The cells response to injury is initiated by growth factors and cytokines that play a key role in wound restoration, and their biological action is achieved via signal transduction. Growth factors and cytokines play distinct roles through all phases of wound healing. In response to injury, they can trigger several strategic signalling transduction pathways that are mostly activated during embryonic skin development. Extracellular signal-regulated kinases (ERKs) and calcium (Ca2+) are the first intracellular signalling molecules for tissue repair response. These signalling molecules regulate several biological activities including cellular migration, proliferation, contractility, survival and many more related to different transcription factors that are usually induced by several other intracellular signalling pathways. This phenomenon makes it difficult to link a specific signalling response to injury.
- signalling pathways
- chronic wound
- diabetes
1. Introduction
2. Wnt/β-Catenin Pathway in Wound Healing
The designation Wnt was created after the name Wingless-linked integration site [11] and identifies a family of glycolipoproteins that regulates embryonic growth and homeostasis in adults. Depending on the type of Wnt ligand, the related signal is via the canonical or non-canonical Wnt signalling pathway. In the canonical Wnt pathway, a co-activator of transcription, β catenin, is the central facilitator (Wnt/β-catenin signalling). Wnt/β-catenin signalling is one of the critical molecular mechanisms for cell proliferation, polarity, determination of fate and tissue restoration. The Wnt/β-catenin signal transduction pathway is blocked when competitive antagonists bind to their specific receptors. Common antagonists of Wnt/β-catenin signalling include Wnt inhibitory factor-1 (WIF 1) and secreted frizzled-related proteins (SFRPs) [12]. Defects in the Wnt/β-catenin pathway are associated with genetic defects, cancer and vascular diseases [13]. There are 19 Wnt members in humans, which include Wnt-1, Wnt-2, Wnt-2b, Wnt-3, Wnt 3a, Wnt-4, Wnt-5a, Wnt-5b, Wnt-6, Wnt-7a, Wnt 7b, Wnt-8a, Wnt-8b, Wnt-9a, Wnt-9b, Wnt-10a, Wnt-10b, Wnt-11 and Wnt-16 [14]. Signal transduction in the Wnt/β catenin pathway (Figure 1) begins with the attachment of Wnt proteins to the seven-pass frizzled (Fz) transmembrane receptors and the co-receptor lipoprotein receptor-related proteins (LRP). When the Wnt ligand is not present (OFF), a protein complex consisting of axin, casein kinase (CK) 1, adenomatous polyposis coli (APC) and glycogen synthase kinase 3 beta (GSK3β) is formed. GSK3β causes the phosphorylation of β-catenin, tagging it for degradation by proteasomes. The attachment of Wnt to receptor Fz (ON) advances the stimulation of the dishevelled (Dvl) protein that is responsible for deactivating the axin protein complex. This results in the accumulation of cytoplasmic β-catenin, favouring its translocation to the nucleus and the formation of an active transcriptional complex with T cell-specific factor (TCF) and lymphoid enhancer-binding factor 1 (LEF1) for protein transcription [5][15]. Largely, Wnt3a is involved in activating the canonical Wnt/β-catenin pathway, and in vitro, synthetic Wnt3a activates the Wnt/β-catenin pathway for cell proliferation and differentiation [16].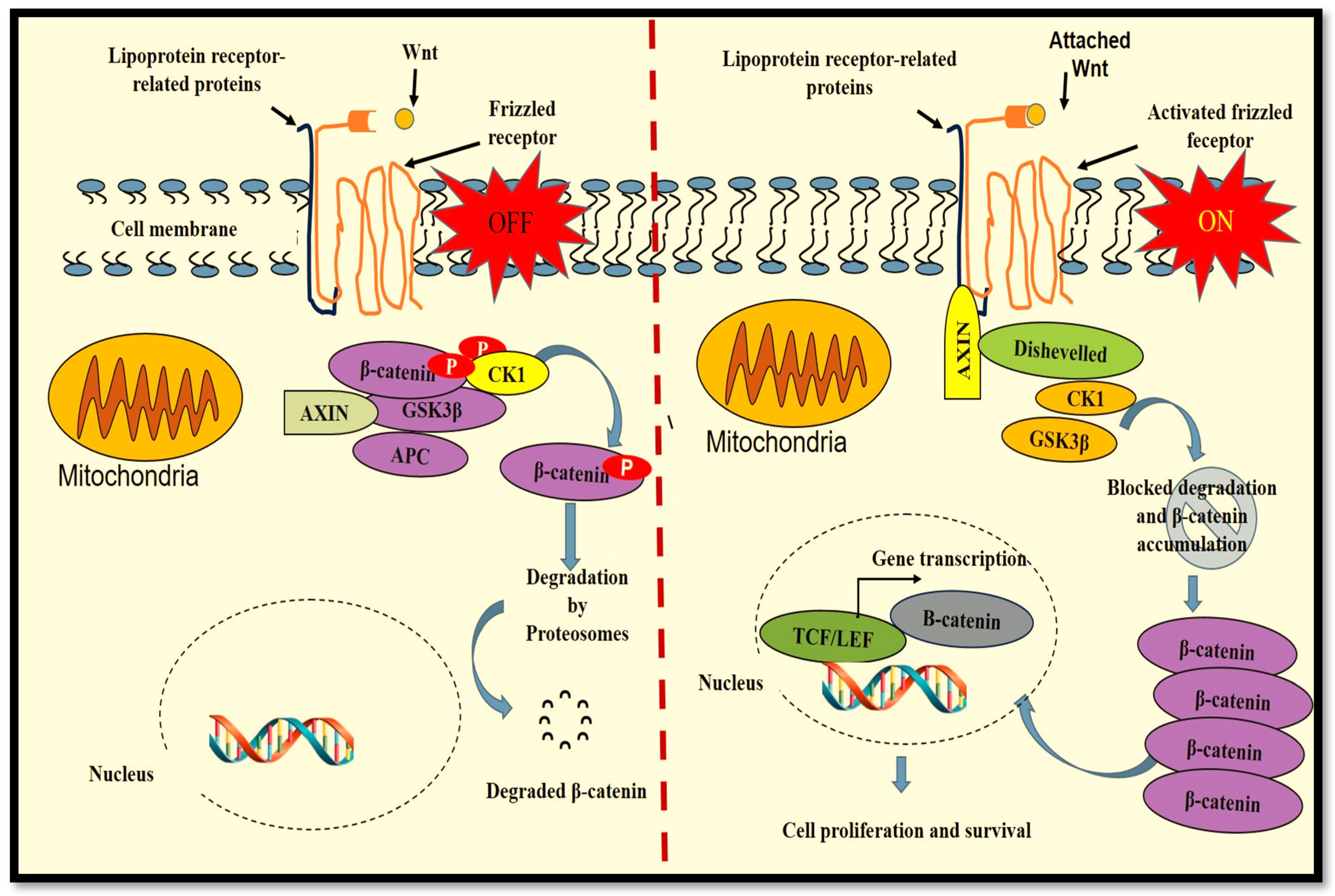
3. Regulation of the Wnt/β-Catenin Pathway in Diabetic Wound Healing
A delay in wound restoration in DM is mainly due to mechanisms related to abnormal inflammation, irregular expression of matrix metalloproteinases (MMPs), reduced cell proliferation, disproportionate cell apoptosis and reduced expression of growth factors and their receptors [3]. High protease levels significantly inhibit dermal reconstruction by reducing ECM components and fibroblast function. Fibroblasts from chronic diabetic wounds are exceedingly senescent, further contributing to reduced ECM deposition [21]. In addition, the reduced healing process in diabetic wounds is worsened by reduced dermal cell neovascularisation, persistent infection and poor cell differentiation within the wound, largely affecting the treatment outcome [16]. The Wnt/β-catenin signalling pathway directly participates in the alteration of various biological processes related to the manifestation and advancement of DM and its complications [12]. During diabetic wound restoration, Wnt/β-catenin signalling stimulates skin thickness and pigmentation, and the literature reports that increased regulation of the Wnt/β-catenin pathway augments the action of high-glucose-suppressed cells [6]. It is suggested that reduced activity of the Wnt/β-catenin pathway is due to decreased R-spondin (RSPO) instigated by DM and is one of the main reasons for the irregularity in diabetic wound healing [10]. The RSPO protein family consist of RSPO 1 to 4 secreted proteins that are enhancers of the Wnt signalling pathway. RSPOs are responsible for the stabilisation of the Wnt receptors and their co-receptors via the inactivation of membrane-bound ubiquitin ligases ZNRF3 (zinc and ring finger 3) and RNF43 (ring finger 43) that antagonize the Wnt pathway by targeting the Wnt receptors for ubiquitylation-mediated disintegration [22]. Adjustment or alteration of the Wnt/β-catenin pathway is known to enhance diabetic wound restoration, and it is suggested that transplanting Wnt signalling-activated cells promotes diabetic wound restoration [16][23]. In diabetic wounds, there is a significant decrease in the activity of GSK3β, caspase 3, NF-κB and β-catenin pathways [24]. GSK3β, a serine/threonine kinase, is ubiquitously expressed as a strategic regulator of various signalling pathways for cellular proliferation and survival and plays a critical role in phosphorylating the Wnt receptors on LRP5/6, in that way causing stabilization of the Wnt/β-catenin pathway [25]. Inhibition of GSK3β is critical in cell proliferation and differentiation during the wound restorative process, and modulation of GSK3β-mediated Wnt/β-catenin pathway advances diabetic wound healing [26].References
- Bielefeld, K.A.; Amini-Nik, S.; Alman, B.A. Cutaneous wound healing: Recruiting developmental pathways for regeneration. Cell. Mol. Life Sci. 2013, 70, 2059–2081.
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265–266.
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223.
- Rosińczuk, J.; Taradaj, J.; Dymarek, R.; Sopel, M. Mechanoregulation of Wound Healing and Skin Homeostasis. BioMed Res. Int. 2016, 2016, 3943481.
- Shi, Y.; Shu, B.; Yang, R.; Xu, Y.; Xing, B.; Liu, J.; Chen, L.; Qi, S.; Liu, X.; Wang, P.; et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res Ther. 2015, 6, 120.
- Zhang, H.; Nie, X.; Shi, X.; Zhao, J.; Chen, Y.; Yao, Q.; Sun, C.; Yang, J. Regulatory Mechanisms of the Wnt/β-Catenin Pathway in Diabetic Cutaneous Ulcers. Front. Pharmacol. 2018, 9, 1114.
- Qing, C. The molecular biology in wound healing & nonhealing wound. Chin. J. Traumatol. 2017, 20, 189–193.
- Lin, B.S.; Chang, C.C.; Su, C.L.; Li, J.R.; Chen, M.L.; Chen, M.Y.; Huang, Y.K. The assessment of Buerger’s exercise on dorsal foot skin circulation in patients with vasculopathic diabetic foot ulcer by using wireless near-infrared spectroscope: A cohort prospective study. Lasers Med. Sci. 2018, 33, 977–982.
- Rigato, M.; Pizzol, D.; Tiago, A.; Putoto, G.; Avogaro, A.; Fadini, G.P. Characteristics, prevalence, and outcomes of diabetic foot ulcers in Africa. A systemic review and meta-analysis. Diabetes Res. Clin. Pract. 2018, 142, 63–73.
- Zhao, Y.; Ming, L.; Wei, Z.; Bin, W.; Yudong, Z.; Haiwen, S.U.; Xiaolan, R.E.N.; Qingzhi, H.A.O. Changes in the expression of Wnt/β-catenin signaling pathway in diabetic ulcers. Chin. J. Pathophysiol. 2015, 17, 2033–2038.
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front. Immunol. 2016, 7, 378.
- Nie, X.; Wei, X.; Ma, H.; Fan, L.; Chen, W.D. The complex role of Wnt ligands in type 2 diabetes mellitus and related complications. J. Cell. Mol. Med. 2021, 25, 6479–6495.
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 1, 9–26.
- Nie, X.; Liu, H.; Liu, L.; Wang, Y.D.; Chen, W.D. Emerging Roles of Wnt Ligands in Human Colorectal Cancer. Front. Oncol. 2020, 10, 1341.
- Tamura, M.; Nemoto, E. Role of the Wnt signaling molecules in the tooth. Jpn. Dent. Sci. Rev. 2016, 52, 75–83.
- Sun, T.J.; Tao, R.; Han, Y.Q.; Xu, G.; Liu, J.; Han, Y.F. Therapeutic potential of umbilical cord mesenchymal stem cells with Wnt/β-catenin signaling pathway pre-activated for the treatment of diabetic wounds. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2460–2464.
- Bastakoty, D.; Young, P.P. Wnt/β-catenin pathway in tissue injury: Roles in pathology and therapeutic opportunities for regeneration. FASEB 2016, 10, 3271–3284.
- Wang, X.; Zhu, Y.; Sun, C.; Wang, T.; Shen, Y.; Cai, W.; Sun, J.; Chi, L.; Wang, H.; Song, N.; et al. Feedback Activation of Basic Fibroblast Growth Factor Signaling via the Wnt/β-Catenin Pathway in Skin Fibroblasts. Front. Pharmacol. 2017, 8, 32.
- Tang, D.; He, Y.; Li, W.; Li, H. Wnt/β-catenin interacts with the FGF pathway to promote proliferation and regenerative cell proliferation in the zebrafish lateral line neuromast. Exp. Mol. Med. 2019, 51, 1–16.
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burn Trauma 2019, 7, 10.
- Lauer, G.; Sollberg, S.; Cole, M.; Flamme, I.; Stürzebecher, J.; Mann, K.; Krieg, T.; Eming, S.A. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J. Investig. Dermatol. 2000, 115, 12–18.
- Zhang, M.; Haughey, M.; Wang, N.Y.; Blease, K.; Kapoun, A.M.; Couto, S.; Belka, I.; Hoey, T.; Groza, M.; Hartke, J.; et al. Targeting the Wnt signaling pathway through R-spondin 3 identifies an anti-fibrosis treatment strategy for multiple organs. PLoS ONE 2020, 3, e0229445.
- Dash, S.N.; Dash, N.R.; Guru, B.; Mohapatra, P.C. Towards reaching the target: Clinical application of mesenchymal stem cells for diabetic foot ulcers. Rejuvenation Res. 2014, 1, 40–53.
- Hoke, G.D.; Ramos, C.; Hoke, N.N.; Crossland, M.C.; Shawler, L.G.; Boykin, J.V. Atypical diabetic foot ulcer keratinocyte protein signaling correlates with impaired wound healing. J. Diabetes Res. 2016, 2016, 1586927.
- Chen, R.F.; Lin, Y.N.; Liu, K.F.; Wang, C.T.; Ramachandran, S.; Wang, C.J.; Kuo, Y. The Acceleration of Diabetic Wound Healing by Low-Intensity Extracorporeal Shockwave Involves in the GSK-3_ Pathway. Biomedicines 2021, 9, 21.
- Lin, C.L.; Wang, J.Y.; Huang, Y.T.; Kuo, Y.H.; Surendran, K.; Wang, F.S. Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J. Am. Soc. Nephrol. 2006, 17, 2812–2820.
