a | Yes |
| Azevedo 2020 [53] | Albumin | n/a | n/a | Yes |
| Bai 2020 [80] | PLGA + glutamic acid conjugated amphiphilic dendrimer | n/a | n/a | Yes |
| Chai 2020 [81] | Poly (acrylamido phenylboronic acid)/sodium alginate | n/a | Cicloborate (Glucose sensing) and glucose oxidase | Yes |
| Chen Z 2020 [82] | Chitosan/Hyaluronic acid | CPP | n/a | Yes |
| Cheng 2020 [83] | Poly (n-butylcyanoacrylate) | Ratio insulin/Poly (n-butylcyanoacrylate) | Ratio insulin/Poly (n-butylcyanoacrylate) | Yes |
| Ding 2020 [84] | amphiphilic cholesterol- phosphate conjugate |
n/a | pH dependent | Yes |
| Han X 2020 [85] | Zwitterionic micelles | Betaine | n/a | Yes |
| Jana 2020 [86] | hyaluronic acid | n/a | Glucose oxidase | n/a |
| Mumuni 2020 [87] | Chitosan/mucin | n/a | n/a | yes |
| Sladek 2020 [88] | Hyaluronic acid/chitosan | Sucrose laurate | n/a | Yes |
| Sudhakar 2020 [89] | Chitosan | n/a | pH dependent | Yes |
| Tan X 2020 [90] | Mesoporous silica | PEG + CPP | n/a | Yes |
| Wang T 2020 [91] | Lipid nanoparticles | n/a | n/a | Yes |
| Zhou S 2020 [92] | Chitosan | PC6 | pH dependent | Yes |
| Zhou X 2020 [93] | Alginate | n/a | Glucose oxidase | Yes |
| Zhou Y 2020 [94] | FeCl3·6H2O + BTC | SDS | pH dependent | Yes |
| Bao X 2021 [95] | Zein/casein-dextran | Cholic acid | n/a | Yes |
| Benyettou 2021 [96] | Nanoscale imine-linked covalent organic frameworks | n/a | pH dependent | Yes |
| Cui 2021 [97] | Chitosan + Hyaluronic acid | Biotin | n/a | Yes |
| Huang X 2021 [54] | layered double hydroxide nanoparticle + hyaluronic acid | Deoxycholic acid | n/a | Yes |
| Kim WJ 2021 [98] | POSS-APBA | n/a | phenylboronic acid | n/a |
| Li H 2021 [99] | polyphosphoesters-based copolymer |
n/a | phenylboronic acid | Yes |
| Li J 2021 [100] | Alginate/chitosan | n/a | pH dependent | Yes |
| Liu X 2021 [101] | PLGA/PEG | Angiopep-2 | n/a | Yes |
| Qin 2021 [102] | Mesoporous silica + Alginate + Boronic acid Mesoporous silica + Chitosan + boronic acid |
n/a | phenylboronic acid | Yes |
| Rao 2021 [103] | Porous silicon nanoparticles | Zwitterionic dodecyl sulfobetaine | n/a | Yes |
| Volpatti 2021 [104] | Polycation | n/a | Glucose oxidase | Yes |
| Wang W 2021 [105] | PLGA | Chitosan + Cholanic acid | n/a | Yes |
| Zhang Y 2021 [106] | mesoporous silica nanoparticles | CPP | n/a | Yes |
| Fu 2022 [107] | Glycopolymer | n/a | phenylboronic acid | Yes |
| Li J 2022 [108] | PLGA-Hyd-PEG | PEG | n/a | Yes |
| Martins 2022 [109] | Lignin-encapsulated silicon | Fc fragment of IgG | pH dependent | n/a |
| Reboredo 2022 [110] | Zein | PEG | n/a | Yes |
| Rohra 2022 [111] | Gold nanoparticle-encapsulated zeolitic imidazolate framework-8 | n/a | Glucose oxidase | n/a |
| Xi Z 2022 [48] | PLGA/PEG | PEG, folate and charge-convertible tripeptide | n/a | Yes |
| Xu 2022 [112] | konjac glucomannan/concanavalin A | n/a | Glucose sensing | Yes |
3. Nanoparticles with Intestinal Targets
3.1. Inflammatory Bowel Diseases
IBD, which include both Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammatory disorders characterized by mucosal immune system dysregulation, which has an impact in the small intestine and colon. In recent decades, the necessity to conceive a novel therapeutic approach to IBD treatment has led to the increased interest in nanobased drug delivery systems [116][117][118]. This is due to the many side effects caused by the commonly used drugs to treat chronic inflammatory disorders, such as IBD. In particular, 5-aminosalicylates (5-ASA), antibiotics, and corticosteroids can cause, in the long-term, several side effects, including bone damage, such as the steroid-induced necrosis of the femoral head. Genome-wide association studies have demonstrated that genetic background is only one of the factors involved in the pathogenesis of the disease together with the environmental ones. The recent advances in understanding the pathways involved in the development of IBD have allowed to provide some more therapies, but since the exact cause is not completely understood, there is currently no cure tackling the primum movens of the disease. However, even classical drugs used to treat IBD could take advantage of new-targeted delivery systems that give the possibility to load drugs, natural compounds, antibodies, and other biological compounds inside functionalized NPs able to reach the colon. On the other hand, as described by Hartwig et al. [119], wresearchers must consider that the research on NPs was usually performed considering colonic drug delivery in healthy individuals and not in a pathological condition, so the data should be regarded with caution in IBD patients. In fact, these individuals have important changes in colon microbiota composition and pH mean values, in addition to the diarrhoea that may affect the gastrointestinal transit time.3.2. Nanoparticles Loading Drugs
Drugs available to treat IBD, such as Budesonide or Prednisolone, could have better efficacy and less side effects if properly conveyed, since a targeted delivery could, in theory, allow to reduce the total amount of drug administered to the patient. Naeem et al. designed a system in which budesonide loading-PLGA NPs were covered by Eudragit®s100, thus generating microparticles (NPinMP). Their findings showed that the orally delivered NPinMP in a mouse model of DSS-induced colitis was able to reduce the number of macrophages and neutrophils assessed by immunofluorescence imaging, reduce TNFα serum levels, and cause a restoration of normal colon length. This treatment was superior to the use of NPs alone, which failed to significantly mitigate inflammation; these data can be explained by the better protection through the gastrointestinal tract provided by the double coating of the drug, which allowed a higher quantity of budesonide to be released in the colon [120]. Zhou et al. created a negatively charged Prednisolone-loading nanogel with a high affinity for the damaged colon tissue due to the positive charges located at the inflamed intestinal site. The persistence of this NPs administered by enema in the large intestine was able to provide, through the gradual release, a reduction in inflammatory parameters in a TNBS-induced colitis rat model [121]. Patients with IBD (in particular UC) can also combine the oral treatment with the enema one to achieve better effectiveness. Date et al., tested both nano-suspensions (NS) and micro-suspensions (MS) of budesonide, embedded in an inert mucus substance (Pluronic f127) for the in vivo enema treatment. The particles were tested in TNBS-induced UC mouse model, demonstrating that both formulations were able to restore the colon length and the weight loss. However, the NS showed a better efficacy in decreasing the inflammatory state of the colon, significantly lowering the number of colon-infiltrating monocytes and the levels of pro-inflammatory cytokines within the tissue [122]. In another study, the authors used different drugs (budesonide, vancomycin, and GM-CSF) loaded in NPs composed of human serum albumin covered with heparin; this second coating was chosen since, in theory, it should be able to selectively bind to the inflamed colon area thanks to the negative charges of its glycosaminoglycan molecule. The formulation was delivered by enema in DSS- induced colitis and showed that the NPs can be efficiently loaded with different drugs at the same time. The authors also observed that smaller particles were better retained in a healthy colon, whereas larger particles preferred the binding in the inflamed area, obtaining a reduction in the inflammatory parameters [123]. Lee et al. used Dexamethasone (Dexa) to create spherical polymeric nano-constructs, composed by PLGA and Dexa core and then covered with PEG, for the treatment of IBD. These NPs were injected performing an intravenous infusion to a mouse model of UC. The near infrared imaging results demonstrated the powerful anti-inflammatory action together with the rapid intracellular release of the NPs [124]. Although i.v. administration could be extremely effective, its use in everyday treatment of IBD patients results very difficult, and this kind of approach should be reserved for biologicals. Ceria NPs (Ce NPs) are defined as nanozymes since they behave as enzymes with the ability of scavenging multiple ROS types, thus providing anti-redox and anti-inflammatory activity [125]. These nanozymes can exist in both reduced (Ce3+) and oxidized (Ce4+) state, mimicking, respectively, catalase and superoxide dismutase enzyme activity. In the work of Zhao et al., PEG-loaded Ce NPs were administered in a mouse model of UC, and showed an important reduction in colonic inflammation, as demonstrated by histology and cytokine analysis [125]. In this regard, in a report by Asgharzade et al., Ce NPs were used to deliver Sulfasalazine [126]; Sulfasalazine is the drug resulting from the combination of a sulphonamide and salicylic acid, that are released after the ingestion. Its main mechanism of action includes intrinsic anti-inflammatory and anti-redox activities, and the important reduction in iNOS levels. In a preclinical mouse model of DSS-induced colitis, these particles improved the disease activity index, as well as the histopathological score, and upregulated antioxidant molecules, such as glutathione [126]. In another work performed by Ahmada et al., Sulfasalazine was encapsulated in gelatin NPs and was then coated with Eudragit®s100. The nanodrug was then orally delivered to mice affected by UC and tested in a cellular model of Caco2 treated with DSS. The major protective effect was observed in 5-ASA NPs compared to the free drug, with an improvement at the histological level, increase in colon length, and decrease in serum inflammatory markers [127]. A model of intestinal organoid has been proposed for the study of IBD, conveying PLGA NPs covered with alginate or chitosan and loaded with 5-ASA. In particular, the alginate and chitosan coating negatively or positively charged the NPs. As expected, chitosan-covered NPs were preferentially transported through the epithelium to the intestinal organoid lumen [128]. Interestingly, the possible application of drugs not currently used to treat IBD, delivered in form of NPs, could open other possibilities in the treatment of the disease. Some of these drugs have a role in the modulation of the inflammatory state, such as Isoniazid (INH), an anti-tuberculosis drug known to have important anti-inflammatory actions and a structure similar to COX II inhibitors. The agent was entrapped into an enteric polymer Eudragit®s100, that was degraded at colon pH 7. In the DSS-induced colitis in mice, the comparison of the effect of the free drug and the drug-loaded NPs, assessed by H&E staining, revealed the restorative effect of the NPs-loaded with INH as compared to the free drug. In addition, the authors also demonstrated a possible synergistic effect of the nanodrug in combination with 5-ASA [129]. Another example is Raloxifene, an anti-cancer drug that modulates the estrogen receptor; Greish et al. proved its inhibitory effect on the pathway of NF-kB, a central player able to regulate the production of inflammatory cytokines in IBD. Their report compared the use of the free drug with the drug loaded on PS co-maleic acid micelles, testing them on in vitro and in vivo IBD models. Both formulations were able to induce a protective effect downregulating the NF-kB-dependent signalling pathway, even though the NPs-associated drugs had a major inhibiting power, particularly in lowering the production of IL-6 and TNFα [130]. Cai et al. studied a pH responsive system based on the administration of Tacrolimus, a calcineurin inhibitor that regulates the expression IL-2 and T cells signalling. The drug was loaded onto chitosan NPs functionalized with tripolyphosphate (TPP), a polyanion linked together by crosslinking, HA (with high affinity for the CD44 receptors), and Eudragit®s100 as enteric coating material. In vivo, their experiments suggested that the orally delivered NPs were able to restore colon length, reduce histological damage and prevent the development of the inflammatory cascade typical of IBD [131]. Antibiotics can also have a role in IBD by changing the microbiota composition, which could be altered in this disease; moreover, a subsequent targeted supplementation could help to restore a normal microbiota diversity. One example is Rifaximin a non-systemic antibiotic with antimicrobial capacity, which was loaded on tamarind gum NPs. These NPs were able to resist the degradation of the upper intestinal tract and showed a mucus adhesive capacity in the colon, allowing a prolonged release of the loaded drug. This experiment was carried out on Wistar rats with TNBS-induced colitis showing that these NPs were able to improve the colon length and decrease the serum levels of inflammatory cytokines as compared to the not-treated rats [132].References
- Cueva, C.; Gil-Sánchez, I.; Tamargo, A.; Miralles, B.; Crespo, J.; Bartolomé, B.; Moreno-Arribas, M.V. Gastrointestinal digestion of food-use silver nanoparticles in the dynamic SIMulator of the GastroIntestinal tract (simgi®). Impact on human gut microbiota. Food Chem. Toxicol. 2019, 132, 110657.
- Amara, S.; Bourlieu, C.; Humbert, L.; Rainteau, D.; Carrière, F. Variations in gastrointestinal lipases, pH and bile acid levels with food intake, age and diseases: Possible impact on oral lipid-based drug delivery systems. Adv. Drug Deliv. Rev. 2019, 142, 3–15.
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570.
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106.
- Lee, M.K. Liposomes for enhanced bioavailability of water-insoluble drugs: In vivo evidence and recent approaches. Pharmaceutics 2020, 12, 264.
- Salah, E.; Abouelfetouh, M.M.; Pan, Y.; Chen, D.; Xie, S. Solid lipid nanoparticles for enhanced oral absorption: A review. Colloids Surf. B Biointerfaces 2020, 196, 111305.
- Anton, N.; Vandamme, T.F. Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm. Res. 2011, 28, 978–985.
- Wu, L.; Shan, W.; Zhang, Z.; Huang, Y. Engineering nanomaterials to overcome the mucosal barrier by modulating surface properties. Adv. Drug Deliv. Rev. 2018, 124, 150–163.
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; Husøy, T.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585.
- Medina-Reyes, E.I.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; Díaz-Urbina, D.; Chirino, Y.I.; Pedraza-Chaverri, J. Food additives containing nanoparticles induce gastrotoxicity, hepatotoxicity and alterations in animal behavior: The unknown role of oxidative stress. Food Chem. Toxicol. 2020, 146, 111814.
- Zhao, R.; Xiang, J.; Wang, B.; Chen, L.; Tan, S. Recent Advances in the Development of Noble Metal NPs for Cancer Therapy. Pharmacol. Ther. 2022, 2022, 2444516.
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473.
- Huang, X.; Tang, M. Review of gut nanotoxicology in mammals: Exposure, transformation, distribution and toxicity. Sci. Total Environ. 2021, 773, 145078.
- Madni, A.; Rehman, S.; Sultan, H.; Khan, M.M.; Ahmad, F.; Raza, M.R.; Rai, N.; Parveen, F. Mechanistic Approaches of Internalization, Subcellular Trafficking, and Cytotoxicity of Nanoparticles for Targeting the Small Intestine. AAPS PharmSciTech 2021, 22, 3.
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267.
- Patra, C.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Futur. J. Pharm. Sci. 2017, 3, 33–45.
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031.
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Udenigwe, C.C. Extraction technology impacts on the structure-function relationship between sodium alginate extracts and their in vitro prebiotic activity. Food Biosci. 2020, 37, 100672.
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Hogan, S.A.; López-Rubio, A.; Brodkorb, A. Nano- and microstructural evolution of alginate beads in simulated gastrointestinal fluids. Impact of M/G ratio, molecular weight and pH. Carbohydr. Polym. 2019, 223, 115121.
- Qin, X.S.; Luo, Z.G.; Li, X.L. An enhanced pH-sensitive carrier based on alginate-Ca-EDTA in a set-type W1/O/W2 double emulsion model stabilized with WPI-EGCG covalent conjugates for probiotics colon-targeted release. Food Hydrocoll. 2021, 113, 106460.
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and interfacial properties. Polymers 2015, 7, 552–579.
- Vllasaliu, D.; Exposito-Harris, R.; Heras, A.; Casettari, L.; Garnett, M.; Illum, L.; Stolnik, S. Tight junction modulation by chitosan nanoparticles: Comparison with chitosan solution. Int. J. Pharm. 2010, 400, 183–193.
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53.
- Chen, C.H.; Lin, Y.S.; Wu, S.J.; Mi, F.L. Mutlifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym. 2018, 193, 163–172.
- Faralli, A.; Shekarforoush, E.; Ajalloueian, F.; Mendes, A.C.; Chronakis, I.S. In vitro permeability enhancement of curcumin across Caco-2 cells monolayers using electrospun xanthan-chitosan nanofibers. Carbohydr. Polym. 2019, 206, 38–47.
- Yeh, T.H.; Hsu, L.W.; Tseng, M.T.; Lee, P.L.; Sonjae, K.; Ho, Y.C.; Sung, H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173.
- Hsu, L.W.; Ho, Y.C.; Chuang, E.Y.; Chen, C.T.; Juang, J.H.; Su, F.Y.; Hwang, S.M.; Sung, H.W. Effects of pH on molecular mechanisms of chitosan-integrin interactions and resulting tight-junction disruptions. Biomaterials 2013, 34, 784–793.
- Pritchard, K.; Lansley, A.B.; Martin, G.P.; Helliwell, M.; Marriott, C.; Benedetti, L.M. Evaluation of the bioadhesive properties of hyaluronan derivatives: Detachment weight and mucociliary transport rate studies. Int. J. Pharm. 1996, 129, 137–145.
- Sandri, G.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Zerrouk, N.; Caramella, C. Mucoadhesive and penetration enhancement properties of three grades of hyaluronic acid using porcine buccal and vaginal tissue, Caco-2 cell lines, and rat jejunum. J. Pharm. Pharmacol. 2004, 56, 1083–1090.
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schön, A.; Gong, X.; Yang, J.C.; Lamb, N.W.; Cai, S.; Yu, T.; Freire, E.; et al. Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS Nano 2015, 9, 9217–9227.
- Inchaurraga, L.; Martín-Arbella, N.; Zabaleta, V.; Quincoces, G.; Peñuelas, I.; Irache, J.M. In vivo study of the mucus-permeating properties of PEG-coated nanoparticles following oral administration. Eur. J. Pharm. Biopharm. 2015, 97, 280–289.
- Liu, M.; Zhang, J.; Zhu, X.; Shan, W.; Li, L.; Zhong, J.; Zhang, Z.; Huang, Y. Efficient mucus permeation and tight junction opening by dissociable “mucus-inert” agent coated trimethyl chitosan nanoparticles for oral insulin delivery. J. Control. Release 2016, 222, 67–77.
- Kosińska, A.; Andlauer, W. Modulation of tight junction integrity by food components. Food Res. Int. 2013, 54, 951–960.
- Haasbroek, A.; Willers, C.; Glyn, M.; Du Plessis, L.; Hamman, J. Intestinal drug absorption enhancement by Aloe vera gel and whole leaf extract: In vitro investigations into the mechanisms of action. Pharmaceutics 2019, 11, 36.
- Chen, X.Y.; Butt, A.M.; Mohd Amin, M.C.I. Molecular Evaluation of Oral Immunogenicity of Hepatitis B Antigen Delivered by Hydrogel Microparticles. Mol. Pharm. 2019, 16, 3853–3872.
- Di Pierro, M.; Lu, R.; Uzzau, S.; Wang, W.; Margaretten, K.; Pazzani, C.; Maimone, F.; Fasano, A. Zonula occludens toxin structure-function analysis: Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 2001, 276, 19160–19165.
- Lee, J.Y.J.H.; Sahu, A.; Choi, W.I.; Lee, J.Y.J.H.; Tae, G. ZOT-derived peptide and chitosan functionalized nanocarrier for oral delivery of protein drug. Biomaterials 2016, 103, 160–169.
- Sonaje, K.; Lin, K.J.; Tseng, M.T.; Wey, S.P.; Su, F.Y.; Chuang, E.Y.; Hsu, C.W.; Chen, C.T.; Sung, H.W. Effects of chitosan-nanoparticle-mediated tight junction opening on the oral absorption of endotoxins. Biomaterials 2011, 32, 8712–8721.
- Zhang, N.; Ping, Q.N.; Huang, G.H.; Xu, W.F. Investigation of lectin-modified insulin liposomes as carriers for oral administration. Int. J. Pharm. 2005, 294, 247–259.
- Jia, Z.; Wignall, A.; Prestidge, C.; Thierry, B. An ex vivo investigation of the intestinal uptake and translocation of nanoparticles targeted to Peyer’s patches microfold cells. Int. J. Pharm. 2021, 594, 120167.
- Garinot, M.; Fiévez, V.; Pourcelle, V.; Stoffelbach, F.; des Rieux, A.; Plapied, L.; Theate, I.; Freichels, H.; Jérôme, C.; Marchand-Brynaert, J.; et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J. Control. Release 2007, 120, 195–204.
- Berillo, D.; Yeskendir, A.; Zharkinbekov, Z.; Raziyeva, K.; Saparov, A. Peptide-Based Drug Delivery Systems. Medicina 2021, 57, 1209.
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524.
- Tian, H.; He, Z.; Sun, C.; Yang, C.; Zhao, P.; Liu, L.; Leong, K.W.; Mao, H.Q.; Liu, Z.; Chen, Y. Uniform Core–Shell Nanoparticles with Thiolated Hyaluronic Acid Coating to Enhance Oral Delivery of Insulin. Adv. Healthc. Mater. 2018, 7, 285.
- Xiao, B.; Viennois, E.; Chen, Q.; Wang, L.; Han, M.K.; Zhang, Y.; Zhang, Z.; Kang, Y.; Wan, Y.; Merlin, D. Silencing of Intestinal Glycoprotein CD98 by Orally Targeted Nanoparticles Enhances Chemosensitization of Colon Cancer. ACS Nano 2018, 12, 5253–5265.
- Liu, P.; Gao, C.; Chen, H.; Vong, C.T.; Wu, X.; Tang, X.; Wang, S.; Wang, Y. Receptor-mediated targeted drug delivery systems for treatment of inflammatory bowel disease: Opportunities and emerging strategies. Acta Pharm. Sin. B 2021, 11, 2798–2818.
- Liu, W.; Zhu, Y.; Wang, F.; Li, X.; Liu, X.; Pang, J.; Pan, W. Galactosylated chitosanfunctionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. R. Soc. Open Sci. 2018, 5, 181027.
- Xi, Z.; Ahmad, E.; Zhang, W.; Li, J.; Wang, A.; Faridoon; Wang, N.; Zhu, C.; Huang, W.; Xu, L.; et al. Dual-modified nanoparticles overcome sequential absorption barriers for oral insulin delivery. J. Control. Release 2022, 342, 1–13.
- Álvarez-González, B.; Rozalen, M.; Fernández-Perales, M.; Álvarez, M.A.; Sánchez-Polo, M. Methotrexate Gold Nanocarriers: Loading and Release Study: Its Activity in Colon and Lung Cancer Cells. Molecules 2020, 25, 6049.
- Yong, J.M.; Mantaj, J.; Cheng, Y.; Vllasaliu, D. Delivery of nanoparticles across the intestinal epithelium via the transferrin transport pathway. Pharmaceutics 2019, 11, 298.
- Zhang, W.; Michalowski, C.B.; Beloqui, A. Oral Delivery of Biologics in Inflammatory Bowel Disease Treatment. Front. Bioeng. Biotechnol. 2021, 9, 675194.
- Liu, L.; Zhang, Y.; Yu, S.; Yang, Z.; He, C.; Chen, X. Dual Stimuli-Responsive Nanoparticle-Incorporated Hydrogels as an Oral Insulin Carrier for Intestine-Targeted Delivery and Enhanced Paracellular Permeation. ACS Biomater. Sci. Eng. 2018, 4, 2889–2902.
- Azevedo, C.; Nilsen, J.; Grevys, A.; Nunes, R.; Andersen, J.T.; Sarmento, B. Engineered albumin-functionalized nanoparticles for improved FcRn binding enhance oral delivery of insulin. J. Control. Release 2020, 327, 161–173.
- Huang, X.; Han, S.; Chen, Z.; Zhao, L.; Wang, C.; Guo, Q.; Li, Y.; Sun, Y. Layered double hydroxide modified with deoxycholic and hyaluronic acids for efficient oral insulin absorption. Int. J. Nanomed. 2021, 16, 7861–7873.
- Urimi, D.; Agrawal, A.K.; Kushwah, V.; Jain, S. Polyglutamic Acid Functionalization of Chitosan Nanoparticles Enhances the Therapeutic Efficacy of Insulin Following Oral Administration. AAPS PharmSciTech 2019, 20, 131.
- Li, R.; Laurent, F.; Taverner, A.; Mackay, J.; De Bank, P.A.; Mrsny, R.J. Intestinal transcytosis of a protein cargo and nanoparticles mediated by a non-toxic form of Pseudomonas aeruginosa exotoxin A. Pharmaceutics 2021, 13, 1171.
- Zhang, X.; Chen, D.; Ba, S.; Zhu, J.; Zhang, J.; Hong, W.; Zhao, X.; Hu, H.; Qiao, M. Poly(l-histidine) based triblock copolymers: PH induced reassembly of copolymer micelles and mechanism underlying endolysosomal escape for intracellular delivery. Biomacromolecules 2014, 15, 4032–4045.
- Li, L.; Yang, L.; Li, M.; Zhang, L. A cell-penetrating peptide mediated chitosan nanocarriers for improving intestinal insulin delivery. Carbohydr. Polym. 2017, 174, 182–189.
- Wu, J.Z.; Williams, G.R.; Li, H.Y.; Wang, D.; Wu, H.; De Li, S.; Zhu, L.M. Glucose- and temperature-sensitive nanoparticles for insulin delivery. Int. J. Nanomed. 2017, 12, 4037–4057.
- Alfatama, M.; Lim, L.Y.; Wong, T.W. Alginate-C18 Conjugate Nanoparticles Loaded in Tripolyphosphate-Cross-Linked Chitosan-Oleic Acid Conjugate-Coated Calcium Alginate Beads as Oral Insulin Carrier. Mol. Pharm. 2018, 15, 3369–3382.
- Czuba, E.; Diop, M.; Mura, C.; Schaschkow, A.; Langlois, A.; Bietiger, W.; Neidl, R.; Virciglio, A.; Auberval, N.; Julien-David, D.; et al. Oral insulin delivery, the challenge to increase insulin bioavailability: Influence of surface charge in nanoparticle system. Int. J. Pharm. 2018, 542, 47–55.
- Fan, W.; Xia, D.; Zhu, Q.; Li, X.; He, S.; Zhu, C.; Guo, S.; Hovgaard, L.; Yang, M.; Gan, Y. Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery. Biomaterials 2018, 151, 13–23.
- Hou, L.; Zheng, Y.; Wang, Y.; Hu, Y.; Shi, J.; Liu, Q.; Zhang, H.; Zhang, Z. Self-Regulated Carboxyphenylboronic Acid-Modified Mesoporous Silica Nanoparticles with “touch Switch” Releasing Property for Insulin Delivery. ACS Appl. Mater. Interfaces 2018, 10, 21927–21938.
- Jamshidi, M.; Ziamajidi, N.; Khodadadi, I.; Dehghan, A.; Kalantarian, G.; Abbasalipourkabir, R. The effect of insulin-loaded trimethylchitosan nanoparticles on rats with diabetes type I. Biomed. Pharmacother. 2018, 97, 729–735.
- Ji, N.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Preparation and Characterization of Insulin-Loaded Zein/Carboxymethylated Short-Chain Amylose Complex Nanoparticles. J. Agric. Food Chem. 2018, 66, 9335–9343.
- Song, M.; Wang, H.; Chen, K.; Zhang, S.; Yu, L.; Elshazly, E.H.; Ke, L.; Gong, R. Oral insulin delivery by carboxymethyl-β-cyclodextrin-grafted chitosan nanoparticles for improving diabetic treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, S774–S782.
- Wang, W.; Liao, L.; Zhang, X.; Lei, F.; Zhang, Y.; Liu, G.; Xie, W. An intelligent nanoscale insulin delivery system. Molecules 2018, 23, 2945.
- Xu, Y.; Zheng, Y.; Wu, L.; Zhu, X.; Zhang, Z.; Huang, Y. Novel Solid Lipid Nanoparticle with Endosomal Escape Function for Oral Delivery of Insulin. ACS Appl. Mater. Interfaces 2018, 10, 9315–9324.
- Zhang, Y.; Zhang, L.; Ban, Q.; Li, J.; Li, C.H.; Guan, Y.Q. Preparation and characterization of hydroxyapatite nanoparticles carrying insulin and gallic acid for insulin oral delivery. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 353–364.
- Zhang, L.; Qin, H.; Li, J.; Qiu, J.N.; Huang, J.M.; Li, M.C.; Guan, Y.Q. Preparation and characterization of layer-by-layer hypoglycemic nanoparticles with pH-sensitivity for oral insulin delivery. J. Mater. Chem. B 2018, 6, 7451–7461.
- Alsulays, B.B.; Anwer, M.K.; Soliman, G.A.; Alshehri, S.M.; Khafagy, E.S. Impact of penetratin stereochemistry on the oral bioavailability of insulin-loaded solid lipid nanoparticles. Int. J. Nanomed. 2019, 14, 9127–9138.
- Guo, F.; Ouyang, T.; Peng, T.; Zhang, X.; Xie, B.; Yang, X.; Liang, D.; Zhong, H. Enhanced oral absorption of insulin using colon-specific nanoparticles co-modified with amphiphilic chitosan derivatives and cell-penetrating peptides. Biomater. Sci. 2019, 7, 1493–1506.
- Hu, X.B.; Tang, T.T.; Li, Y.J.; Wu, J.Y.; Wang, J.M.; Liu, X.Y.; Xiang, D.X. Phospholipid complex based nanoemulsion system for oral insulin delivery: Preparation, in vitro, and in vivo evaluations. Int. J. Nanomed. 2019, 14, 3055–3067.
- Jamwal, S.; Ram, B.; Ranote, S.; Dharela, R.; Chauhan, G.S. New glucose oxidase-immobilized stimuli-responsive dextran nanoparticles for insulin delivery. Int. J. Biol. Macromol. 2019, 123, 968–978.
- Ji, N.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Chitosan coating of zein-carboxymethylated short-chain amylose nanocomposites improves oral bioavailability of insulin in vitro and in vivo. J. Control. Release 2019, 313, 1–13.
- Mohammadpour, F.; Hadizadeh, F.; Tafaghodi, M.; Sadri, K.; Mohammadpour, A.H.; Kalani, M.R.; Gholami, L.; Mahmoudi, A.; Chamani, J. Preparation, in vitro and in vivo evaluation of PLGA/Chitosan based nano-complex as a novel insulin delivery formulation. Int. J. Pharm. 2019, 572, 118710.
- Muntoni, E.; Marini, E.; Ahmadi, N.; Milla, P.; Ghè, C.; Bargoni, A.; Capucchio, M.T.; Biasibetti, E.; Battaglia, L. Lipid nanoparticles as vehicles for oral delivery of insulin and insulin analogs: Preliminary ex vivo and in vivo studies. Acta Diabetol. 2019, 56, 1283–1292.
- Mudassir, J.; Darwis, Y.; Muhamad, S.; Khan, A.A. Self-assembled insulin and nanogels polyelectrolyte complex (Ins/NGs-PEC) for oral insulin delivery: Characterization, lyophilization and in-vivo evaluation. Int. J. Nanomed. 2019, 14, 4895–4909.
- Tsai, L.C.; Chen, C.H.; Lin, C.W.; Ho, Y.C.; Mi, F.L. Development of mutlifunctional nanoparticles self-assembled from trimethyl chitosan and fucoidan for enhanced oral delivery of insulin. Int. J. Biol. Macromol. 2019, 126, 141–150.
- Bai, Y.; Zhou, R.; Wu, L.; Zheng, Y.; Liu, X.; Wu, R.; Li, X.; Huang, Y. Nanoparticles with surface features of dendritic oligopeptides as potential oral drug delivery systems. J. Mater. Chem. B 2020, 8, 2636–2649.
- Chai, Z.; Dong, H.; Sun, X.; Fan, Y.; Wang, Y.; Huang, F. Development of glucose oxidase-immobilized alginate nanoparticles for enhanced glucose-triggered insulin delivery in diabetic mice. Int. J. Biol. Macromol. 2020, 159, 640–647.
- Chen, Z.; Han, S.; Yang, X.; Xu, L.; Qi, H.; Hao, G.; Cao, J.; Liang, Y.; Ma, Q.; Zhang, G.; et al. Overcoming multiple absorption barrier for insulin oral delivery using multifunctional nanoparticles based on chitosan derivatives and hyaluronic acid. Int. J. Nanomed. 2020, 15, 4877–4898.
- Cheng, H.; Zhang, X.; Qin, L.; Huo, Y.; Cui, Z.; Liu, C.; Sun, Y.; Guan, J.; Mao, S. Design of self-polymerized insulin loaded poly(n-butylcyanoacrylate) nanoparticles for tunable oral delivery. J. Control. Release 2020, 321, 641–653.
- Ding, Y.; Wang, Q.; Liu, G.; Feng, Y.; Zhou, W. Cholesterol moieties as building blocks for assembling nanoparticles to achieve effective oral delivery of insulin. Biomater. Sci. 2020, 8, 3979–3993.
- Han, X.; Lu, Y.; Xie, J.; Zhang, E.; Zhu, H.; Du, H.; Wang, K.; Song, B.; Yang, C.; Shi, Y.; et al. Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nat. Nanotechnol. 2020, 15, 605–614.
- Jana, B.A.; Shinde, U.; Wadhwani, A. Synthetic enzyme-based nanoparticles act as smart catalyst for glucose responsive release of insulin. J. Biotechnol. 2020, 324, 1–6.
- Mumuni, M.A.; Kenechukwu, F.C.; Ofokansi, K.C.; Attama, A.A.; Díaz, D.D. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr. Polym. 2020, 229, 115506.
- Sladek, S.; McCartney, F.; Eskander, M.; Dunne, D.J.; Santos-Martinez, M.J.; Benetti, F.; Tajber, L.; Brayden, D.J. An enteric-coated polyelectrolyte nanocomplex delivers insulin in rat intestinal instillations when combined with a permeation enhancer. Pharmaceutics 2020, 12, 259.
- Sudhakar, S.; Chandran, S.V.; Selvamurugan, N.; Nazeer, R.A. Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo. Int. J. Biol. Macromol. 2020, 150, 281–288.
- Tan, X.; Yin, N.; Liu, Z.; Sun, R.; Gou, J.; Yin, T.; Zhang, Y.; He, H.; Tang, X. Hydrophilic and Electroneutral Nanoparticles to Overcome Mucus Trapping and Enhance Oral Delivery of Insulin. Mol. Pharm. 2020, 17, 3177–3191.
- Wang, T.; Shen, L.; Zhang, Y.; Li, H.; Wang, Y.; Quan, D. “Oil-Soluble” Reversed Lipid Nanoparticles for Oral Insulin Delivery. J. Nanobiotechnol. 2020, 18, 98.
- Zhou, S.; Deng, H.; Zhang, Y.; Wu, P.; He, B.; Dai, W.; Zhang, H.; Zhang, Q.; Zhao, R.; Wang, X. Thiolated Nanoparticles Overcome the Mucus Barrier and Epithelial Barrier for Oral Delivery of Insulin. Mol. Pharm. 2020, 17, 239–250.
- Zhou, X.; Wu, H.; Long, R.; Wang, S.; Huang, H.; Xia, Y.; Wang, P.; Lei, Y.; Cai, Y.; Cai, D.; et al. Oral delivery of insulin with intelligent glucose-responsive switch for blood glucose regulation. J. Nanobiotechnol. 2020, 18, 96.
- Zhou, Y.; Liu, L.; Cao, Y.; Yu, S.; He, C.; Chen, X. A Nanocomposite Vehicle Based on Metal-Organic Framework Nanoparticle Incorporated Biodegradable Microspheres for Enhanced Oral Insulin Delivery. ACS Appl. Mater. Interfaces 2020, 12, 22581–22592.
- Bao, X.; Qian, K.; Yao, P. Insulin- And cholic acid-loaded zein/casein-dextran nanoparticles enhance the oral absorption and hypoglycemic effect of insulin. J. Mater. Chem. B 2021, 9, 6234–6245.
- Benyettou, F.; Kaddour, N.; Prakasam, T.; Das, G.; Sharma, S.K.; Thomas, S.A.; Bekhti-Sari, F.; Whelan, J.; Alkhalifah, M.A.; Khair, M.; et al. In vivo oral insulin delivery via covalent organic frameworks. Chem. Sci. 2021, 12, 6037–6047.
- Cui, Z.; Qin, L.; Guo, S.; Cheng, H.; Zhang, X.; Guan, J.; Mao, S. Design of biotin decorated enterocyte targeting muco-inert nanocomplexes for enhanced oral insulin delivery. Carbohydr. Polym. 2021, 261, 117873.
- Kim, W.J.; Kwon, Y.J.; Cho, C.H.; Ye, S.K.; Kim, K.O. Insulin smart drug delivery nanoparticles of aminophenylboronic acid–POSS molecule at neutral pH. Sci. Rep. 2021, 11, 21894.
- Li, H.; Zhou, R.; He, J.; Zhang, M.; Liu, J.; Sun, X.; Ni, P. Glucose-Sensitive Core-Cross-Linked Nanoparticles Constructed with Polyphosphoester Diblock Copolymer for Controlling Insulin Delivery. Bioconjug. Chem. 2021, 32, 2095–2107.
- Li, J.; Wu, H.; Jiang, K.; Liu, Y.; Yang, L.; Park, H.J. Alginate Calcium Microbeads Containing Chitosan Nanoparticles for Controlled Insulin Release. Appl. Biochem. Biotechnol. 2021, 193, 463–478.
- Liu, X.; Wu, R.; Li, Y.; Wang, L.; Zhou, R.; Li, L.; Xiang, Y.; Wu, J.; Xing, L.; Huang, Y. Angiopep-2-functionalized nanoparticles enhance transport of protein drugs across intestinal epithelia by self-regulation of targeted receptors. Biomater. Sci. 2021, 9, 2903–2916.
- Qin, T.; Yan, L.; Wang, X.; Lin, S.; Zeng, Q. Glucose-Responsive Polyelectrolyte Complexes Based on Dendritic Mesoporous Silica for Oral Insulin Delivery. AAPS PharmSciTech 2021, 22, 226.
- Rao, R.; Liu, X.; Li, Y.; Tan, X.; Zhou, H.; Bai, X.; Yang, X.; Liu, W. Bioinspired zwitterionic polyphosphoester modified porous silicon nanoparticles for efficient oral insulin delivery. Biomater. Sci. 2021, 9, 685–699.
- Volpatti, L.R.; Burns, D.M.; Basu, A.; Langer, R.; Anderson, D.G. Engineered insulin-polycation complexes for glucose-responsive delivery with high insulin loading. J. Control. Release 2021, 338, 71–79.
- Wang, W.; Yu, C.; Zhang, F.; Li, Y.; Zhang, B.; Huang, J.; Zhang, Z.; Jin, L. Improved oral delivery of insulin by PLGA nanoparticles coated with 5β-cholanic acid conjugated glycol chitosan. Biomed. Mater. 2021, 16, 064103.
- Zhang, Y.; Xiong, M.; Ni, X.; Wang, J.; Rong, H.; Su, Y.; Yu, S.; Mohammad, I.S.; Leung, S.S.Y.; Hu, H. Virus-Mimicking Mesoporous Silica Nanoparticles with an Electrically Neutral and Hydrophilic Surface to Improve the Oral Absorption of Insulin by Breaking through Dual Barriers of the Mucus Layer and the Intestinal Epithelium. ACS Appl. Mater. Interfaces 2021, 13, 18077–18088.
- Fu, Y.; Sun, Y.; Chen, M.; Xing, W.; Xu, Y.; Qian, X.; Zhu, W. Glycopolymer Nanoparticles with On-Demand Glucose-Responsive Insulin Delivery and Low-Hypoglycemia Risks for Type 1 Diabetic Treatment. Biomacromolecules 2022, 23, 1251–1258.
- Li, J.; Qiang, H.; Yang, W.; Xu, Y.; Feng, T.; Cai, H.; Wang, S.; Liu, Z.; Zhang, Z.; Zhang, J. Oral insulin delivery by epithelium microenvironment-adaptive nanoparticles. J. Control. Release 2022, 341, 31–43.
- Martins, J.P.; Figueiredo, P.; Wang, S.; Espo, E.; Celi, E.; Martins, B.; Kemell, M.; Moslova, K.; Mäkilä, E.; Salonen, J.; et al. Neonatal Fc receptor-targeted lignin-encapsulated porous silicon nanoparticles for enhanced cellular interactions and insulin permeation across the intestinal epithelium. Bioact. Mater. 2022, 9, 299–315.
- Reboredo, C.; González-Navarro, C.J.; Martínez-López, A.L.; Martínez-Ohárriz, C.; Sarmento, B.; Irache, J.M. Zein-Based Nanoparticles as Oral Carriers for Insulin Delivery. Pharmaceutics 2022, 14, 39.
- Rohra, N.; Gaikwad, G.; Dandekar, P.; Jain, R. Microfluidic Synthesis of a Bioactive Metal–Organic Framework for Glucose-Responsive Insulin Delivery. ACS Appl. Mater. Interfaces 2022, 14, 8251–8265.
- Xu, M.; Huang, J.; Jiang, S.; He, J.; Wang, Z.; Qin, H.; Guan, Y.Q. Glucose sensitive konjac glucomannan/concanavalin A nanoparticles as oral insulin delivery system. Int. J. Biol. Macromol. 2022, 202, 296–308.
- Pandita, D.; Kumar, S.; Lather, V. Hybrid poly(lactic-co-glycolic acid) nanoparticles: Design and delivery prospectives. Drug Discov. Today 2015, 20, 95–104.
- Qi, W.; Yan, X.; Duan, L.; Cui, Y.; Yang, Y.; Li, J. Glucose-sensitive microcapsules from glutaraldehyde cross-linked hemoglobin and glucose oxidase. Biomacromolecules 2009, 10, 1212–1216.
- Ma, Q.; Zhao, X.; Shi, A.; Wu, J. Bioresponsive functional phenylboronic acid-based delivery system as an emerging platform for diabetic therapy. Int. J. Nanomed. 2021, 16, 297–314.
- Naeem, M.; Awan, U.A.; Subhan, F.; Cao, J.; Hlaing, S.P.; Lee, J.; Im, E.; Jung, Y.; Yoo, J.W. Advances in colon-targeted nano-drug delivery systems: Challenges and solutions. Arch. Pharm. Res. 2020, 43, 153–169.
- Feng, Z.; Jiao, L.; Wu, Z.; Xu, J.; Gu, P.; Xu, S.; Liu, Z.; Hu, Y.; Liu, J.; Wu, Y.; et al. A Novel Nanomedicine Ameliorates Acute Inflammatory Bowel Disease by Regulating Macrophages and T-Cells. Mol. Pharm. 2021, 18, 3484–3495.
- Nedelcu, A.; Mosteanu, O.; Pop, T.; Mocan, T.; Mocan, L. Recent advances in nanoparticle-mediated treatment of inflammatory bowel diseases. Appl. Sci. 2021, 11, 438.
- Hartwig, O.; Shetab Boushehri, M.A.; Shalaby, K.S.; Loretz, B.; Lamprecht, A.; Lehr, C.M. Drug delivery to the inflamed intestinal mucosa—Targeting technologies and human cell culture models for better therapies of IBD. Adv. Drug Deliv. Rev. 2021, 175, 113828.
- Naeem, M.; Lee, J.; Oshi, M.A.; Cao, J.; Hlaing, S.P.; Im, E.; Jung, Y.; Yoo, J.W. Colitis-targeted hybrid nanoparticles-in-microparticles system for the treatment of ulcerative colitis. Acta Biomater. 2020, 116, 368–382.
- Zhou, H.; Ikeuchi-Takahashi, Y.; Hattori, Y.; Onishi, H. Nanogels of a succinylated glycol chitosan-succinyl prednisolone conjugate: Release behavior, gastrointestinal distribution, and systemic absorption. Int. J. Mol. Sci. 2020, 21, 2376.
- Date, A.A.; Halpert, G.; Babu, T.; Ortiz, J.; Kanvinde, P.; Dimitrion, P.; Narayan, J.; Zierden, H.; Betageri, K.; Musmanno, O.; et al. Mucus-penetrating budesonide nanosuspension enema for local treatment of inflammatory bowel disease. Biomaterials 2018, 185, 97–105.
- Zhang, S.; Cho, W.J.; Jin, A.T.; Kok, L.Y.; Shi, Y.; Heller, D.E.; Lee, Y.A.L.; Zhou, Y.; Xie, X.; Korzenik, J.R.; et al. Heparin-Coated Albumin Nanoparticles for Drug Combination in Targeting Inflamed Intestine. Adv. Healthc. Mater. 2020, 9, 2000536.
- Lee, A.; De Mei, C.; Fereira, M.; Marotta, R.; Yoon, H.Y.; Kim, K.; Kwon, I.C.; Decuzzi, P. Dexamethasone-loaded polymeric nanoconstructs for monitoring and treating inflammatory bowel disease. Theranostics 2017, 7, 3653–3666.
- Zhao, J.; Gao, W.; Cai, X.; Xu, J.; Zou, D.; Li, Z.; Hu, B.; Zheng, Y. Nanozyme-mediated catalytic nanotherapy for inflammatory bowel disease. Theranostics 2019, 9, 2843–2855.
- Asgharzadeh, F.; Hashemzadeh, A.; Rahmani, F.; Yaghoubi, A.; Nazari, S.E.; Avan, A.; Mehr, S.M.H.; Soleimanpour, S.; Khazaei, M. Cerium oxide nanoparticles acts as a novel therapeutic agent for ulcerative colitis through anti-oxidative mechanism. Life Sci. 2021, 278, 119500.
- Ahmad, A.; Ansari, M.M.; Mishra, R.K.; Kumar, A.; Vyawahare, A.; Verma, R.K.; Raza, S.S.; Khan, R. Enteric-coated gelatin nanoparticles mediated oral delivery of 5-aminosalicylic acid alleviates severity of DSS-induced ulcerative colitis. Mater. Sci. Eng. C 2021, 119, 111582.
- Davoudi, Z.; Peroutka-Bigus, N.; Bellaire, B.; Jergens, A.; Wannemuehler, M.; Wang, Q. Gut organoid as a new platform to study alginate and chitosan mediated plga nanoparticles for drug delivery. Mar. Drugs 2021, 19, 282.
- Yaghoubi, A.; Davoodi, J.; Asgharzadeh, F.; Rezaie, S.; Nazari, E.; Khazaei, M.; Soleimanpour, S. Therapeutic effect of an anti-tuberculosis agent, isoniazid, and its nano-isoform in ulcerative colitis. Int. Immunopharmacol. 2021, 96, 107577.
- Greish, K.; Taha, S.; Jasim, A.; Elghany, S.A.; Sultan, A.; AlKhateeb, A.; Othman, M.; Jun, F.; Taurin, S.; Bakhiet, M. Styrene maleic acid encapsulated raloxifene micelles for management of inflammatory bowel disease. Clin. Transl. Med. 2017, 6, 28.
- Cai, X.; Wang, X.; He, M.; Wang, Y.; Lan, M.; Zhao, Y.; Gao, F. Colon-targeted delivery of tacrolimus using pH-responsive polymeric nanoparticles for murine colitis therapy. Int. J. Pharm. 2021, 606, 120836.
- Amaldoss, M.J.N.; Najar, I.A.; Kumar, J.; Sharma, A. Therapeutic efficacy of rifaximin loaded tamarind gum polysaccharide nanoparticles in TNBS induced IBD model Wistar rats. Rep. Pract. Oncol. Radiother. 2021, 26, 712–729.
