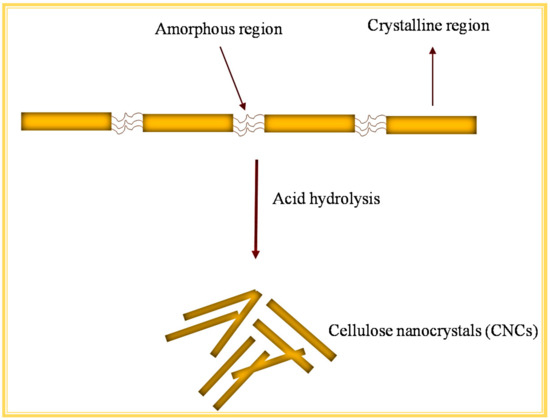
Cellulose has both highly ordered crystalline and amorphous regions in varying proportions, depending on its source. Removing the amorphous region influences the structure and crystallinity of the cellulose, resulting in the formation of CNCs. CNCs are needle-like particles made up of cellulose chain segments that have been organized in an almost defect-free crystalline structure with at least one dimension less-than-or-equal-to 100 nm. CNCs are also known as cellulose nanowhiskers, cellulose whiskers, and nanocrystalline cellulose, but CNCs is the most used term. CNCs have a high thermal stability, surface area, and crystallinity compared to bulk cellulose, which has more amorphous fractions. Different types of LCB waste have been used to extract CNCs such as cotton, pineapple leaf, sugarcane bagasse, walnut shell, soy hulls, bamboo fibre, and many more.
- cellulose nanocrystals
- agricultural waste
- lignocellulosic biomass
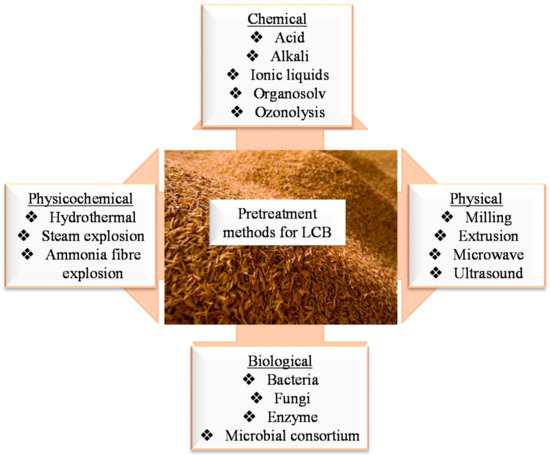
1. Pre-Treatment of Agricultural Waste
2. Extraction of CNCs
2.1. Acid Hydrolysis
2.2. Oxidation
2.3. Other Methods
| Agricultural Residue | Pre-Treatment Conditions | Extraction Conditions | CNC Diameter | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mengkuang leaves | Alkaline treatment using 4% NaOH at 125 °C for 2 h, bleaching using 1.7 w/v% NaClO2 at pH 4.5 and 125 °C for 4 h. | Acid hydrolysis was carried out using 60 wt% H2SO4 solution at 45 °C | 2–5 nm | [29][37] | |||||||
| Ball milling machine for 2 h in DMSO | 41.21 | 70.58 | 174.27 ± 4.32 | [ | 81 | ][47] | Mango seed | Alkali treatment using 2% NaOH at 100 °C for 4 h, bleaching with a solution made up of equal parts (v:v) of acetate buffer (27 g of NaOH and 75 mL of glacial acetic acid, diluted to 1 L of distilled water) and aqueous chlorite (1.7 wt% NaClO2 in water) at 80 °C for 6 h. | Acid hydrolysis was performed at 40 °C for 10 min using H2SO4 (11.21 M). |
4.59 ± 2.22 nm | [ |
| Corncob | Cellulose 45.01 ± 0.9%, hemicellulose33.12 ± 1.1%, lignin 13.81 ± 1.3%, ash 3.1 ± 0.5, other extractives 4.96 ± 1.1%. | 60% H2SO4 at 45 °C for 1 h. | 72 | ][38] | |||||||
| - | 72.36 | 131.4 | [ | 79 | ] | [45] | Agave tequilana and barley | The ground fibres were dispersed in an acid solution (0.2 wt% of acetic acid) of 0.27 wt% of NaClO2 and 0.7 wt% NaOH kept at 70 °C and stirred for 1.5 h. The sample was then treated with 17.5 wt% NaOH for 30 min. | Acid hydrolysis was performed at 50 °C with 65 wt% of H2SO4. | MCC 16 ± 6 nm A. Tequilana 11 ± 4 nm | |
| Corncob | Cellulose 63.5%, xylan 2.7%, lignin 25.8%, ash 2.1%. | 64% H2SO4 | Barley 10 ± 4 nm |
[46][7] | |||||||
| at 45 °C for 1 h | 34.5 | 55.9 | 198 ± 51 | [ | 38 | ][48] | Tomato peels | Tomato peels were placed in oluene/ethanol (2:1, v/v) in a Soxhlet apparatus for 20 h to remove wax, phenolics, pigments, and oils. The bleaching was achieved by 1.4% NaClO at pH 3.5 adjusted with acetic acid kept at 70 °C for 5 h, then treated with 5% KOH solution at room temperature for 24 h and then heated at 90 °C for 2 h. | Acid hydrolysis was performed using H2SO4 (64 wt%) at 45 °C for 30 min. | 3.5 ± 5 nm | [44][5] |
| Corncob | Cellulose 63.5%, xylan 2.7%, lignin 25.8%, ash 2.1%. | 0.5% HCl and 88% CH2O2 at 95 °C for 30 min. | 66.3 | 63.8 | 421 ± 112 | [38][48] | Sugarcane bagasse | The sugarcane bagasse was pre-treated with ethanol/water (1:1 v/v) solution at a solid/liquid ratio of 1:10 at 190 °C for 2 h. The pulped bagasse was then bleached using 24% H2O2 and 4% NaOH at 70–80 °C for 1 h. | Acid hydrolysis was performed at 50 °C in preheated 65 wt% H2SO4 for 40 min. | 6 ± 1 nm | [73] |
| Corncob | Cellulose 63.5%, xylan 2.7%, lignin 25.8%, ash 2.1%. | TEMPO (1 mmol/L) and sodium bromide (10 mmol/L) at pH 10. | [ | 39 | 78.4] | ||||||
| 49.9 | 438 ± 173 | [ | 38 | ] | [ | Groundnut shells | The shells were put under Soxhlet extraction for 8 h using benzene: methanol (2:1 ratio) as solvent. The de-waxed shells were subsequently bleached by treatment at 70 °C for 2 h with 1.5% (w/v) NaClO solution at pH 3–4 adjusted by 5% glacial acetic acid. The sample was then treated with 1 M NaOH solution at 65 °C for 2 h. | Acid hydrolysis process using 65 wt% H2SO4 for 75 min at 45 °C. | 9 nm | [74][40] | |
| 48 | ] | Coffee husk | Alkali treatment was carried out with a 4 wt% NaOH solution for 3 h, the sample was then bleached using equal parts of acetate buffer solution, NaClO (1.7 wt%), and water for 4 h. | Acid hydrolysis treatment was performed using 64%, wt H2SO4 at 50 °C for 40 min |
20 ± 4 nm | [75][41] | |||||
| Pineapple crown waste | Alkaline treatment was performed using 5% NaOH solution at 90 °C for 1 h, the sample was then subjected to bleaching using a mixture of 16% (v/v) H2O2 and 5% NaOH at 55 °C for 90 min. | Acid-catalyzed hydrolysis method using 60 wt% H2SO4 at 45 °C for 1 h. |
39 ± 12 nm | [76][42] | |||||||
| Cucumber peels | The sample was pre-treated with 1 M HCl solution for 1 h at 80–85 °C, followed by alkali treatment using 1 M NaOH for 1 h at 80–85 °C. The sample was bleached using 4% (w/v) NaOCl for 1 h at 90–95 °C. | Acid-catalyzed hydrolysis method using 60 wt% H2SO4 at 45 °C for 1 h. | 32.9 nm | [45][6] | |||||||
| Corncob | The sample was pre-treated with 1 M HCl solution for 1 h at 80–85 °C. Followed by alkali treatment using 1 M NaOH for 1 h at 80–85 °C. The sample was bleached using 4% (w/v) NaOCl for 1 h at 90–95 °C. | Acid-catalyzed hydrolysis method using 60 wt% H2SO4 at 45 °C for 1 h. | - | [77][43] |
3. CNCs from Corncobs
| Source | Components | Isolation Conditions | Yield (%) | CI* (%) | Average Size (nm) | References |
|---|---|---|---|---|---|---|
| Corncob | Cellulose 34.11 ± 1.47%, lignin 15.08 ± 1.32%, hemicellulose 20.17 ± 2.43%, ash 30.06 ± 1.36%, other components 0.58 ± 0.11%. |
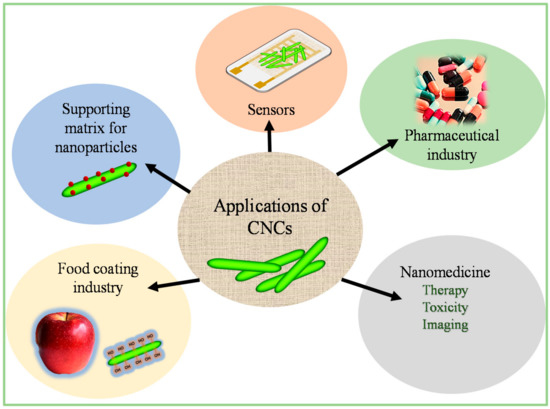
4. Prospective Applications of CNCs
References
- Doh, H.; Lee, M.H.; Whiteside, W.S. Physicochemical characteristics of cellulose nanocrystals isolated from seaweed biomass. Food Hydrocoll. 2019, 102, 105542.
- Xu, J.; Krietemeyer, E.F.; Boddu, V.M.; Liu, S.X.; Liu, W.-C. Production and characterization of cellulose nanofibril (CNF) from agricultural waste corn stover. Carbohydr. Polym. 2018, 192, 202–207.
- Slavutsky, A.M.; Bertuzzi, M.A. Water barrier properties of starch films reinforced with cellulose nanocrystals obtained from sugarcane bagasse. Carbohydr. Polym. 2014, 110, 53–61.
- Dos Santos, R.M.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Dantas, N.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714.
- Jiang, F.; Hsieh, Y.-L. Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydr. Polym. 2015, 122, 60–68.
- Prasanna, N.S.; Mitra, J. Isolation and characterization of cellulose nanocrystals from Cucumis sativus peels. Carbohydr. Polym. 2020, 247, 116706.
- Espino, E.; Cakir, M.; Domenek, S.; Roman-Gutierrez, A.D.; Belgacem, M.N.; Bras, J. Isolation and characterization of cellulose nanocrystals from industrial by-products of Agave tequilana and barley. Ind. Crops Prod. 2014, 62, 552–559.
- Mazlita, Y.; Lee, H.; Hamid, S. Preparation of Cellulose Nanocrystals Bio-Polymer from Agro-Industrial Wastes: Separation and Characterization. Polym. Polym. Compos. 2016, 24, 719–728.
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709.
- Hafemann, E.; Battisti, R.; Bresolin, D.; Marangoni, C.; Machado, R.A.F. Enhancing Chlorine-Free Purification Routes of Rice Husk Biomass Waste to Obtain Cellulose Nanocrystals. Waste Biomass Valorization 2020, 11, 6595–6611.
- Mishra, S.; Kharkar, P.S.; Pethe, A.M. Biomass and waste materials as potential sources of nanocrystalline cellulose: Comparative review of preparation methods. Carbohydr. Polym. 2018, 207, 418–427.
- Huang, S.T.; Liu, X.H.; Chang, C.Y.; Wang, Y.X. Recent developments and prospective food-related applications of cellulose nanocrystals: A review. Cellulose 2020, 27, 2991–3011.
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806.
- Kassab, Z.; Kassem, I.; Hannache, H.; Bouhfid, R.; Qaiss, A.E.K.; El Achaby, M. Tomato plant residue as new renewable source for cellulose production: Extraction of cellulose nanocrystals with different surface functionalities. Cellulose 2020, 27, 4287–4303.
- Wang, Z.; Yao, Z.; Zhou, J.; He, M.; Jiang, Q.; Li, S.; Ma, Y.; Liu, M.; Luo, S. Isolation and characterization of cellulose nanocrystals from pueraria root residue. Int. J. Biol. Macromol. 2018, 129, 1081–1089.
- Silvério, H.A.; Neto, W.P.F.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites. Ind. Crops Prod. 2013, 44, 427–436.
- Pal, N.; Banerjee, S.; Roy, P.; Pal, K. Reduced graphene oxide and PEG-grafted TEMPO-oxidized cellulose nanocrystal reinforced poly-lactic acid nanocomposite film for biomedical application. Mater. Sci. Eng. C 2019, 104, 109956.
- Li, B.; Xu, W.; Kronlund, D.; Määttänen, A.; Liu, J.; Smått, J.-H.; Peltonen, J.; Willför, S.; Mu, X.; Xu, C. Cellulose nanocrystals prepared via formic acid hydrolysis followed by TEMPO-mediated oxidation. Carbohydr. Polym. 2015, 133, 605–612.
- Rohaizu, R.; Wanrosli, W. Sono-assisted TEMPO oxidation of oil palm lignocellulosic biomass for isolation of nanocrystalline cellulose. Ultrason. Sonochem. 2017, 34, 631–639.
- Zhang, K.; Sun, P.; Liu, H.; Shang, S.; Song, J.; Wang, D. Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr. Polym. 2016, 138, 237–243.
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 18, 2333–2342.
- Sharma, P.R.; Zheng, B.; Sharma, S.K.; Zhan, C.; Wang, R.; Bhatia, S.R.; Hsiao, B.S. High Aspect Ratio Carboxycellulose Nanofibers Prepared by Nitro-Oxidation Method and Their Nanopaper Properties. ACS Appl. Nano Mater. 2018, 1, 3969–3980.
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Review: Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog. Polym. Sci. 2018, 86, 122–148.
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180.
- Koshani, R.; van de Ven, T.G.M.; Madadlou, A. Characterization of Carboxylated Cellulose Nanocrytals Isolated through Catalyst-Assisted H2O2 Oxidation in a One-Step Procedure. J. Agric. Food Chem. 2018, 66, 7692–7700.
- Zhan, C.B.; Sharma, P.R.; Geng, L.H.; Sharma, S.K.; Wang, R.F.; Joshi, R.; Hsiao, B.S. Structural characterization of carboxyl cellulose nanofibers extracted from underutilized sources. Sci. China Technol. Sci. 2019, 62, 971–981.
- Song, X.; Zhou, L.; Ding, B.; Cui, X.; Duan, Y.; Zhang, J. Simultaneous improvement of thermal stability and redispersibility of cellulose nanocrystals by using ionic liquids. Carbohydr. Polym. 2018, 186, 252–259.
- Haron, G.A.S.; Mahmood, H.; Noh, M.H.; Alam, Z.; Moniruzzaman, M. Ionic Liquids as a Sustainable Platform for Nanocellulose Processing from Bioresources: Overview and Current Status. ACS Sustain. Chem. Eng. 2021, 9, 1008–1034.
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786.
- Rovera, C.; Ghaani, M.; Santo, N.; Trabattoni, S.; Olsson, R.T.; Romano, D.; Farris, S. Enzymatic Hydrolysis in the Green Production of Bacterial Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2018, 6, 7725–7734.
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Raghav, G.R. A comprehensive review on cellulose nanocrystals and cellulose nanofibers: Pretreatment, preparation, and characterization. Polym. Compos. 2021, 42, 1588–1630.
- Li, J.; Wei, X.; Wang, Q.; Chen, J.; Chang, G.; Kong, L.; Su, J.; Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 2012, 90, 1609–1613.
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25.
- Kawee, N.; Lam, N.T.; Sukyai, P. Homogenous isolation of individualized bacterial nanofibrillated cellulose by high pressure homogenization. Carbohydr. Polym. 2018, 179, 394–401.
- Yang, W.; Feng, Y.; He, H.; Yang, Z. Environmentally-Friendly Extraction of Cellulose Nanofibers from Steam-Explosion Pretreated Sugar Beet Pulp. Materials 2018, 11, 1160.
- Damay, J.; Duret, X.; Ghislain, T.; Lalonde, O.; Lavoie, J.-M. Steam explosion of sweet sorghum stems: Optimisation of the production of sugars by response surface methodology combined with the severity factor. Ind. Crops Prod. 2018, 111, 482–493.
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779.
- Henrique, M.A.; Neto, W.P.F.; Silverio, H.A.; Martins, D.F.; Gurgel, L.V.A.; Barud, H.S.; de Morais, L.C.; Pasquini, D. Kinetic study of the thermal decomposition of cellulose nanocrystals with different polymorphs, cellulose I and II, extracted from different sources and using different types of acids. Ind. Crops Prod. 2015, 76, 128–140.
- Oliveira, F.B.; Bras, J.; Pimenta, M.T.B.; Curvelo, A.A.S.; Belgacem, M.N. Production of cellulose nanocrystals from sugarcane bagasse fibers and pith. Ind. Crops Prod. 2016, 93, 48–57.
- Bano, S.; Negi, Y.S. Studies on cellulose nanocrystals isolated from groundnut shells. Cabohydr. Polym. 2017, 157, 1041–1049.
- Collazo-Bgliardi, S.; Ortega-Toro, R.; Boix, A.C. Isolation and characterisation of microcrystalline cellulose and cellulose nanocrystals from coffee husk and comparative study with rice husk. Cabohydr. Polym. 2017, 191, 205–215.
- Prado, K.S.; Spinace, M.A.S. Isolation and characterization of cellulose nanocrystals from pineapple crown waste and their potential uses. Int. J. Biol. Macromol. 2019, 122, 410–416.
- Magagula, L.P.; Moloto, N.; Gqoba, S.; Kooyman, P.J.; Motaung, T.E.; Linganiso, E.C. Synthesis of fluorescent nitrogen-doped carbon spheres from corncob residue for the detection of Fe (III) in aqueous solutions. 2021 IEEE Sens. 2021, 1–4.
- Saifaddin, G. Maize Production in South Africa by Province in 2019/2020 (Stats SA). 2021. Available online: https://www.statista.com/statistics/1135488/maize-production-in-south-africa-by-province/#:~:text=In%202019%2F2020%2C%20the%20total,Cape%20producing%2034%20thousand%20maize (accessed on 8 July 2021).
- Louis, A.C.F.; Venkatachalam, S. Energy efficient process for valorization of corn cob as a source for nanocrystalline cellulose and hemicellulose production. Int. J. Biol. Macromol. 2020, 163, 260–269.
- Adejumo, A.L.; Azeez, L.; Oyedeji, A.O.; Adetoro, R.O.; Aderibigbe, F.A. Nanostructured and surface functionalized corncob as unique adsorbents for anionic dye remediation. SN Appl. Sci. 2020, 2, 301.
- Harini, K.; Mohan, C.C. Isolation and characterization of micro and nanocrystalline cellulose fibers from the walnut shell, corncob and sugarcane bagasse. Int. J. Biol. Macromol. 2020, 163, 1375–1383.
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016, 151, 716–724.
- Du, H.S.; Liu, W.M.; Zhang, M.L.; Si, C.L.; Zhang, X.Y.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144.
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, M.K. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45.
- Dhar, P.; Bhardwaj, U.; Kumar, A.; Katiyar, V. Poly (3-hydroxybutyrate)/cellulose nanocrystal films for food packaging applications: Barrier and migration studies. Polym. Eng. Sci. 2015, 55, 2388–2395.
- Peng, B.; Tang, J.; Wang, P.; Luo, J.; Xiao, P.; Lin, Y.; Tam, K.C. Rheological properties of cellulose nanocrystal-polymeric systems. Cellulose 2018, 25, 3229–3240.
- Souza, D.R.D.S.; de Mesquita, J.P.; Lago, R.M.; Caminhas, L.D.; Pereira, F.V. Cellulose nanocrystals: A versatile precursor for the preparation of different carbon structures and luminescent carbon dots. Ind. Crops Prod. 2016, 93, 121–128.
