Considering the biological activities of both flavonoids and 1,2,3-triazole ring, as well as the metabolic stability associated to this heterocyclic ring, hybridization of flavonoids with a 1,2,3-triazole ring has been widely reported over the last years. The most common way to obtain these hybrids is through the copper (I) catalyzed azide-alkyne cycloaddition (CuAAC), also known as Click Chemistry reaction. ThisIt reviewwas highlightsed the flavonoid hybrids linked by the1,2,3-triazole ring obtained since 2017, including chalcones, flavones, flavanones and flavonols, among others, with antitumor, antimicrobial, antidiabetic, neuroprotective, anti-inflammatory, antioxidant, and antifouling activities.
- flavonoids
- Click Chemistry
- 1,2,3-triazole
- biological activities
- Introduction
1. Introduction
The 1,2,3-triazole ring in Medicinal Chemistry has been gaining increased attention over the past years. The ability of this heterocycle moiety to participate in hydrogen bonding and dipole interactions provides an improvement of solubility and binding to biomolecular targets. Triazole ring is stable towards hydrolysis, oxidative, and reductive conditions and enzymatic degradation [1]. Moreover, triazole can be used as a linker and as a bioisostere of different functional groups for the synthesis of new active compounds [2][3]. Besides that, therapeutic properties have been attributed to 1,2,3-triazole hybrids, including antibacterial [4], anticancer [5][6], anti-inflammatory [7] activities, among others [3].
The most common reaction to synthesize the 1,2,3-triazole ring is the Copper (I) catalyzed azide-alkyne cycloaddition (CuAAC). CuAAC, commonly referred to as Click Chemistry, was first introduced by Sharpless and Meldal groups in 2002 and involves the reaction of a terminal alkyne and an aliphatic azide using copper as a catalyst to give 1,4-disubstituted-triazoles [8][9]. Huisgen 1,3-dipolar cycloaddition (Figure 1A) presented several limitations, including long reaction time, high temperature, and the formation of a mixture of 1,4 and 1,5-disubstitution products. In contrast, CuAAC (Figure 1B) allows selective, high-yield, faster and mild reaction conditions, with the formation of few side products [10][11].
Moreover being widely used in drug discovery [12][13], CuAAC has been applied in other areas, including bioimaging [14][15], biological [16], and biomedical[17] applications, synthesis of polymers [18][19] and dendrimers [20][21], and preparation of chemosensors[22], among others.
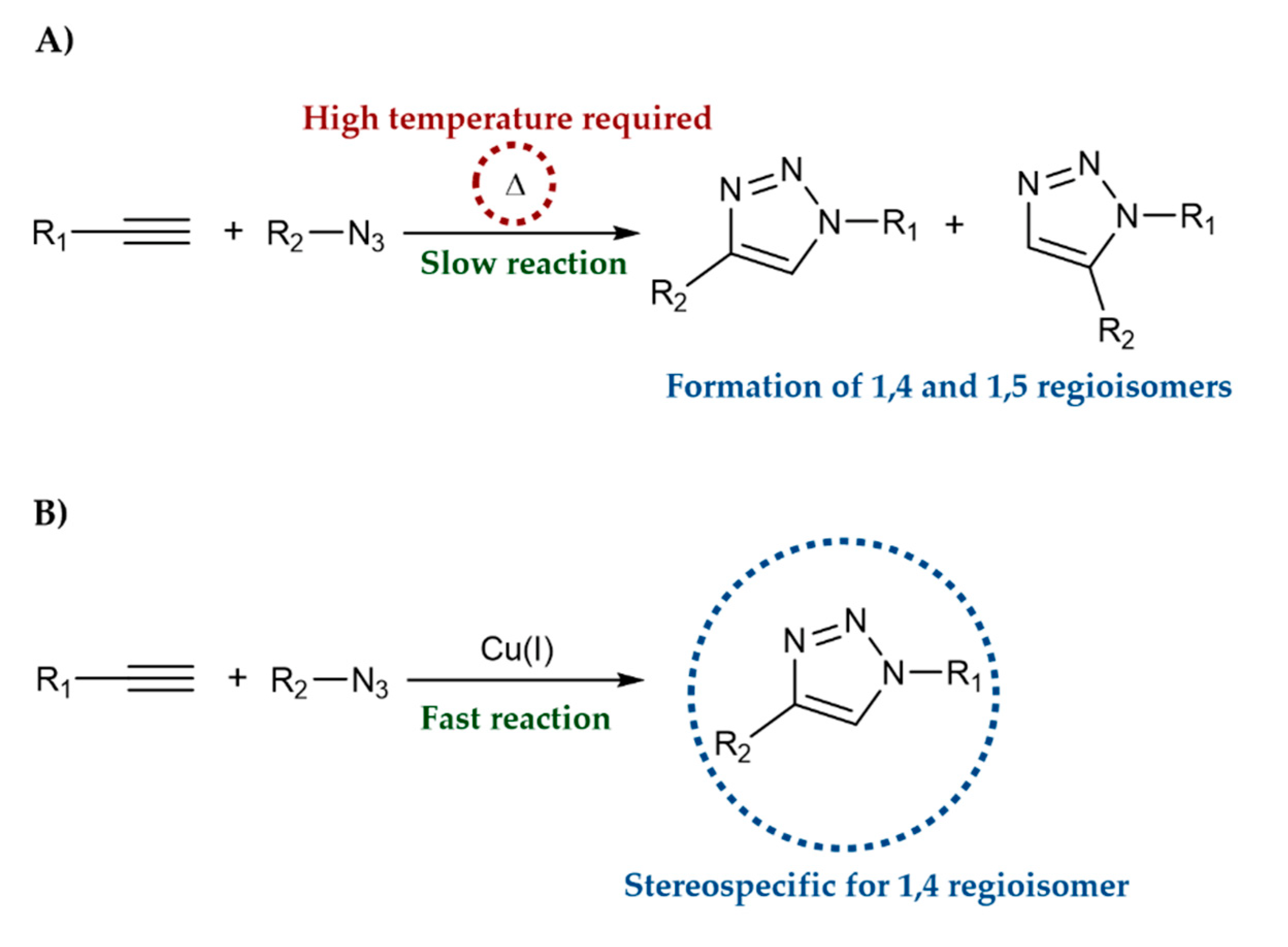
Figure 1. A) Huisgen 1,3-dipolar cycloaddition. B) Copper (I) catalyzed azide-alkyne cycloaddition (CuAAC).
Flavonoids are well-known for their wide range of pharmacological activities, such as anti-inflammatory, cardioprotective, antimicrobial, anticancer, and neuroprotective [23][24][25][26][27][28][29][30][31][32]. Beyond their potential in Medicinal Chemistry, flavonoids have been reported for their antioxidant, antiaging, anti-browning, anticorrosive, and antifouling activities which have highlighted the potential of flavonoids for several industrial applications (food, cosmetic, marine industries) [33][34][35][36][37][38]. Hybridization of flavonoids with other pharmacophoric moieties has become a strategy in Medicinal Chemistry for the development of compounds with higher potency by combining two or more pharmacophores into a single entity [39][40]. The incorporation of 1,2,3-triazole ring in flavonoid hybridization has become a strategy due to its bioactivity, metabolic stability, and convenient synthesis [41].
The purpose of Ithis review i is to highlight the biological activities of flavonoid hybrids linked by the 1,2,3-triazole ring synthesized in the last five years, emphasizing the mechanism of action and SAR studies. Most of the flavonoid hybrids were obtained by CuAAC using copper sulphate and sodium ascorbate as catalysts, with slight modifications in the solvent system, since the in situ generation of Cu(I) salts from copper sulphate through reduction by sodium ascorbate is more convenient and more commonly used than Cu(I) salts, which needs the presence of a base as stabilizer and to assist in the ionization of the terminal acetylene [42]. Nevertheless, the utilization of copper iodide (CuI) as a catalyst is also widely reported. Some research papers report the use of other catalysts, such as bromotris(triphenylphosphine)copper(I). Prior to the CuAAC step, a terminal alkyne or an azide is introduced in the flavonoid scaffold. Interestingly, for the synthesis of chalcones, most of the research papers reported the propargylation on the building blocks acetophenone or benzaldehyde (Figure 2A), followed by Claisen–Schmidt condensation, whereas for other flavonoids the propargylation generally occurred on the flavonoid scaffold (FFigure 2B).
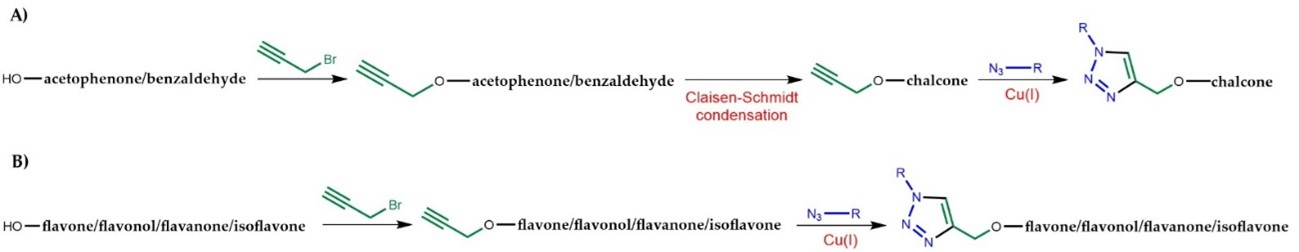
Figure 2. General steps for the synthesis of flavonoid hybrids conjugure 2B)ated with 1,2,3-triazole. A) Synthesis of chalcone-1,2,3-triazole hybrids. B) Synthesis of flavone/flavonol/flavanone/isoflavone-1,2,3-triazole hybrids.

Figure 2. General steps for the synthesis of flavonoid hybrids conjugated with 1,2,3-triazole. A) Synthesis of chalcone-1,2,3-triazole hybrids. B) Synthesis of flavone/flavonol/flavanone/isoflavone-1,2,3-triazole hybrids.
The biological activities of the different subclasses of flavonoid hybrids, synthesized since 2017 are following presented, beginning with antitumor activity for which higher number of compounds were reported.
- 1,2,3-Triazole linked Flavonoid Hybrids with antitumor activity
2. 1,2,3-Triazole linked Flavonoid Hybrids with Antitumor Activity
In this chapter, more than 300 synthetic 1,2,3-triazole linked flavonoid hybrids with antitumor activity, including chalcones, flavanones, flavones, flavonols and isoflavones, have been reported since 2017. The conjugation of flavonoids by CuAAC giving rise to bioactive flavonoid dimers was also described. As shown in the Figure 3, chalcones was the most common flavonoid subclass.
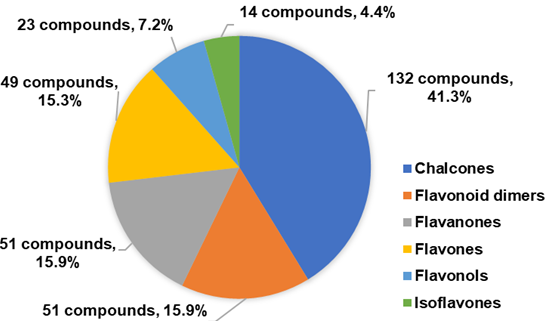
Figure 3. Distribution of the flavonoid hybrids with antitumor activity according to flavonoid subclasses.
- 1,2,3-Triazole-Linked Flavonoid Hybrids with Antimicrobial Activity
3. 1,2,3-Triazole-Linked Flavonoid Hybrids with Antimicrobial Activity
In this chapter, the synthesis and antimicrobial activities of more than 200 flavonoid hybrids linked by the 1,2,3-triazole ring, reported since 2017, are described. Most of these compounds correspond to chalcone derivatives, as shown in FigureFigure 4. 4. Nevertheless, other classes of flavonoids, such as flavanones, flavones, and isoflavones, were also reported. Moreover, most of the described flavonoid hybrids showed antibacterial and antifungal activity. However, some reports about flavonoids with antiparasitic and antiviral activity can also be found in literature. It should be noted that several compounds simultaneously exhibited antibacterial, antifungal, and antiparasitic activities.
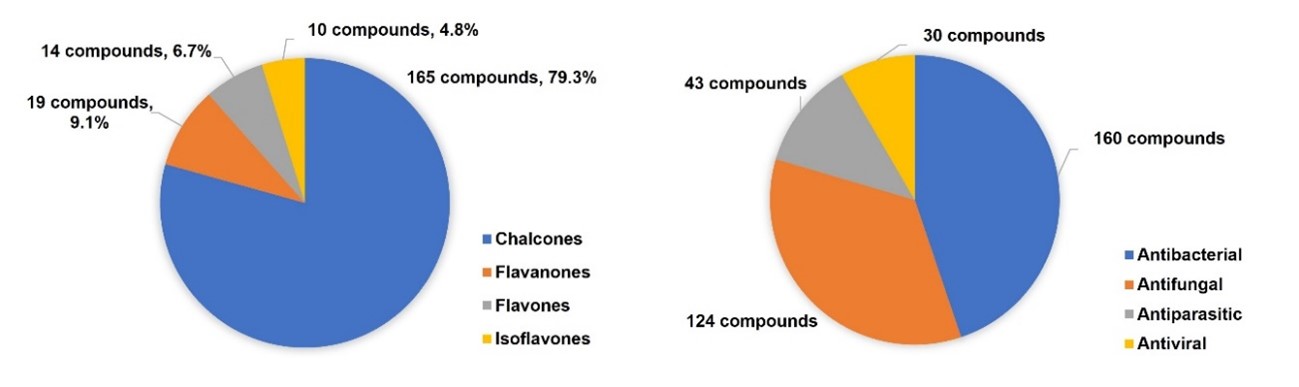
Figure 4. Distribution of the flavonoid hybrids according to the reported antimicrobial activity.
- 1,2,3-Triazole-Linked Flavonoid Hybrids with other activities
4. 1,2,3-Triazole-Linked Flavonoid Hybrids with Other Activities
Besides antitumor and antimicrobial activities, a total of 163 flavonoid hybrids conjugated by the 1,2,3-triazole ring with other activities, including neuroprotective, anti-inflammatory, antioxidant, antidiabetic, and antifouling, were also reported since 2017 and recorded in this review. Moreover, some of these compounds have been shown to be modulators of other enzymes, namely carbonic anhydrase, serine proteases and glutatione S-transferase. The distribution of the different classes of flavonoids is highlighted in Figure 5.
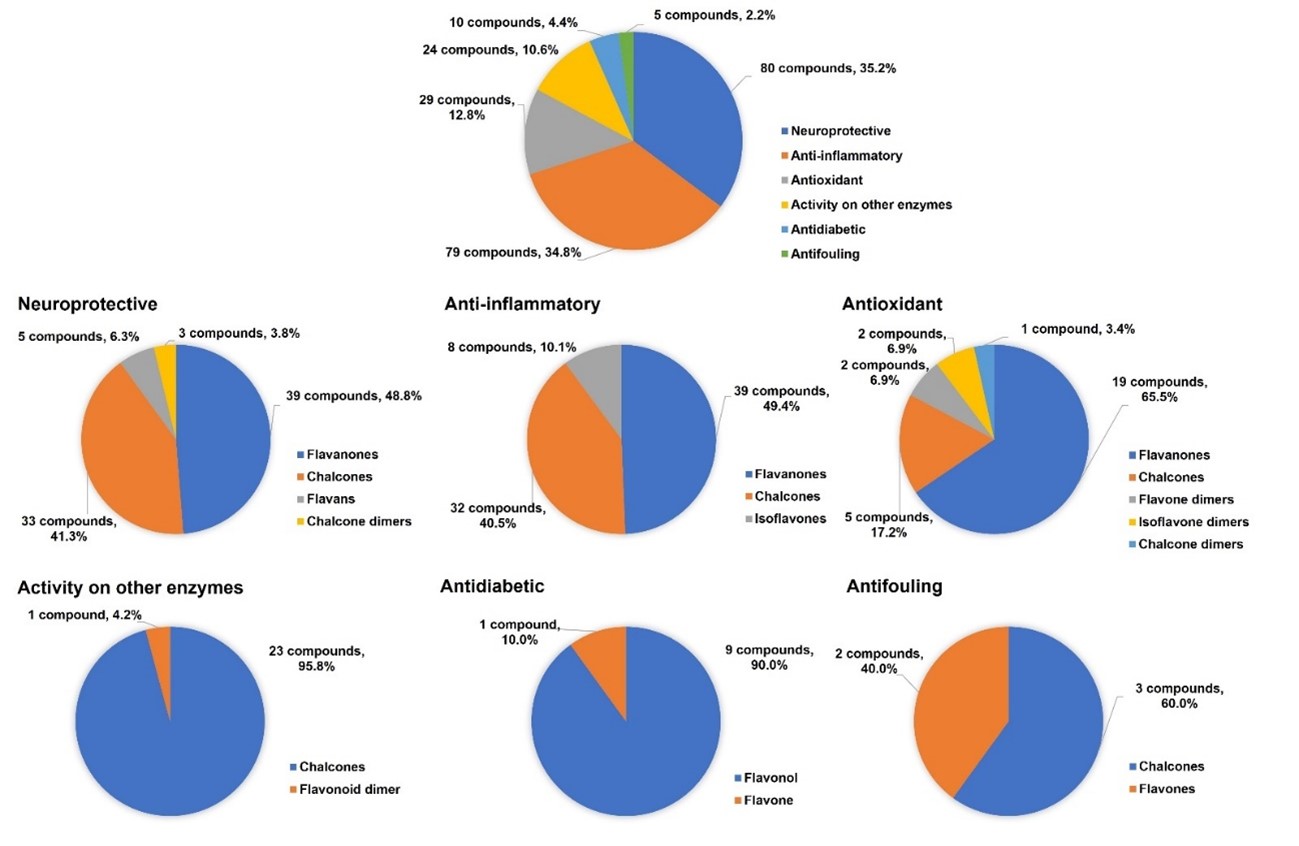
Figure 5. Other activities attributed to 1,2,3-triazole linked flavonoid hybrids.
- Conclusion
5. Conclusion
In this review, mMany compounds (691) belonging to the 1,2,3-triazole linked flavonoid hybrids reported since 2017, were highlighted, namely concerning their synthesis and biological activities. Concerning the synthesis of the triazole ring, it was found that for most studies, the Click Chemistry step was accomplished at room temperature using copper sulphate as a catalyst and sodium ascorbate as a reducing agent, with slight modifications on the solvent system, generally giving rise to the desired compounds in high yields. Nevertheless, the use of conventional heating or MW irradiation was also reported, with the advantage of the decrease of reaction time. Instead of copper sulphate, the use of other copper catalysts such as copper iodide or bromotris(triphenylphosphine)copper(I) was also described with overall good results. Interestingly, some reports focused the Click Chemistry step using cellulose supported copper iodide nanoparticles as a reusable catalyst without loss of efficiency.
Among biological activities, antitumor and antimicrobial activities have been the most studilearned, mainly for chalcone derivatives. Some of derivatives of flavonoids with these activities revealed to be more potent than parent compounds or even commercially available drugs, using in vitro assays.
Considering the flavonoids with antimicrobial activity, several hybrids showed comparable or even higher antibacterial activity to reference drug ciprofloxacin, currently used for the treatment of microbial diseases. Moreover, some hybrids revealed promising antiplasmodial activity against multidrug resistant strains. These data suggest the potential of some flavonoid-hybrids linked by 1,2,3-triazole ring to circumvent microbiological diseases, especially those with multidrug resistance.
In addition to the antitumor and antimicrobial activity, several hybrids of flavonoids exhibited quite promising anti-inflammatory and antidiabetic activities. Interestingly, some of these compounds also showed suitable pharmacokinetic and toxicity characteristics in in silico ADMET studiones, reinforcing that the flavonoid hybridization using 1,2,3-triazole as linker may allow not only to improve the potency, but also the pharmacokinetic profile of flavonoids.
References
- F. Silva, M. Cardoso, P. Ferreira, V. Ferreira, Biological Properties of 1H-1,2,3- and 2H-1,2,3-Triazoles, in: W. Dehaen, V.A. Bakulev (Eds.) Chemistry of 1,2,3-triazoles, Springer International Publishing, 2014, pp. 117-165.
- E. Bonandi, M.S. Christodoulou, G. Fumagalli, D. Perdicchia, G. Rastelli, D. Passarella, The 1,2,3-triazole ring as a bioisostere in medicinal chemistry, Drug Discov. Today, 22 (2017) 1572-1581.
- D. Dheer, V. Singh, R. Shankar, Medicinal attributes of 1,2,3-triazoles: Current developments, Bioorg. Chem., 71 (2017) 30-54.
- F. Bi, S. Ji, H. Venter, J. Liu, S.J. Semple, S. Ma, Substitution of terminal amide with 1H-1,2,3-triazole: Identification of unexpected class of potent antibacterial agents, Bioorg. Med. Chem. Lett., 28 (2018) 884-891
- R. Lakkakula, A. Roy, K. Mukkanti, G. Sridhar, Synthesis and Anticancer Activity of 1,2,3-Triazole Fused N-Arylpyrazole Derivatives, Russ. J. Gen. Chem., 89 (2019) 831-835.
- Z. Xu, S.-J. Zhao, Y. Liu, 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships, Eur. J. Med. Chem., 183 (2019) 111700.
- K.K. Angajala, S. Vianala, R. Macha, M. Raghavender, M.K. Thupurani, P.J. Pathi, Synthesis, anti-inflammatory, bactericidal activities and docking studies of novel 1,2,3-triazoles derived from ibuprofen using click chemistry, SpringerPlus, 5 (2016) 423.
- J. Totobenazara, A.J. Burke, New click-chemistry methods for 1,2,3-triazoles synthesis: recent advances and applications, Tetrahedron Lett., 56 (2015) 2853-2859.
- M.S. Singh, S. Chowdhury, S. Koley, Advances of azide-alkyne cycloaddition-click chemistry over the recent decade, Tetrahedron, 72 (2016) 5257-5283.
- E. Haldón, M.C. Nicasio, P.J. Pérez, Copper-catalysed azide–alkyne cycloadditions (CuAAC): an update, Org. Biomol. Chem., 13 (2015) 9528-9550.
- L. Liang, D. Astruc, The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview, Coord. Chem. Rev., 255 (2011) 2933-2945.
- A. Keivanloo, M. Fakharian, S. Sepehri, 1,2,3-Triazoles based 3-substituted 2-thioquinoxalines: Synthesis, anti-bacterial activities, and molecular docking studies, J. Mol. Struct., 1202 (2020) 127262.
- J. Qiu, C.-M. Yuan, M. Wen, Y.-N. Li, J. Chen, J.-Y. Jian, L.-J. Huang, W. Gu, Y.-M. Li, X.-J. Hao, Design, synthesis, and cytotoxic activities of novel hybrids of parthenolide and thiazolidinedione via click chemistry, J. Asian Nat. Prod. Res., 22 (2020) 425-433.
- Z. Du, D. Yu, X. Du, P. Scott, J. Ren, X. Qu, Self-triggered click reaction in an Alzheimer's disease model: in situ bifunctional drug synthesis catalyzed by neurotoxic copper accumulated in amyloid-β plaques, Chem. Sci., 10 (2019) 10343-10350.
- E.P.S. Shankar, D. Bahulayan, Chemistry, chemical biology and photophysics of certain new chromene–triazole–coumarin triads as fluorescent inhibitors of CDK2 and CDK4 induced cancers, New J. Chem., 43 (2019) 13863-13872.
- P. Yue, Y. Zhang, Z.F. Guo, A.C. Cao, Z.L. Lu, Y.G. Zhai, Synthesis of bifunctional molecules containing [12]aneN3 and coumarin moieties as effective DNA condensation agents and new non-viral gene vectors, Org. Biomol. Chem., 13 (2015) 4494-4505.
- M. Araújo, S.J. Bidarra, P.M. Alves, J. Valcarcel, J.A. Vázquez, C.C. Barrias, Coumarin-grafted blue-emitting fluorescent alginate as a potentially valuable tool for biomedical applications, J. Mater. Chem. B, 8 (2020) 813-825.
- Y. Huang, Y. Liu, S. Muhammad, Q. Jia, Y. Ding, Y. Chen, A facile end-functionalization of polystyrene by ATRP and click chemistry: Chain end effect on the glass transition temperature, React. Funct. Polym., 151 (2020) 104566.
- M. Zeng, X. Cao, H. Xu, W. Gan, B.D. Smith, H. Gao, J. Yuan, Synthesis and direct assembly of linear–dendritic copolymers via CuAAC click polymerization-induced self-assembly (CPISA), Polym. Chem., 11 (2020) 936-943.
- A. Concellón, R. Termine, A. Golemme, P. Romero, M. Marcos, J.L. Serrano, High hole mobility and light-harvesting in discotic nematic dendrimers prepared via ‘click’ chemistry, J. Mater. Chem. C, 7 (2019) 2911-2918.
- K. Rajavelu, P. Rajakumar, Synthesis, photophysical, and electrochemical properties of triazolyl dendrimers with thiazolylchalcone surface unit, Synth. Commun., 48 (2018) 38-49.
- T. Puthiyedath, D. Bahulayan, A click derived triazole-coumarin derivative as fluorescence on-off PET based sensor for Ca2+and Fe3+ ions, Sens. Actuators B Chem., 272 (2018) 110-117.
- P. Brandão, J.B. Loureiro, S. Carvalho, M.H. Hamadou, S. Cravo, J. Moreira, D. Pereira, A. Palmeira, M. Pinto, L. Saraiva, H. Cidade, Targeting the MDM2-p53 protein-protein interaction with prenylchalcones: Synthesis of a small library and evaluation of potential antitumor activity, Eur. J. Med. Chem., 156 (2018) 711-721.
- M.M. Jucá, F.M.S. Cysne Filho, J.C. de Almeida, D.d.S. Mesquita, J.R.d.M. Barriga, K.C.F. Dias, T.M. Barbosa, L.C. Vasconcelos, L.K.A.M. Leal, J.E. Ribeiro, S.M.M. Vasconcelos, Flavonoids: biological activities and therapeutic potential, Nat. Prod. Res., 34 (2020) 692-705.
- P. Maher, The Potential of Flavonoids for the Treatment of Neurodegenerative Diseases, Int. J. Mol. Sci., 20 (2019) 19.
- B.T. Martins, M. Correia da Silva, M. Pinto, H. Cidade, A. Kijjoa, Marine natural flavonoids: chemistry and biological activities, Nat. Prod. Res., 33 (2019) 3260-3272.
- D. Mendanha, J. Vieira de Castro, J. Moreira, B.M. Costa, H. Cidade, M. Pinto, H. Ferreira, N.M. Neves, A New Chalcone Derivative with Promising Antiproliferative and Anti-Invasion Activities in Glioblastoma Cells, Molecules, 26 (2021).
- J. Moreira, J. Almeida, L. Saraiva, H. Cidade, M. Pinto, Chalcones as Promising Antitumor Agents by Targeting the p53 Pathway: An Overview and New Insights in Drug-Likeness, Molecules, 26 (2021).
- J. Moreira, D. Ribeiro, P.M.A. Silva, N. Nazareth, M. Monteiro, A. Palmeira, L. Saraiva, M. Pinto, H. Bousbaa, H. Cidade, New Alkoxy Flavone Derivatives Targeting Caspases: Synthesis and Antitumor Activity Evaluation, Molecules, 24 (2019).
- A.N. Panche, A.D. Diwan, S.R. Chandra, Flavonoids: an overview, J. Nutr. Sci., 5 (2016) e47.
- D. Pereira, R.T. Lima, A. Palmeira, H. Seca, J. Soares, S. Gomes, L. Raimundo, C. Maciel, M. Pinto, E. Sousa, M. Helena Vasconcelos, L. Saraiva, H. Cidade, Design and synthesis of new inhibitors of p53–MDM2 interaction with a chalcone scaffold, Arab. J. Chem., 12 (2019) 4150-4161.
- P. Pinto, C.M. Machado, J. Moreira, J.D.P. Almeida, P.M.A. Silva, A.C. Henriques, J.X. Soares, J.A.R. Salvador, C. Afonso, M. Pinto, H. Bousbaa, H. Cidade, Chalcone derivatives targeting mitosis: synthesis, evaluation of antitumor activity and lipophilicity, Eur. J. Med. Chem., 184 (2019) 111752.
- J.R. Almeida, J. Moreira, D. Pereira, S. Pereira, J. Antunes, A. Palmeira, V. Vasconcelos, M. Pinto, M. Correia-da-Silva, H. Cidade, Potential of synthetic chalcone derivatives to prevent marine biofouling, Sci. Total Environ., 643 (2018) 98-106.
- D.J. de Lima Cherubim, C.V. Buzanello Martins, L. Oliveira Fariña, R.A. da Silva de Lucca, Polyphenols as natural antioxidants in cosmetics applications, J. Cosmet. Dermatol., 19 (2020) 33-37.
- A. Espinoza Vázquez, I.A. Figueroa, F.J.R. Gómez, A.P. Vásquez, R. Mata, D. Ángeles Beltrán, A. Miralrio, M. Castro, (–) – Epicatechin gallate as a corrosion inhibitor for bronze in a saline medium and theoretical study, J. Mol. Struct., 1227 (2021) 129416.
- A. Kurek-Górecka, M. Górecki, A. Rzepecka-Stojko, R. Balwierz, J. Stojko, Bee Products in Dermatology and Skin Care, Molecules, 25 (2020).
- S. Maqsood, O. Adiamo, M. Ahmad, P. Mudgil, Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients, Food Chem., 308 (2020) 125522.
- R.J. Obaid, E.U. Mughal, N. Naeem, A. Sadiq, R.I. Alsantali, R.S. Jassas, Z. Moussa, S.A. Ahmed, Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: a systematic review, RSC Adv., 11 (2021) 22159-22198.
- S. Kumar, N. Verma, N. Kumar, A. Patel, P. Roy, V. Pruthi, N. Ahmed, Design, synthesis, molecular docking, and biological studies of novel phytoestrogen-tanaproget hybrids, Synth. Commun., 46 (2016) 460-474.
- Z. Sang, K. Wang, J. Shi, X. Cheng, G. Zhu, R. Wei, Q. Ma, L. Yu, Y. Zhao, Z. Tan, W. Liu, Apigenin-rivastigmine hybrids as multi-target-directed liagnds for the treatment of Alzheimer’s disease, Eur. J. Med. Chem., 187 (2020) 111958.
- L. Zhao, L. Mao, G. Hong, X. Yang, T. Liu, Design, synthesis and anticancer activity of matrine–1H-1,2,3-triazole–chalcone conjugates, Bioorg. Med. Chem. Lett., 25 (2015) 2540-2544.
- M.R.E.S. Aly, H.A. Saad, M.A.M. Mohamed, Click reaction based synthesis, antimicrobial, and cytotoxic activities of new 1,2,3-triazoles, Bioorg. Med. Chem. Lett., 25 (2015) 2824-2830.
