Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mariana Monteiro and Version 2 by Vivi Li.
Oleuropein (OLE) and hydroxytyrosol (HT) are olive-derived phenols recognised as health-promoting agents with antioxidant, anti-inflammatory, cardioprotective, antifungal, antimicrobial, and antitumor activities, providing a wide range of applications as functional food ingredients. HT is Generally Recognised as Safe (GRAS) by the European Food Safety Authority (EFSA) and the Food and Drug Administration (FDA), whereas OLE is included in EFSA daily consumptions recommendations, albeit there is no official GRAS status for its pure form.
- olive phenols
- delivery
- encapsulation
- complexation
- emulsions
- chemical functionalisation
- food application
1. Introduction
Biophenols derived from olives are used as traditional remedies for a variety of conditions, including inflammatory states and cardiovascular diseases. Oleuropein (OLE) and hydroxytyrosol (HT) are the most well-known compounds of this family. OLE is the main phenol in olive-derived olive products, being composed of an elenolic acid linked to an o-diphenol, hydroxytyrosol, and a glucose residue (Figure 1a). It is present in olive tree leaves and drupes. Its aglycone form is also found in olive oil (oleuropein is not soluble in oil due to the superior polarity) [1]. HT (Figure 1b), also known as 3,4-dihydroxyphenylethanol (DOPET) or 3,4-dihydroxyphenolethanol (3,4-DHPEA) or 4-(2-hydroxyethyl)-1,2-benzenediol, results from OLE degradation. It is found mainly in olive oil and cured olives and, in lower amounts, in olive leaves and other products such as grapes, wine and olive by-products [2][3][4][2,3,4]. Its occurrence in wine has been proposed to result from alcoholic fermentation or from the enzymatic oxidation of tyrosol (TYR) [2][5][2,5]. OLE is responsible for the bitter taste of fresh olives. To become acceptable by consumers, fresh olives are submitted to a de-bittering process in which OLE is hydrolysed to HT and elenolic acid, two non-bitter compounds [6]. Degradation of OLE occurs both during the maturation of the olives and oil storage [7].
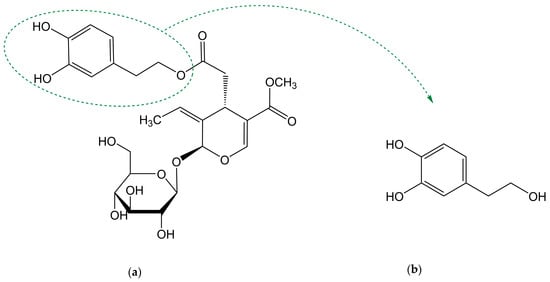
Figure 1.
Chemical structure of (
a
) oleuropein and (
b
) hydroxytyrosol.
The amount of these compounds in their natural sources is variable. A database on polyphenol content in foods reports that OLE average content in olive oils varies from 0.17 mg/100 g for extra virgin oil to less than 1 µg/100 g for virgin ones, while in black and green olives its mean content is 72 and 56 mg/100 g [8]. On the other hand, the average content of HT in olives and derivatives varies, in mg/kg of product, from 3.5 in virgin olive oils, to 7.7 in extra-virgin olive oils, 659 in black olives and 556 in green olives [9].
The antioxidant activity of OLE and HT is, as observed for other phenolic compounds, dictated by the presence of an o-diphenolic group in their structures. Such fact was corroborated by many authors through in vitro and in vivo studies [10][11][10,11]. These compounds are also claimed to exert anti-inflammatory, cardioprotective, antifungal, antimicrobial and antitumor activities, and to have potential physiological benefits on plasma lipoproteins, platelets and cellular function [12]. Moreover, HT is also mentioned to contribute to bone health [7].
The claimed health-promoting properties of OLE and HT, allied to their easy and affordable recovery from olive by-products, make them excellent ingredients for application in functional foods [13]. HT is Generally Recognised as Safe (GRAS) by the European Food Safety Authority (EFSA) and the Food and Drug Administration (FDA) and claimed to benefit human health when ingested at a daily dose of at least 5 mg (this includes related compounds such as OLE) (EC No. 432/2012) [14][15][14,15]. Moreover, HT is authorised as a novel food ingredient by the European Commission (EC) under the regulation EC No 258/97 of 2017 [16]. OLE is included in EFSA’s daily consumption recommendations, albeit there is no official GRAS status for its pure form. Notably, an olive leaf extract Bonolive®, containing 80% OLE, was approved for use as an ingredient in a range of food categories, including yoghurt, confectionery, fine bakery wares and beverages (EC No 258/97), and received Self-affirmed GRAS status in the United States in 2016 [17]. Note that Self affirmed GRAS status refers to an independent GRAS determination not involving the FDA. In this status, the stakeholder is not legally obligated to notify the FDA of its determination before adding the substance to food, but may voluntarily submit their independent GRAS determination to the FDA for review and response [18]. These facts support the use of OLE as a food ingredient.
Since the amount of OLE and HT obtained through their natural sources’ consumption is far below the recommended daily intake, it is important to promote their intake by incorporating them in other types of foods. Examples include beverages, fish-based products and vegetable oils, as already reported for OLE [19] and for HT [20][21][20,21].
The use of OLE and HT as a food ingredient at the industrial scale faces various challenges. OLE is unstable in water or ethanol if under UV light, decomposing through hydrolysis or transesterification [22]. Moreover, its application often causes sensorial constraints due to its bitter taste, requiring the use of taste-masking compounds such as sodium cyclamate and sucrose [23]. HT is highly sensitive to air and light due to its hydroxy groups and its amphiphilic character brings difficulties in mixing it into foods. Additionally, HT may interact with proteins and other food nutrients, which may affect the extension of the metabolisation and, therefore, its bioavailability in the body [24]. Thus, the food matrix must also be considered when aiming to use OLE and HT as food ingredients. The following sections highlight relevant strategies that have been tried by distinct authors to overcome such challenges, particularly the encapsulation in delivery systems and the synthesis of OLE and HT derivatives.
2. Encapsulation of OLE and HT
Encapsulation is a technique in which one or more ingredients (active material or core) are trapped within some form of a matrix (wall, encapsulating agent, shell or carrier material) that can vary in form and size, including solid or liquid, homogeneous or heterogeneous and microscopic or macroscopic [25]. Nanoencapsulation often refers to capsules with dimensions on the scale of 1–1000 nm, although some authors usually consider those with dimensions above 100 nm as microcapsules [26]. The maximum size limit for microcapsules is more consensual, being generally considered at 800 µm [27]. The wall material of these capsules may include lipids, proteins, hydrocarbons, or other polymers [28]. Olive-derived biophenols have been encapsulated in different types of delivery systems with a variety of purposes: protecting them from degradation, increasing solubility in particular media, allowing their controlled delivery, and enhancing their bioavailability. It is unquestionable that of the two olive biophenols, HT is by far the most investigated, possibly not only due to its antioxidant power and tasteless character, but also as a product of OLE degradation (a favorable aspect to obtain this compound in considerable amounts).2.1. Lipids
The main advantage of using lipids as components of nano and microcapsules is that they are biocompatible and biodegradable [29]. Liposomes are among the first lipid nanoparticles ever developed and they result from the self-assembly of the amphiphilic phospholipids in an aqueous medium into one or several concentric closed bilayers. Relevant studies focusing on the application of these structures to olive biophenols are summarised in Table 1. OLE was shown to have superior encapsulation efficiency (30%) in liposomes than HT and tyrosol (TYR) (12% and 4% respectively), with improved physical stability and less tendency to aggregate [30]. In addition, no cytotoxic effects were noticed for the encapsulated OLE and HT on human chondrocyte cells cells at concentrations tested: max 1.4 × 10−1 and 6.0 × 10−2 mM, respectively [30]. Still, Yuan and coworkers [31] were able to encapsulate HT in nanoliposomes (average size of 200 nm) with a 45% efficiency. The authors highlighted the enhanced stability of encapsulated HT in aqueous media, which increased from 7 to 30 days, at 25 °C, allowing its prolonged release without any loss of α-diphenyl-β-picrylhydrazyl (DPPH) radical scavenging activity. In another study, Evans and Compton [32] produced liposomes enclosing an HT derivative, namely phosphatidyl-HT, at a subnanomolar range of concentrations. The liposomes had a size of approximately 85 nm, a surface charge of −25 mV, and they were suggested to afford formulations with good stability. Moreover, OLE was encapsulated in 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) liposomes and interaction studies showed that OLE can strongly interact with phospholipid headgroups. Additionally, in the same study, olive leaf extracts rich with OLE were encapsulated in DPPC liposomes with a mean encapsulation efficiency of 34% and a mean particle size of 405 nm, which show good stability in commercial lemonade drink over long periods (47 days) at refrigeration temperatures (5 °C) [33]. Another study used ufasomes (unsaturated fatty acid liposomes made up of oleic and linoleic acids) to encapsulate OLE. The in vitro studies on CaCo-2 cells demonstrated the great ability of ufasomes to interact and to be internalised into cell model and the improvement of natural antioxidant activity of OLE against oxidative stress induced by H2O2 on cell model [34].Table 1.
Reports of lipid-based encapsulation systems for Oleuropein (OLE) and hydroxytyrosol (HT).
| Formulation | Application | Main Findings | Ref. |
|---|---|---|---|
| Liposomes | |||
| Liposomes with OLE, HT and TYR | Drug-delivery system | ↑ EE% for OLE No cytotoxic effects on human chondrocyte cells |
[30] |
| DPPC liposomes with OLE | Beverages | EE: 34% Particle size: 405 nm Stable in commercial lemonade drink over 47 days at 5 °C |
[33] |
| Ufasomes with OLE | Claim for food application | ↑ antioxidant activity of encapsulated OLE against oxidative stress induced by H2O2 on CaCo-2 cells | [34] |
| Liposomes with phosphatidyl-HT | Claim for food application | Particle size 85 nm; Surface charge: <−25 mV (stable liposomes) | [32] |
| Nanostructured lipid carriers | |||
| OLE-loaded NLC | Claim for food application | OLE leakage was not observed in the nanocarriers within the 3 months of storage Good stability of OLE-loaded NLC |
[35] |
| Emulsions | |||
| Lipid emulsions and microemulsions | Claim for food application | Digestibility assay: ↓ Gastric lipolysis of microemulsion compared to emulsions. ↓ Effect of duodenal lipolysis by the dispersion type. | [36][37][36,37] |
| OLE-loaded W/O/W | Claim for food application | Emulsions were stabilised for + than 40 days of storage with ↑ hydrophobic emulsifier concentration and ↓ OLE concentration | [38] |
| OLE-loaded O/W | Claim for food application | Stable monodisperse oil-in-water O/W was produced when higher hydrophobic triglyceride oils are used | [39] |
| OLE-loaded O/W | Claim for food application | ↑ stability due to the surface activity of OLE | [40] |
| Nano OLE-loaded W/O/W | Claim for food application | Optimum conditions for formulation: 8% WPC, 1.97% pectin and 8.74% Span 80 EE: 91%; Droplet size: 191 nm; Surface charge: −26.8 mV |
[41] |
| O/W, W/O/W and GDE with HT and perilla oil | Claim for food applications | Emulsions structurally stable at 4 °C up to 22 days. HT losses up to 24% throughout the storage of GDE → ↓ antioxidant activity of the emulsion. No lipid oxidation during storage. |
[42] |
| GDE with HT | Animal fat replacing | Physical properties: ↑ formation of weaker gels; no significant loss levels until 30 days; minimal changes in colour and pH of W/O/W during storage. Oxidation: systems little prone to oxidation even at 30 days. Biological activity: ↑ antioxidant and ↑ antimicrobial activity |
[43] |
| HT in W/O/W enriched in chia oil | Meat supplementation | Presence of HT: ↑ oxidative stability: ↑ DPPH free radicals scavenging; ↑ FRAP; ↓ TBARS | [44] |
EE—Encapsulated efficiency; DPPH—α-diphenyl-β-picrylhydrazyl; GDE—Gelled double emulsion; FRAP—Ferric reducing antioxidant power; HT—Hydroxytyrosol; NLC—Nanostructure lipid carriers; OLE—Oleuropein; O/W—Simple emulsion; and TBARS—Thiobarbituric acid reactive substances; TYR—Tyrosol; W/O/W—Double emulsion.
Solid lipid nanoparticles, which contain lipid in the solid stage at room and body temperature, have some limitations such as leakage of encapsulated compounds. This lead to the development of nanostructured lipid carriers (NLC), in which the lipid phase contains both solid (fat) and liquid (oil) lipids at room temperature [45]. Soleimanifard et al. [35] demonstrated that OLE-loaded NLC was stable for a period of 3 months, without leakage from the nanocarriers when kept at −18 °C and room temperature.
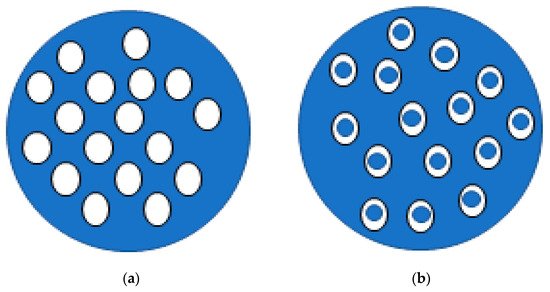
Lipids can also be used in the formulation of microemulsions. Conventional emulsions (often having micro and macrosized droplets) are dispersions of one liquid phase into another liquid phase (immiscible), forming droplets with high interfacial tension that are, therefore, thermodynamically unstable. In turn, lipid microemulsions (emulsions with microscopic droplets) are known to be thermodynamically stable mixtures formed by an aqueous phase, an oil phase and a surfactant [46]. They differ from other lipid particles due to the absence of a solid lipid in the oil phase [47][48][47,48], and can be organised as simple or double emulsions (W/O/W) (Figure 2). Double emulsions, in which the oil droplets are dispersed in the aqueous phase, also contain smaller water droplets in their interior [49]. In general, these are more suitable for the encapsulation of compounds with high hydrophilicity, avoiding the loss of compound to the continuous phase [50][51][50,51]. Some studies are listed in Table 1.
 Chatzidaki et al. [36][37][36,37] developed a microemulsion (droplet size of 20 nm) and an emulsion (droplet size of 354 nm) for HT encapsulation, having found that both systems were able to keep the high antioxidant activity of this compound. The authors also noted that the microemulsion was more resistant to gastric digestion, probably due to lower gastric lipolysis.
W/O/W emulsions have also been used to encapsulate OLE and HT. Souilem et al. [38] produced food-grade W/O/W loaded with OLE, noticing that it only remained stable (for 40 days) for low loading ratios of OLE (0.1–0.3 wt.%). Later, the same authors demonstrated that due to OLE’s interfacial activity, it was possible to produce stable oil-in-water (O/W) emulsions in high hydrophobic triglyceride oils, such as refined soybean oil, extra virgin olive oil and refined olive oil [39]. Further, through molecular dynamics studies, these authors also proved the influence of the surface activity of OLE in the preparation, stability and delivery of the food emulsions. The amphipathic character of OLE decreased the interfacial activity between oil and water phases, showing an emulsifying ability that stabilizes O/W droplets [40]. On the other hand, Gharehbeglou et al. [41] produced double nano-emulsions with OLE stabilised by pectin–whey protein concentrate (WPC) complexes, having set the optimum conditions for this formulation as 8% WPC, 1.97% pectin, 8.74% Span 80 (sorbitan mono-oleate, a non-ionic surfactant), 1:4 ratio of inner-to-outer phase and pH 6.1. The obtained nano W/O/W was characterised by a droplet size of 191 nm, zeta potential of −26.8 mV, and encapsulation efficiency of 91%.
HT was also previously shown to be loaded in W/O/W, albeit less retained than in simple systems, according to Flaiz et al. [42]. In addition, gelled W/O/W, i.e., W/O/W gellified at 4 °C for 24 h, offers an interesting possibility for the food industry to encapsulate bioactive compounds and provide certain plastic properties. Although the formation of gelled W/O/W (in this case, gelled by 4% gelatin and 2% transglutaminase) is advantageous in decreasing phase separation, the HT losses were still above those of simple emulsions during storage [42]. Nevertheless, the same research group showed that gelled W/O/W with HT were stable for up to 30 days and could be used as a healthier replacement for meat fat [43], allowing to retard microbial growth and oxidation. In addition, Cofrades et al. [44] reported that W/O/W of chia seed oil and perilla oil with HT were highly stable. When used to supplement meat at 100 mg/kg meat, this microemulsion system improved the antioxidant properties of the supplemented food products, and simultaneously reduced their oxidation levels. This tendency was even more evident when a simple HT emulsion was used [44].
Chatzidaki et al. [36][37][36,37] developed a microemulsion (droplet size of 20 nm) and an emulsion (droplet size of 354 nm) for HT encapsulation, having found that both systems were able to keep the high antioxidant activity of this compound. The authors also noted that the microemulsion was more resistant to gastric digestion, probably due to lower gastric lipolysis.
W/O/W emulsions have also been used to encapsulate OLE and HT. Souilem et al. [38] produced food-grade W/O/W loaded with OLE, noticing that it only remained stable (for 40 days) for low loading ratios of OLE (0.1–0.3 wt.%). Later, the same authors demonstrated that due to OLE’s interfacial activity, it was possible to produce stable oil-in-water (O/W) emulsions in high hydrophobic triglyceride oils, such as refined soybean oil, extra virgin olive oil and refined olive oil [39]. Further, through molecular dynamics studies, these authors also proved the influence of the surface activity of OLE in the preparation, stability and delivery of the food emulsions. The amphipathic character of OLE decreased the interfacial activity between oil and water phases, showing an emulsifying ability that stabilizes O/W droplets [40]. On the other hand, Gharehbeglou et al. [41] produced double nano-emulsions with OLE stabilised by pectin–whey protein concentrate (WPC) complexes, having set the optimum conditions for this formulation as 8% WPC, 1.97% pectin, 8.74% Span 80 (sorbitan mono-oleate, a non-ionic surfactant), 1:4 ratio of inner-to-outer phase and pH 6.1. The obtained nano W/O/W was characterised by a droplet size of 191 nm, zeta potential of −26.8 mV, and encapsulation efficiency of 91%.
HT was also previously shown to be loaded in W/O/W, albeit less retained than in simple systems, according to Flaiz et al. [42]. In addition, gelled W/O/W, i.e., W/O/W gellified at 4 °C for 24 h, offers an interesting possibility for the food industry to encapsulate bioactive compounds and provide certain plastic properties. Although the formation of gelled W/O/W (in this case, gelled by 4% gelatin and 2% transglutaminase) is advantageous in decreasing phase separation, the HT losses were still above those of simple emulsions during storage [42]. Nevertheless, the same research group showed that gelled W/O/W with HT were stable for up to 30 days and could be used as a healthier replacement for meat fat [43], allowing to retard microbial growth and oxidation. In addition, Cofrades et al. [44] reported that W/O/W of chia seed oil and perilla oil with HT were highly stable. When used to supplement meat at 100 mg/kg meat, this microemulsion system improved the antioxidant properties of the supplemented food products, and simultaneously reduced their oxidation levels. This tendency was even more evident when a simple HT emulsion was used [44].

Figure 2. Schematic representation of simple (a) and double (b) emulsions: white fill correspond to the oil phase and blue fill represents water phase.
2.2. Biopolymer-Based Systems
The production of colloidal delivery systems with food-grade biopolymers such as polysaccharides and proteins is an affordable and practical strategy for encapsulation in food applications. The encapsulant material may vary in composition (protein and/or polysaccharide type), structure (homogenous, core–shell and dispersion) and dimensions, thus achieving distinct systems categories, including biopolymer nanoparticles, hydrogel particles and filled hydrogel particles [52]. As with other systems, most authors focused on HT instead of OLE (Table 2).Table 2.
Reports of biopolymer-bases systems on the delivery of OLE and HT: cellulose, starch, pectin and biocomposites.
| Formulation | Application | Main Findings | Ref. |
| Cellulose microcapsules with HT | Claim for food application | EE: 82.4–88.1% Particle size: 156.6–304.0 µm Microcapsules with HT are gastro-resistant and retain > 50% of their antioxidant capacity in simulated GI fluids. |
[53] |
| Starch granules with HT and probiotics | Nutraceuticals | Resistant against GI tract conditions and stable up to 6 months of storage under refrigeration. ↓ HT bioavailability by the administration of live L. plantarum bacteria with the olive phenol-containing extract, compared to the extract alone. |
[54] |
| Starch nanocrystals or nanoparticles in a PVA film with HT | Active packaging | HT migrated values for all formulations ≤ migration limits for food contact materials. Gradual release of HT during 21 days. Highest gradual release for films with starch nanoparticles. ↑ antioxidant activity for all ternary formulations over time. |
[55] |
| Poly(ε-caprolactone)-based NC and montmorillonite, Cloisite30B films with HT | Active packaging | HT ↑ poly(ε-caprolactone) crystallinity, ↓ thermal stability and plasticizing effect. Interaction of HT-Cloisite30B led to a prolonged release of the HT. |
[56] |
| Pectin plus fish gelatin composite films with HT and DHPG | Strawberry preservation | ↑ stretching capacity and resistance to breakage. The edible film preserved strawberries with a significant delay in visible decay. | [57] |
| Meat preservation | ↓ lipid oxidation in raw beef meat during refrigerated storage. Film with adequate mechanical and oxygen barrier properties. Film with beeswax ↓ lipid oxidation and ↓ the oxygen barrier capacity. | [58] | |
| MD-OLE and IN-OLE | Claim for food application | Protection of OLE from GI conditions. | [59] |
| Eudraguard® protect with HT | Claim for food application | Spherical non-aggregate particle (particle size: 230 nm) Loading capacity of HT: 38% |
[60] |
DHPG—3,4-dihydroxyphenylglycol; EE—Encapsulated efficiency; GI—Gastrointestinal; HT—Hydroxytyrosol; IN—Inulin; MD—Maltodextrin; MF—multifunctional; NC—Nanocomposite; OLE—Oleuropein; PVA—poly(vinyl alcohol).
Owing to their neglectable toxicity and high biodegradability, cellulose and its derivatives have been extensively used for compound delivery in several fields [61][62][63][64][61,62,63,64]. Paulo and Santos [53] tested the use of ethyl cellulose to provide HT in polymeric microcapsules (mean particle size 156.6–304.0 µm), concluding that the capsules remained stable over a wide temperature range and had good encapsulation efficiency (≈88%, regarding the proportion of HT encapsulated versus the amount added) despite a low loading of 5% (ratio between the amount of encapsulated compound and amount of vehicle). In addition, the authors demonstrated that the formulated capsules were gastro-resistant and retained over 50% of the initial amount of HT’s antioxidant activity in simulated gastrointestinal fluid [53].
Starch granules and nanostructures were investigated as alternatives to cellulose for encapsulation of compounds in food applications. Aponte and colleagues [54] formulated starch granules for the combined delivery of HT and probiotics, highlighting their stability and gastro-resistance, as well as the ability to improve the amount of bioavailable HT. Moreover, HT was incorporated in starch nanostructures and applied in active packaging films of poly(vinyl alcohol) (PVA), allowing the gradual release of HT while preserving its antioxidant properties [55]. The same type of application was also achieved by Beltrán et al. [56], in poly(ε-caprolactone) and Cloisite 30B films containing 5–10 wt.% HT. The presence of Cloisite 30B decreased the release of HT, while the presence of HT improved crystallinity but decreased thermal stability, acting simultaneously as plasticizing [56].
A composite film made with pectin and gelatin with HT and 3,4-dihydroxyphenylglycol (DHPG) was applied for the preservation of strawberries [57] and raw meat [58]. Notably, these films had superior stretching capacity and resistance to breakage in comparison with films containing no olive-derived biophenols. Furthermore, the films effectively delayed both mould growth and lipid oxidation on the food products, for at least 7 days of storage. Maltodextrin (MD) and inulin (IN) are also used as delivery systems for bioactive compounds. MD, a saccharide that contains D-glucose units linked with α-(1→4) glycosidic bonds, has high solubility in water, low viscosity, and it forms colourless solutions. Moreover, it is a very digestible polymer from which bioactive compounds may be quickly released during digestion [65]. In turn, IN, a branched fructo-oligosaccharide composed of β-(2→1) glycosidic linked fructose units, can pass relatively intact through the gastric tract [66]. To ouresearchers' knowledge, the application of MD and IN to deliver olive-derived biophenols is yet rather rare to date. Even so, as pointed by González et al. [59], MD-OLE and IN-OLE encapsulations allow protecting OLE from gastrointestinal conditions and it significantly improves their bioaccessibility (15% and 12% for MD-OLE and IN-OLE, respectively, vs. 1.5% in non-encapsulated OLE). The food-approved-biopolymer Eudraguard® protect (composed of methacrylic acid and methyl methacrylate monomer units) was used to encapsulate HT-rich olive oil by supercritical fluid extraction of emulsions, leading to the formation of spherical, non-aggregated particles of an average size of 230 nm and resulted in HT-oil loads of 38% [60].
2.3. Complexation Methods
Molecular inclusion is a process in which a “host” molecule has a cavity into which a “guest” molecule can be accommodated to form an inclusion complex [25]. Cyclodextrins (CDs) are the most commonly used host molecules in foods due to their GRAS status by the FDA. A few studies report CDs as hosts for OLE or HT inclusion (Table 3) [67][68][67,68]. CDs, obtained from the enzymatic degradation of starch [67], are macrocyclic oligosaccharides having 6, 7, or 8 α-D-glucose units, being called, respectively, α-, β-, and γ-CD [69]. CDs have a hollow, truncated conical shape and are the encapsulating agents of choice to improve the stability, activity and solubility of various bioactive compounds [70].Table 3.
Reports regarding the molecular encapsulation of OLE and HT.
| Formulation | Application | Results | Ref. |
|---|---|---|---|
| Oleuropein | |||
| α-CD·OLE, β-CD·OLE and Ɣ-CD·OLE | Claim for food application | OLE form binary complexes (1:1) with the three types of CDs β-CD is the most effective for complexation. |
[71] |
| β-LG·OLE | Claim for food application | ↑ stability of formed complexes and validity of docking results for β-LG·OLE. | [72] |
| OLE·ALA | Claim for food application | OLE binds to ALA mainly via electrostatic, van der Waals and hydrogen bonds. | [73] |
| Hydroxytyrosol and Oleuropein | |||
| β-CD·HT, β-CD·OLE and β-CD·TYR | Claim for food application | No OH group of HT and OLE is shielded in the β-CD cavity Antioxidant activity: β-CD·HT > β-CD·OLE > β-CD·TYR. |
[74] |
| Hydroxytyrosol | |||
| β-CD·olive biophenols | Claim for food application | ↓ bitter taste and preserves them against chemical and physical decomposition reactions during storage. | [75] |
| β-CD·HT, HP-β-CD·HT |
Claim for food application | Insertion of the HT through the narrower face of the CDs. ↑ antioxidant capacity and photoprotection of HT. |
[76] |
| β-CD·HT | Food industry | ↓ HT bioaccessibility (−20%) and absorption (−10%) in presence of foods (7 mg of HT in the meal). β-CD did not affect bioaccessibility and absorption. |
[77] |
| β-CD·HT | Claim for food application | β-CD and drying processes do not affect the efficiency of HT to reduce the DPPH radical. | [78] |
| HT/DHPG-soluble and insoluble dietary fiber of apple cell wall | Dietary fiber | Non-covalent interaction between phenols and the apple cell wall fibers. Antioxidant activity of HT/DHPG was not altered after complexation with apple cell wall fibers and after a simulated gastrointestinal digestion. | [79] |
ALA—α-lactalbumin; CDs—Cyclodextrins; DPPH—α-Diphenyl-β-picrylhydrazyl; DHPG—3,4-Dihydroxyphenylglycol; HP-β-CD—2-(Hydroxy)propyl-β-cyclodextrin; HT—Hydroxytyrosol; OH—Hydroxy group; OLE—Oleuropein; TYR—Tyrosol; α-CD—α-Cyclodextrin; β-CD—β-Cyclodextrin; β-LG—β-Lactoglobulin.
Structure analysis of complexes between OLE and CDs (OLE·CD) has been studied by Efmorfopoulou et al. [71], who proved that OLE forms binary systems (1:1) in neutral aqueous solutions with the three types of CDs and that β-CD was the most effective for complexation. Proteins such as β-lactoglobulin (β-LG) and α-lactalbumin (ALA) were also investigated regarding their interaction with OLE [72]; these proteins have the advantage of providing desirable textures in food. Vanaei and co-workers studied the formation of a complex between β-LG and OLE by molecular docking, having shown its high stability [72]. In the case of OLE·ALA complexes, Katouzian et al. [73] revealed that OLE binds to ALA mainly via electrostatic, van der Waals interactions and hydrogen bonds, as confirmed by Fourier-transform infrared spectroscopy (FTIR), fluorescent spectroscopy, dynamic light scattering, circular dichroism spectroscopy and molecular docking analysis.
