High energy demand from the market due to the rapid increment of the human population worldwide has urged society to explore alternatives to replace non-renewable energy. Renewable diesel produced from biomass could be the next potential energy source for its high stability, long-term storage, and comparable performance with diesel fuels. In producing renewable diesel, the application of catalyst is essential, and the catalyst support is synthesized with the catalyst to enhance the reaction rate and catalytic properties. The application of the supported catalyst in increasing the selectivity and yield of renewable diesel is significant, in which the catalytic properties depend on the interaction between catalyst and catalyst support. The supported catalyst as a favorable substance to assist in enhancing renewable diesel yield could lead to a sustainable and greener future for the biofuel industry in Malaysia.
- catalyst support
- renewable diesel
- recyclability
- stability
- enhancement
1. Introduction
2. Renewable Diesel and Biodiesel Production
Both renewable diesel and biodiesel are categorized as biofuel as they are produced from renewable feedstocks. To differentiate them, renewable diesel is also known as green diesel or hydrotreated vegetable oil. Although the feedstocks of renewable diesel and biodiesel are similar, the production process is varied as biodiesel is mainly produced by transesterification and renewable diesel is mainly produced by hydroprocessing (hydrocracking and hydrotreatment) with the presence of hydrogen. The molecular structure of renewable diesel, biodiesel, and petroleum diesel are varied and shown in Figure 1.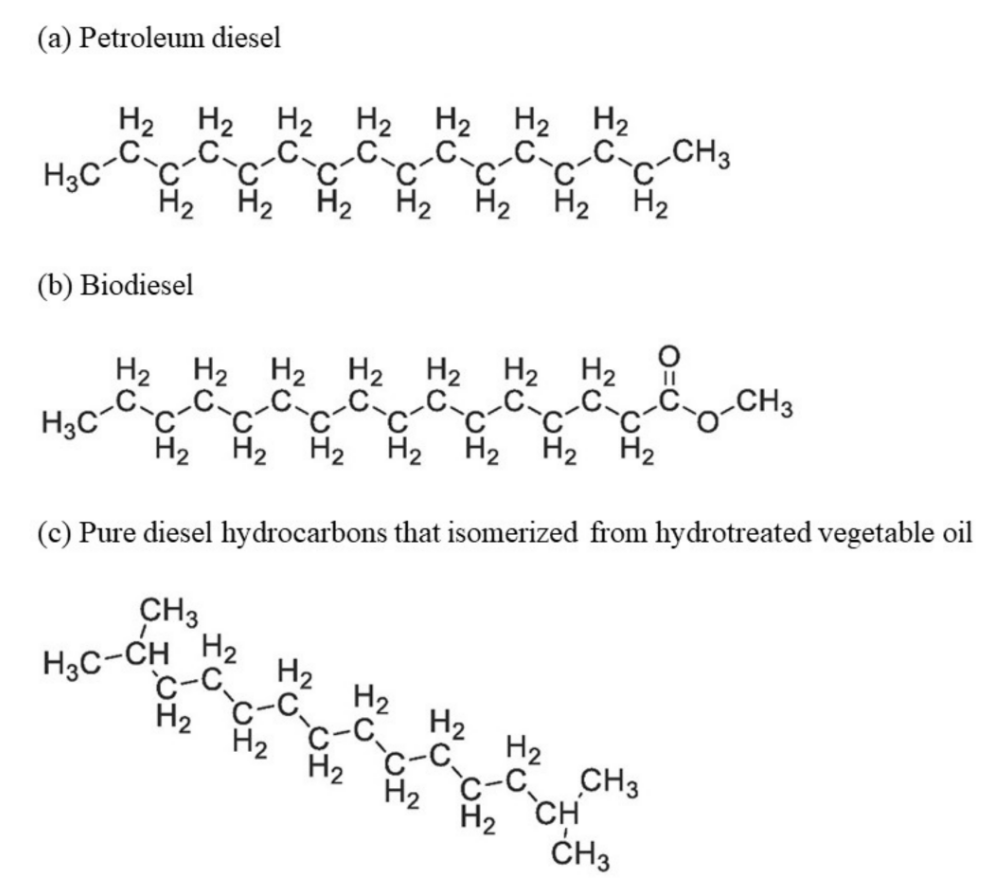
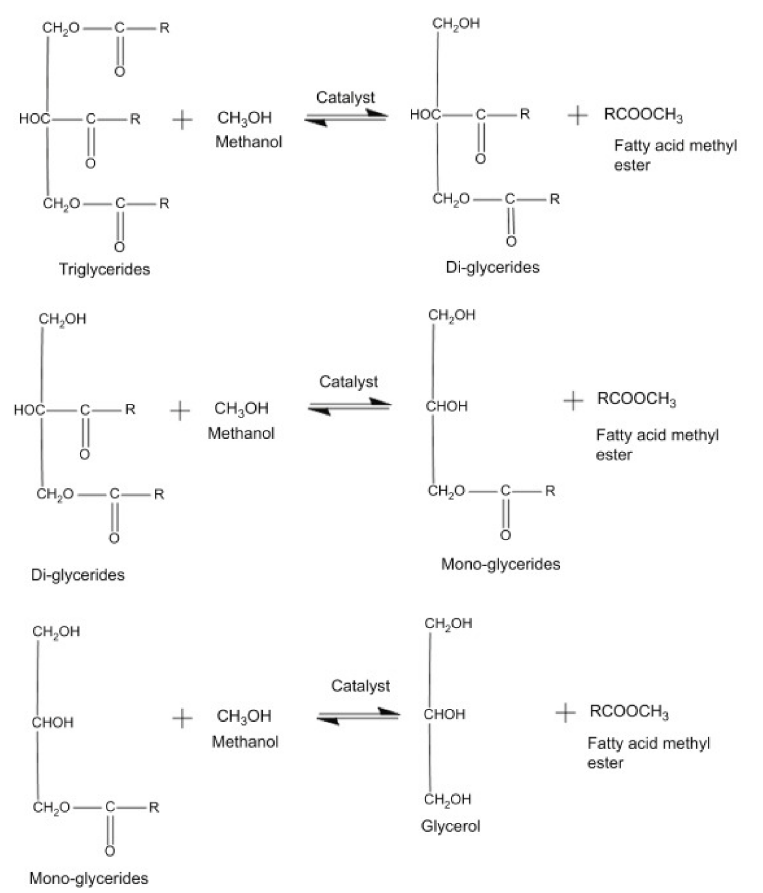 The history of biodiesel begins with the attempts of biofuel engine operation by Rudolph Diesel using peanut oil (1900) and vegetable oil (the 1930s) in running engines with no modifications conducted [1214]. However, the high kinematic viscosity, low volatility, and large molecular mass of vegetable oil imply the impracticality of direct usage in the diesel engine as the performance of the engine is affected [1315]. Hence, transesterification is applied to convert v1 molegetable oil into biodiese of triglyceride into 3 moles of mono-alkyl ester and 1 mole of glycerol with the assistance of 3 moles of alcohol. Transesterification can be conducted with or without the presence of catalysts, where homogeneous catalysts are the traditionally used catalysts for industrial production [1416]. However, the disadvantages of homogeneous catalysts such as difficulty in separation of catalysts and products as well as incapability of reusing catalysts have led to the application of heterogeneous catalysts in biodiesel production. A trimetallic oxide catalyst, SrO-CaO-Al2O3, was studied recently and successfully overcame ther tha issue of active species leaching into the reaction solution while reusing CaO as a catalyst [17]. In theterogeneoat study, the outstanding catalytic stability of the mentioned catalyst was discovered even after the fifth reused cycle and is capable of achieving a fatty acid methyl ester (FAME) yield of around 93%. Other than heterogeneous catalysts, biocatalysts such as immobilized lipase from Bacillus mycoides and Ophiostoma piceae strains, as well as fermented macaúba cake are used to assist in enzymatic transesterification [1518][1619][1720]. Biocatalysts are studied as they are eco-friendly, generate minimal waste while consuming less energy, and function well under mild process conditions [1720]. Other than the type of catalyst, the common alcohol used for biodiesel is methanol and ethanol as short-chain alcohol provides better conversion in the same reaction time [21].
The history of biodiesel begins with the attempts of biofuel engine operation by Rudolph Diesel using peanut oil (1900) and vegetable oil (the 1930s) in running engines with no modifications conducted [1214]. However, the high kinematic viscosity, low volatility, and large molecular mass of vegetable oil imply the impracticality of direct usage in the diesel engine as the performance of the engine is affected [1315]. Hence, transesterification is applied to convert v1 molegetable oil into biodiese of triglyceride into 3 moles of mono-alkyl ester and 1 mole of glycerol with the assistance of 3 moles of alcohol. Transesterification can be conducted with or without the presence of catalysts, where homogeneous catalysts are the traditionally used catalysts for industrial production [1416]. However, the disadvantages of homogeneous catalysts such as difficulty in separation of catalysts and products as well as incapability of reusing catalysts have led to the application of heterogeneous catalysts in biodiesel production. A trimetallic oxide catalyst, SrO-CaO-Al2O3, was studied recently and successfully overcame ther tha issue of active species leaching into the reaction solution while reusing CaO as a catalyst [17]. In theterogeneoat study, the outstanding catalytic stability of the mentioned catalyst was discovered even after the fifth reused cycle and is capable of achieving a fatty acid methyl ester (FAME) yield of around 93%. Other than heterogeneous catalysts, biocatalysts such as immobilized lipase from Bacillus mycoides and Ophiostoma piceae strains, as well as fermented macaúba cake are used to assist in enzymatic transesterification [1518][1619][1720]. Biocatalysts are studied as they are eco-friendly, generate minimal waste while consuming less energy, and function well under mild process conditions [1720]. Other than the type of catalyst, the common alcohol used for biodiesel is methanol and ethanol as short-chain alcohol provides better conversion in the same reaction time [21]. 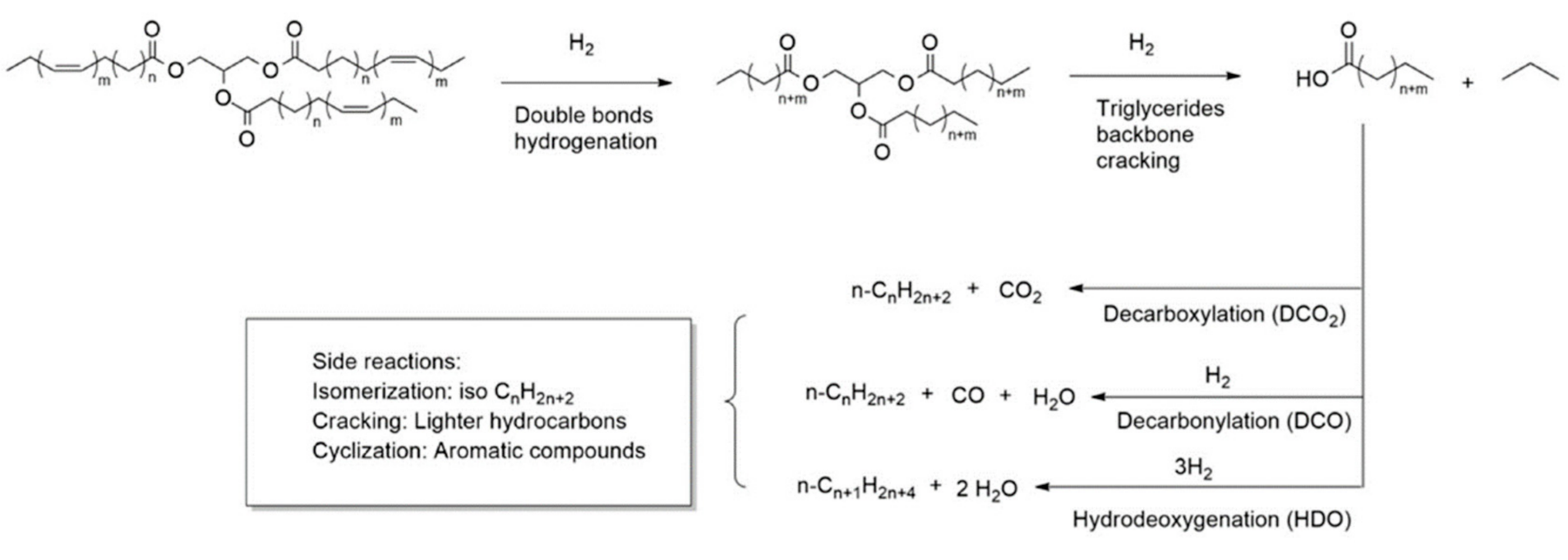
3. Activated Carbon as Catalyst Supports
3.1. Activated Carbon
| Type of support | ||||||
| Type of Support | ||||||
|---|---|---|---|---|---|---|
| Type of catalyst | ||||||
| Elemental Composition | ||||||
| Composition of the active phase | ||||||
| Surface Area | ||||||
| Surface area | ||||||
| Pore Volume | ||||||
| Pore volume | ||||||
| Remarks | ||||||
| Remarks | ||||||
| Reference | ||||||
| Reference | ||||||
| AC | NiP | Ni: 5.14 wt% P: 2.23 wt% |
Micropore: 739 m2/g External: 15 m2/g |
Micro: 0.22 cm3/g Total: 0.25 cm3/g |
Charcoals from Iwasaki kiln | [25] |
| AC | NiP | Ni: 4.66 wt% P: 2.24 wt% |
Micropore: 851 m2/g External: 16 m2/g |
Micro: 0.26 cm3/g Total: 0.31 cm3/g |
Charcoals from tube furnace | [25] |
| AC | Ni2P | - | BET: 612 m2/g | - | Total acidity: 1.3 mmol/g | [31] |
| AC | Ni | O (on the surface): 9.4% | BET: 807.26 cm2/g | Total: 0.185 cm3/g | - | |
| AC | Mo2C | Mo(II): 52% Mo(IV): 8% Mo(VI): 40% |
Total: 417.02 m2/g | Total: 0.22 cm3/g | - | [32] |
| AC | Mo2C | Mo2C (II): 52.17% MoO2 (IV): 8.2% MoO3 (VI): 39.63% |
BET: 322.20 m2/g | Total: 0.202 cm3/g | - | [28] |
| AC | Co-Ag | C: 63.41 wt% O: 13.26 wt% P: 1.45 wt% Co: 9.57 wt% Ag: 12.31 wt% |
BET: 793 m2/g | Total: 1.67 cm3/g | Acidity: 8502.3 µmol/g Total basicity: 6220.2 µmol/g |
[29] |
| AC | CoP | - | BET: 822.9 m2/g | Micro: 68.79% Meso: 31.21% Total 0.43 cm3/g |
Acidity: 52.5 µmol/g | [30] |
| AC | C: 90.03 % H: 0.557% N: 0.367% S: 0.069% O: 8.98% C/H: 161.6 |
Micropore: 775 m2/g External: 15 m2/g |
Micro: 0.23 cm3/g Total: 0.26 cm3/g |
Charcoals from Iwasaki kiln | [37] | |
| AC | C: 80.71 % H: 1.146% N: 1.094% S: 0.078% O: 16.97% C/H: 70.4 |
Micropore: 1202 m2/g External: 20 m2/g |
Micro: 0.39 cm3/g Total: 0.42 cm3/g |
Charcoals from tube furnace | [37] | |
| AC | - | BET: 1484.33 cm2/g | Total: 1.038 cm3/g | Acid sites: 3.96 mmol NH3/g catalysts | [45] | |
| AC | - | BET: 266.1 m2/g | Total: 0.17 cm3/g | Pre-treated with a nitric acid solution | [46] | |
| AC | C: 88.57 wt% O: 8.01 wt% P: 3.42 wt% |
BET: 350 m2/g | Total: 1.88 cm3/g | Total acidity (144 °C): 1055.3 µmol/g Total acidity (852 °C): 2064.7 µmol/g Total basicity (902 °C): 1086.6 µmol/g |
[47] | |
| AC | C: 79.1 w/w% H: 0.9 w/w% N: 0.9 w/w% O: 19.2 w/w% |
BET: 964 m2/g | Micro: 77.92% Meso: 22.08% Total: 0.57 cm3/g |
- | [48] | |
| Type of support | Type of catalyst | Composition of the active phase | Surface area | Pore volume | Remarks | Reference |
| AC | NiP | Ni: 5.14 wt% P: 2.23 wt% |
Micropore: 739 m2/g External: 15 m2/g |
Micro: 0.22 cm3/g Total: 0.25 cm3/g |
Charcoals from Iwasaki kiln | [37] |
| AC | NiP | Ni: 4.66 wt% P: 2.24 wt% |
Micropore: 851 m2/g External: 16 m2/g |
Micro: 0.26 cm3/g Total: 0.31 cm3/g |
Charcoals from tube furnace | [37] |
| AC | Ni2P | - | BET: 612 m2/g | - | Total acidity: 1.3 mmol/g | [49] |
| AC | Ni | O (on the surface): 9.4% | BET: 807.26 cm2/g | Total: 0.185 cm3/g | - | [45] |
| AC | Co-Fe | Co: 8.67 wt% Fe: 3.52 wt% |
Micropore: 459.91 m2/g | Micro: 0.22 cm3/g Total: 0.44 cm3/g |
- | [43] |
| AC | Mo2C | Mo(II): 52% Mo(IV): 8% Mo(VI): 40% |
Total: 417.02 m2/g | Total: 0.22 cm3/g | - | [50] |
| AC | Mo2C | Mo2C (II): 52.17% MoO2 (IV): 8.2% MoO3 (VI): 39.63% |
BET: 322.20 m2/g | Total: 0.202 cm3/g | - | [46] |
| AC | Co-Ag | C: 63.41 wt% O: 13.26 wt% P: 1.45 wt% Co: 9.57 wt% Ag: 12.31 wt% |
BET: 793 m2/g | Total: 1.67 cm3/g | Acidity: 8502.3 µmol/g Total basicity: 6220.2 µmol/g |
[47] |
| AC | CoP | - | BET: 822.9 m2/g | Micro: 68.79% Meso: 31.21% Total 0.43 cm3/g |
Acidity: 52.5 µmol/g | [48] |
3.2. Metal Oxides
4. Recyclability and Stability of Supported Catalysts
4.1. Recyclability
To produce renewable diesel on a commercial scale, the performance of catalysts involved in the reaction is of utmost important. The determination in the recyclability of supported catalysts is often conducted by researchers from the view of economics and sustainability as more cost can be saved and the process is greener if the efficiency of catalysts remains after multiple runs of production. The catalysts utilized for renewable diesel production without support are difficult to recycle, especially for those that are nanosized. Therefore, supported catalysts are more advantageous than unsupported catalysts for their stability and ease of recovery. As the catalysts take up a considerable amount of operating cost, the loss of catalyst has to be minimized and it is favourable to have long lifetime catalysts to reuse and recycle for several runs.Metal oxides are well known as catalyst supports for industrial catalysts, for example, alumina, zirconia and silica
[3351]. They often consist of a metal cation and oxide anion, which form bases through reaction with water and form salts through reaction with acids. The nature of metal oxides varied with the oxidation states, where the metal oxides are more stable and more acidic with higher oxidation states as compared to lower oxidation states. The properties of metal oxides such as stability, strength and chemical nature are influenced by the type of metal/element as shown in the periodic table, with increasing basic nature and solubility of metal oxides observed down the column
[3452]. The metallic elements also dominate their wide range of attractive physicochemical properties, from the aspects of morphological, electronic, textural, structural, and redox
The catalyst support, alumina (Al2O3) also known as the aluminium oxides, is often found compatible with a mixture of catalysts (containing two or more than two types of catalysts). It was reported that the catalyst, 4Pt-8MOx (where M includes Sn, W, Mo and Re) has exhibited improved catalytic activity on hydrodeoxygenation compared to “neat” Pt supported on alumina
[361]. These metal oxides impregnated with Pt have affected the electronic and textural properties of Pt, resulting in hydrodeoxygenation reaction rather than decarboxylation or decarbonylation reaction during deoxygenation. The metal oxides such as MoOx and ReOx have higher weak (100-200 oC) and moderate (200-350 oC) acid sites than “neat” Pt; while the SnOx and WOx have less weak acid sites and higher strong (>350 oC) acid sites than the “neat” Pt. The good compatibility of NiMo with Al
2
3 was proved in another study as high product yield (80 wt%), with relatively stable activity if NiMo is sulphided even after 5 cycles of re-use [37]. Parameters such as reaction pressure and time possess a positive effect on the yield of the product while the negative effect was observed in temperature.
Silicon oxide known as silica (SiO4.2. Stability
The lifespan of supported catalysts not only depends on their reusability; their stability is one of the important characteristics. The interaction between the catalyst and the support defines the stability of supported catalysts, as higher stability of supported catalysts is achieved with stronger interaction. The catalytic stability of supported catalysts can be determined with longer reaction time under optimum processing conditions of other parameters (e.g., pressure or H3.3. Zeolites
3.3.1 Natural Zeolites
The up-gradation of biodiesel to renewable diesel can be performed using zeolites supported catalysts. The study of Fani et al. concluded that Ni catalysts supported on activated natural MOR led to the production of 25 wt% renewable diesel using the highest loading of Ni as catalyst
[4557]. The highest loading of Ni leads to the balanced amount of weak and strong acid sites along with the highest Ni surface area, to produce the highest efficiency for renewable diesel production. Another study reported on the application of Fe/natural zeolite (NZ) showed enhancement in catalytic properties and selectivity towards hydrocarbons with straight-chain alkanes (C15-C18), and the presence of Fe did not change the morphology and crystal structure of zeolites supports [46]. The Fe particles were found to be well-dispersed on the natural zeolite support although the Fe/NZ tends to be agglomerated. It was observed that the impregnation of Fe on NZ led to reduced BET surface area and pore volumes due to the micropore blocking, which was in agreement with the study of Rostamizadeh et al. (2016) [46][47]. The mentioned study has achieved 89% conversion of palm oil into renewable diesel using Fe/natural zeolites as compared to pure natural zeolites as catalysts (58%), showing the maleficent results of natural zeolites as catalyst support.
3.3.2. Synthetic Zeolites
Synthetic zeolites with optimized structure and surface characteristics can be produced via a thermal process by controlling the composition of materials and process temperature during synthesis. The synthesis of synthetic zeolites can be completed using natural raw materials via microwave-assisted synthesis, dialysis, fusion method, ultrasonic method, molten salt method, alkali activation and hydrothermal synthesis
[501]. However, the synthesis of synthetic zeolites from the chemical source of alumina and silica is expensive. Therefore, the raw material namely kaolin can be studied as a precursor of zeolite due to their silica and alumina ratio.
Short synthesizing time, the versatility of catalytic properties in synthetic zeolites and the possibility to generate desired zeolite structures are the advantages of synthetic zeolites over natural zeolites as catalyst support. Ni was found a versatile catalyst working well with synthetic zeolites as catalyst support. The compatibility of Ni with zeolites is found in agreement with the study of Li et al., where 79% to 90% of diesel range alkanes were produced using both H-ZSM-5 and H-MOR as catalyst supportReferences
- Khalit, W.N.A.W.; Askin-Mijan, N.; Marliza, T.S.; Gamal, M.S.; Shamsuddin, M.R.; Saiman, M.I.; Taufiq-Yap, Y.H. Catalytic deoxygenation of waste cooking oil utilizing nickel oxide catalysts over various supports to produce renewable diesel fuel. Biomass Bioenergy 2021, 154, 106248.
- Garraín, D.; Herrera, I.; Lechόn, Y.; Lago, C. Well-to-Tank environmental analysis of a renewable diesel fuel from vegetable oil through co-processing in a hydrotreatment unit. Biomass Bioenergy 2014, 63, 239–249.
- Patel, M.; Oyedun, A.O.; Kumar, A.; Gupta, R. A Techno-Economic Assessment of Renewable Diesel and Gasoline Production from Aspen Hardwood. Waste Biomass Valorizat. 2019, 10, 2745–2760.
- Aatola, H.; Larmi, M.; Sarjovaara, T.; Mikkonen, S. Hydrotreated Vegetable Oil (HVO) as a Renewable Diesel Fuel: Trade-off between NOₓ, Particulate Emission, and Fuel Consumption of a Heavy Duty Engine. SAE Int. J. Engines 2009, 1, 1251–1262.
- Kalnes, T.; Koers, K.P.; Marker, T.; Shonnard, D.R. Green Diesel and Biodiesel: A technoeconomic and Life Cycle Comparison. In Proceedings of the 1st Alternative Fuels Technology Conference, Prague, Czechoslovakia; 2008. Available online: https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/ep.10319 (accessed on 11 October 2021).
- Hill, S.; Shi, E.; Colletti, P.U.S.; Renewable Diesel Capacity Could Increase due to Announced and Developing Projects. Today in Energy. 2021. Available online: https://www.eia.gov/todayinenergy/detail.php?id=48916 (accessed on 13 October 2021).
- Nickel, R.; Kelly, S.; Plume, K. Plume Renewable Diesel Boom Highlights Challenges in Clean-Energy Transition. 2021. Available online: https://www.reuters.com/article/us-global-oil-biofuels-insight-idUSKBN2AV1BS (accessed on 11 October 2021).
- Niemantsverdriet, J.W. Spectroscopy in Catalysis: An Introduction; John Wiley & Sons: Weinheim, Germany, 2007; Printed in Federal Republic of Germany.
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938.
- Sakata, Y.; Tamaura, Y.; Imamura, H.; Watanabe, M. Preparation of a New Type of CaSiO3 with High Surface Area and Property as a Catalyst Support. In Studies in Surface Science and Catalysis; Gaigneaux, E.M., Devillers, M., De Vos, D.E., Hermans, S., Jacobs, P.A., Martens, J.A., Ruiz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 331–338.
- Gerhard, K.; Krahl, J.; Van Gerpen, J. The Biodiesel Handbook; AOCS Press: Urbana, IL, USA, 2010; Available online: https://www.sciencedirect.com/book/9781893997622/the-biodiesel-handbook?via=ihub=#book-description (accessed on 14 October 2021).Chia, S.R.; Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.L. Renewable diesel as fossil fuel substitution in Malaysia: A review. Fuel 2022, 314, 123137.
- Ziolkowska, J.R. Biofuels technologies: An overview of feedstocks, processes, and technologies. In Biofuels for a More Sustainable Future; Ren, J., Scipioni, A., Manzardo, A., Liang, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 1; pp. 1–19. Gerhard, K.; Krahl, J.; Van Gerpen, J. The Biodiesel Handbook; AOCS Press: Urbana, IL, USA, 2010; Available online: https://www.sciencedirect.com/book/9781893997622/the-biodiesel-handbook?via=ihub=#book-description (accessed on 14 October 2021).
- Venkatesan, M.; Vikram, C.J.; Naveenchandran, P. Performance and emission analysis of pongamia oil methyl ester with diesel blend. Middle East J. Sci. Res. 2012, 12, 1758–1765. Thangarasu, V.; Anand, R. Comparative evaluation of corrosion behavior of Aegle Marmelos Correa diesel, biodiesel, and their blends on aluminum and mild steel metals. In Advanced Biofuels; Azad, A.K., Rasul, M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; Chapter 17; pp. 443–471.
- Zahan, K.A.; Kano, M. Biodiesel Production from Palm Oil, Its By-Products and Mill Effluent: A Review. Energies 2018, 11, 2132. Ziolkowska, J.R. Biofuels technologies: An overview of feedstocks, processes, and technologies. In Biofuels for a More Sustainable Future; Ren, J., Scipioni, A., Manzardo, A., Liang, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 1; pp. 1–19.
- Molina-Gutiérrez, M.; Alcaraz, L.; Lόpez, F.A.; Rodríguez-Sánchez, L.; Martínez, M.J.; Prieto, A. Immobilized Forms of the Ophiostoma piceae Lipase for Green Synthesis of Biodiesel. Comparison with Eversa Transform 2.0 and Cal A. J. Fungi 2021, 7, 822. Venkatesan, M.; Vikram, C.J.; Naveenchandran, P. Performance and emission analysis of pongamia oil methyl ester with diesel blend. Middle East J. Sci. Res. 2012, 12, 1758–1765.
- Ávila, S.N.S.; Collaço, A.C.A.; Greco-Duarte, J.; Aguieiras, E.C.G.; de Castro, A.M.; Gutarra, M.L.E.; Cavalcanti, E.D.C.; Freire, D.M.G. Development of a green integrated process for biodiesel esters production: Use of fermented macaúba cake as biocatalyst for macaúba acid oil transesterification. J. Am. Oil Chem. Soc. 2021, 98, 825–835. Zahan, K.A.; Kano, M. Biodiesel Production from Palm Oil, Its By-Products and Mill Effluent: A Review. Energies 2018, 11, 2132.
- Kumar, R.; Pal, P. Lipase immobilized graphene oxide biocatalyst assisted enzymatic transesterification of Pongamia pinnata (Karanja) oil and downstream enrichment of biodiesel by solar-driven direct contact membrane distillation followed by ultrafiltration. Fuel Process. Technol. 2021, 211, 106577. Zhang, Y.; Niu, S.; Han, K.; Li, Y.; Lu, C. Synthesis of the SrO–CaO–Al2O3 trimetallic oxide catalyst for transesterification to produce biodiesel. Renew. Energy 2021, 168, 981–990.
- European Technology and Innovation Platform. Hydrotreatment to HVO. 2021. Available online: https://www.etipbioenergy.eu/value-chains/conversion-technologies/conventional-technologies/hydrotreatment-to-hvo (accessed on 15 October 2021).Molina-Gutiérrez, M.; Alcaraz, L.; Lόpez, F.A.; Rodríguez-Sánchez, L.; Martínez, M.J.; Prieto, A. Immobilized Forms of the Ophiostoma piceae Lipase for Green Synthesis of Biodiesel. Comparison with Eversa Transform 2.0 and Cal A. J. Fungi 2021, 7, 822.
- Papadopoulos, C.; Kordouli, E.; Sygellou, L.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. W promoted Ni-Al2O3 co-precipitated catalysts for green diesel production. Fuel Process. Technol. 2021, 217, 106820. Ávila, S.N.S.; Collaço, A.C.A.; Greco-Duarte, J.; Aguieiras, E.C.G.; de Castro, A.M.; Gutarra, M.L.E.; Cavalcanti, E.D.C.; Freire, D.M.G. Development of a green integrated process for biodiesel esters production: Use of fermented macaúba cake as biocatalyst for macaúba acid oil transesterification. J. Am. Oil Chem. Soc. 2021, 98, 825–835.
- Burimsitthigul, T.; Yoosuk, B.; Ngamcharussrivichai, C.; Prasassarakich, P. Hydrocarbon biofuel from hydrotreating of palm oil over unsupported Ni–Mo sulfide catalysts. Renew. Energy 2021, 163, 1648–1659. Kumar, R.; Pal, P. Lipase immobilized graphene oxide biocatalyst assisted enzymatic transesterification of Pongamia pinnata (Karanja) oil and downstream enrichment of biodiesel by solar-driven direct contact membrane distillation followed by ultrafiltration. Fuel Process. Technol. 2021, 211, 106577.
- Nikolopoulos, I.; Kogkos, G.; Andriopoulou, C.; Kordouli, E.; Dracopoulos, V.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Cobalt–Alumina Coprecipitated Catalysts for Green Diesel Production. Ind. Eng. Chem. Res. 2021, 60, 18672–18683. Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31.
- Wisniowski, H.; Zhang, Y. Using Activated Carbon as a Precious Metal Catalyst Carrier. 2021. Available online: https://www.sigmaaldrich.com/MY/en/technical-documents/technical-article/materials-science-and-engineering/solid-state-synthesis/activated-carbon (accessed on 9 November 2021).European Technology and Innovation Platform. Hydrotreatment to HVO. 2021. Available online: https://www.etipbioenergy.eu/value-chains/conversion-technologies/conventional-technologies/hydrotreatment-to-hvo (accessed on 15 October 2021).
- Chandrasekhar, K. Chapter 3.5—Effective and Nonprecious Cathode Catalysts for Oxygen Reduction Reaction in Microbial Fuel Cells. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 485–501. Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Lee, H.V.; Rashid, U.; Islam, A.; Taufiq-Yap, Y.H. A Review on Thermal Conversion of Plant Oil (Edible and Inedible) into Green Fuel Using Carbon-Based Nanocatalyst. Catalysts 2019, 9, 350.
- Gamal, M.S.; Asikin-Mijan, N.; Khalit, W.N.A.W.; Arumugam, M.; Izham, S.M.; Taufiq-Yap, Y.H. Effective catalytic deoxygenation of palm fatty acid distillate for green diesel production under hydrogen-free atmosphere over bimetallic catalyst CoMo supported on activated carbon. Fuel Process. Technol. 2020, 208, 106519. Papadopoulos, C.; Kordouli, E.; Sygellou, L.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. W promoted Ni-Al2O3 co-precipitated catalysts for green diesel production. Fuel Process. Technol. 2021, 217, 106820.
- Ruangudomsakul, M.; Osakoo, N.; Keawkumay, C.; Kongmanklang, C.; Butburee, T.; Kiatphuengporn, S.; Faungnawakij, K.; Chanlek, N.; Wittayakun, J.; Khemthong, P. Influential properties of activated carbon on dispersion of nickel phosphides and catalytic performance in hydrodeoxygenation of palm oil. Catal. Today 2021, 367, 153–164. Burimsitthigul, T.; Yoosuk, B.; Ngamcharussrivichai, C.; Prasassarakich, P. Hydrocarbon biofuel from hydrotreating of palm oil over unsupported Ni–Mo sulfide catalysts. Renew. Energy 2021, 163, 1648–1659.
- Tapia, J.; Acelas, N.Y.; Lόpez, D.; Moreno, A. NiMo-sulfide supported on activated carbon to produce renewable diesel. Univ. Sci. 2017, 22, 71–85. Nikolopoulos, I.; Kogkos, G.; Andriopoulou, C.; Kordouli, E.; Dracopoulos, V.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Cobalt–Alumina Coprecipitated Catalysts for Green Diesel Production. Ind. Eng. Chem. Res. 2021, 60, 18672–18683.
- Safa Gamal, M.; Asikin-Mijan, N.; Arumugam, M.; Rashid, U.; Taufiq-Yap, Y.H. Solvent-free catalytic deoxygenation of palm fatty acid distillate over cobalt and manganese supported on activated carbon originating from waste coconut shell. J. Anal. Appl. Pyrolysis 2019, 144, 104690. Liu, P.; Chen, C.; Zhou, M.; Xu, J.; Xia, H.; Shang, S.; Jiang, J. Metal–organic framework-derived Ni-based catalyst for the hydrotreatment of triolein into green diesel. Sustain. Energy Fuels 2021, 5, 1809–1820.
- Wang, F.; Jiang, J.; Wang, K.; Zhai, Q.; Sun, H.; Liu, P.; Feng, J.; Xia, H.; Ye, J.; Li, Z.; et al. Activated carbon supported molybdenum and tungsten carbides for hydrotreatment of fatty acids into green diesel. Fuel 2018, 228, 103–111. Lycourghiotis, S.; Kordouli, E.; Kordulis, C.; Bourikas, K. Transformation of residual fatty raw materials into third generation green diesel over a nickel catalyst supported on mineral palygorskite. Renew. Energy 2021, 180, 773–786.
- Safa-Gamal, M.; Asikin-Mijan, N.; Arumugam, M.; Khalit, W.N.A.W.; Nur Azreena, I.; Hafez, F.S.; Taufiq-Yap, Y.H. Catalytic deoxygenation by H2-free single-step conversion of free fatty acid feedstock over a Co-Ag carbon-based catalyst for green diesel production. J. Anal. Appl. Pyrolysis 2021, 160, 105334. Fani, K.; Lycourghiotis, S.; Bourikas, K.; Kordouli, E. Biodiesel Upgrading to Renewable Diesel over Nickel Supported on Natural Mordenite Catalysts. Ind. Eng. Chem. Res. 2021, 60, 18695–18706.
- Kaewtrakulchai, N.; Kaewmeesri, R.; Itthibenchapong, V.; Eiad-Ua, A.; Faungnawakij, K. Palm oil conversion to bio-jet and green diesel fuels over cobalt phosphide on porous carbons derived from palm male flowers. Catalysts 2020, 10, 694. Ameen, M.; Azizan, M.T.; Ramli, A.; Yusup, S.; Alnarabiji, M.S. Catalytic hydrodeoxygenation of rubber seed oil over sonochemically synthesized Ni-Mo/γ-Al2O3 catalyst for green diesel production. Ultrason. Sonochem. 2019, 51, 90–102.
- Pham, L.K.H.; Tran, T.T.V.; Kongparakul, S.; Reubroycharoen, P.; Karnjanakom, S.; Guan, G.; Samart, C. Formation and activity of activated carbon supported Ni2P catalysts for atmospheric deoxygenation of waste cooking oil. Fuel Process. Technol. 2019, 185, 117–125. Wisniowski, H.; Zhang, Y. Using Activated Carbon as a Precious Metal Catalyst Carrier. 2021. Available online: https://www.sigmaaldrich.com/MY/en/technical-documents/technical-article/materials-science-and-engineering/solid-state-synthesis/activated-carbon (accessed on 9 November 2021).
- Wang, F.; Xu, J.; Jiang, J.; Liu, P.; Li, F.; Ye, J.; Zhou, M. Hydrotreatment of vegetable oil for green diesel over activated carbon supported molybdenum carbide catalyst. Fuel 2018, 216, 738–746. Chandrasekhar, K. Chapter 3.5—Effective and Nonprecious Cathode Catalysts for Oxygen Reduction Reaction in Microbial Fuel Cells. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 485–501.
- Sagoff, J. and J. Harmon A catalytic support material takes a leading role. 2018.Nabihah-Fauzi, N.; Asikin-Mijan, N.; Ibrahim, M.L.; Hashim, H.; Yusup, S.; Taufiq-Yap, Y.H.; Mastuli, M.S. Sulfonated SnO 2 nanocatalysts via a self-propagating combustion method for esterification of palm fatty acid distillate. RSC Adv. 2020, 10, 29187–29201.
- Aakash BYJU, Oxides. 2021. p. https://byjus.com/jee/oxide/.Jin, W.; Pastor-Pérez, L.; Villora-Pico, J.J.; Pastor-Blas, M.M.; Sepúlveda-Escribano, A.; Gu, S.; Charisiou, N.D.; Papageridis, K.; Goula, M.A.; Reina, T.R. Catalytic Conversion of Palm Oil to Bio-Hydrogenated Diesel over Novel N-Doped Activated Carbon Supported Pt Nanoparticles. Energies 2020, 13, 132.
- Cortés Corberán, V., V. Rives, and V. Stathopoulos. Chapter 7 - Recent Applications of Nanometal Oxide Catalysts, in Advanced Nanomaterials for Catalysis and Energy in Oxidation Reactions; V.A. Sadykov, Eds.; Elsevier: Amsterdam, 2019; pp. 227-293.Nie, R.; Yang, H.; Zhang, H.; Yu, X.; Lu, X.; Zhou, D.; Xia, Q. Mild-temperature hydrodeoxygenation of vanillin over porous nitrogen-doped carbon black supported nickel nanoparticles. Green Chem. 2017, 19, 3126–3134.
- Sagar Janampelli; Srinivas Darbha; Metal Oxide-Promoted Hydrodeoxygenation Activity of Platinum in Pt-MOx/Al2O3 Catalysts for Green Diesel Production. Energy & Fuels 2018, 32, 12630-12643, 10.1021/acs.energyfuels.8b03588.Gamal, M.S.; Asikin-Mijan, N.; Khalit, W.N.A.W.; Arumugam, M.; Izham, S.M.; Taufiq-Yap, Y.H. Effective catalytic deoxygenation of palm fatty acid distillate for green diesel production under hydrogen-free atmosphere over bimetallic catalyst CoMo supported on activated carbon. Fuel Process. Technol. 2020, 208, 106519.
- Juan Ignacio DEL Rio; Fernando Cardeño; William Perez; Juan D. Peña; Luis A. Rios; Catalytic hydrotreating of jatropha oil into non-isomerized renewable diesel: Effect of catalyst type and process conditions. Chemical Engineering Journal 2018, 352, 232-240, 10.1016/j.cej.2018.07.021.Ruangudomsakul, M.; Osakoo, N.; Keawkumay, C.; Kongmanklang, C.; Butburee, T.; Kiatphuengporn, S.; Faungnawakij, K.; Chanlek, N.; Wittayakun, J.; Khemthong, P. Influential properties of activated carbon on dispersion of nickel phosphides and catalytic performance in hydrodeoxygenation of palm oil. Catal. Today 2021, 367, 153–164.
- Rogelio Sotelo-Boyás; Yanyong Liu; Tomoaki Minowa; Renewable Diesel Production from the Hydrotreating of Rapeseed Oil with Pt/Zeolite and NiMo/Al2O3 Catalysts. Industrial & Engineering Chemistry Research 2010, 50, 2791-2799, 10.1021/ie100824d.Schröder, E.; Thomauske, K.; Weber, C.; Hornung, A.; Tumiatti, V. Experiments on the generation of activated carbon from biomass. J. Anal. Appl. Pyrolysis 2007, 79, 106–111.
- Preeti Shinde; Pradnya Suryawanshi; Kanchan Patil; Vedika Belekar; Sandeep Sankpal; Sagar Delekar; SushilKumar Jadhav; A Brief Overview of Recent Progress in Porous Silica as Catalyst Supports. Journal of Composites Science 2021, 5, 75, 10.3390/jcs5030075.Schrder, E.; Thomauske, K.; Oechsler, B.; Herberger, S. Activated Carbon from Waste Biomass; InTech. Available online: https://www.intechopen.com/chapters/16653 (accessed on 9 November 2021).
- Shir Reen Chia; Saifuddin Nomanbhay; Mei Yin Ong; Kit Wayne Chew; Kuan Shiong Khoo; Hassan Karimi-Maleh; Pau Loke Show; Recent Development of Renewable Diesel Production Using Bimetallic Catalysts. Frontiers in Energy Research 2021, 9, 621, 10.3389/fenrg.2021.769485.Edeh, I.; Overton, T.; Bowra, S. Catalytic hydrothermal deoxygenation of fatty acids over palladium on activated carbon catalyst (Pd/C) for renewable diesel production. Biofuels 2021, 12, 1075–1082.
- Liu, S.; Simonetti, T.; Zheng, W.; Saha, B.; Selective Hydrodeoxygenation of Vegetable Oils and Waste Cooking Oils to Green Diesel Using a Silica-Supported Ir–ReOx Bimetallic Catalyst. ChemSusChem 2018, 11, 1446-1454.Tapia, J.; Acelas, N.Y.; Lόpez, D.; Moreno, A. NiMo-sulfide supported on activated carbon to produce renewable diesel. Univ. Sci. 2017, 22, 71–85.
- Pelemo, Josiah; Omojola, Awogbemi; Freddie, Inambao; Emmanuel, I. Onuh; Development and characterization of coal fly ash reinforced with silica oxide for catalytic green diesel production. International Journal of Mechanical and Production Engineering Research and Development 2021, 11, 405-420.Safa Gamal, M.; Asikin-Mijan, N.; Arumugam, M.; Rashid, U.; Taufiq-Yap, Y.H. Solvent-free catalytic deoxygenation of palm fatty acid distillate over cobalt and manganese supported on activated carbon originating from waste coconut shell. J. Anal. Appl. Pyrolysis 2019, 144, 104690.
- Kristiani, A.; Sudiyarmanto, S.; Aulia, F.; Hidayati, L. N.; Abimanyu, H. (2017). Metal supported on natural zeolite as catalysts for conversion of ethanol to gasoline. In MATEC Web of Conferences (Vol. 101, p. 01001). EDP Sciences.Thangadurai, T.; Tye, C.T. Performance of Activated Carbon Supported Cobalt Oxides and Iron Oxide Catalysts in Catalytic Cracking of Waste Cooking Oil. Period. Polytech. Chem. Eng. 2021, 65, 350–360.
- Wise, W.S.. MINERALS | Zeolites☆ in Reference Module in Earth Systems and Environmental Sciences; N.A., Eds.; Elsevier: Amsterdam, 2013; pp. N.A..Mayorga, M.; Cadavid, J.; Suarez, O.; Vargas, J.; Gonzalez, J.; Narvaez, P. Production of Renewable Diesel by Hydrotreating of Palm Oil with Noble Metallic Catalysts. Chem. Eng. Trans. 2019, 74, 7–12.
- Konstantina Fani; Sotiris Lycourghiotis; Kyriakos Bourikas; Eleana Kordouli; Biodiesel Upgrading to Renewable Diesel over Nickel Supported on Natural Mordenite Catalysts. Industrial & Engineering Chemistry Research 2021, 60, 18695-18706, 10.1021/acs.iecr.1c02560.Hongloi, N.; Prapainainar, P.; Seubsai, A.; Sudsakorn, K.; Prapainainar, C. Nickel catalyst with different supports for green diesel production. Energy 2019, 182, 306–320.
- Riandy Putra; Witri Wahyu Lestari; Fajar Rakhman Wibowo; Bambang Heru Susanto; Fe/Indonesian Natural Zeolite as Hydrodeoxygenation Catalyst in Green Diesel Production from Palm Oil. BULLETIN OF CHEMICAL REACTION ENGINEERING AND CATALYSIS 2018, 13, 245-255, 10.9767/bcrec.13.2.1382.245-255.Wang, F.; Jiang, J.; Wang, K.; Zhai, Q.; Sun, H.; Liu, P.; Feng, J.; Xia, H.; Ye, J.; Li, Z.; et al. Activated carbon supported molybdenum and tungsten carbides for hydrotreatment of fatty acids into green diesel. Fuel 2018, 228, 103–111.
- Mohammad Rostamizadeh; Fereydoon Yaripour; Bifunctional and bimetallic Fe/ZSM-5 nanocatalysts for methanol to olefin reaction. Fuel 2016, 181, 537-546, 10.1016/j.fuel.2016.05.019.Safa-Gamal, M.; Asikin-Mijan, N.; Arumugam, M.; Khalit, W.N.A.W.; Nur Azreena, I.; Hafez, F.S.; Taufiq-Yap, Y.H. Catalytic deoxygenation by H2-free single-step conversion of free fatty acid feedstock over a Co-Ag carbon-based catalyst for green diesel production. J. Anal. Appl. Pyrolysis 2021, 160, 105334.
- Jorge D. Monzón; Maximiliano R. Gonzalez; Lucas E. Mardones; Maria Susana Conconi; Andrea M. Pereyra; Elena I. Basaldella; The role of alkaline activation in the structural transformations of aluminosiliceous industrial wastes towards zeolite production. Materials Today Communications 2019, 21, 100624, 10.1016/j.mtcomm.2019.100624.Kaewtrakulchai, N.; Kaewmeesri, R.; Itthibenchapong, V.; Eiad-Ua, A.; Faungnawakij, K. Palm oil conversion to bio-jet and green diesel fuels over cobalt phosphide on porous carbons derived from palm male flowers. Catalysts 2020, 10, 694.
- Tunde V. Ojumu; Pieter W. Du Plessis; Leslie F. Petrik; Synthesis of zeolite A from coal fly ash using ultrasonic treatment – A replacement for fusion step. Ultrasonics Sonochemistry 2016, 31, 342-349, 10.1016/j.ultsonch.2016.01.016.Pham, L.K.H.; Tran, T.T.V.; Kongparakul, S.; Reubroycharoen, P.; Karnjanakom, S.; Guan, G.; Samart, C. Formation and activity of activated carbon supported Ni2P catalysts for atmospheric deoxygenation of waste cooking oil. Fuel Process. Technol. 2019, 185, 117–125.
- Krisnandi, Y. K.; Parmanti, I. Y.; Yunarti, R. T.; Sihombing, R.; Saragi, I. R. (2018, September). Synthesis and Characterization of Zeolite NaY from kaolin Bangka Belitung with variation of synthesis composition and crystallization time. In Journal of Physics: Conference Series (Vol. 1095, No. 1, p. 012043). IOP Publishing.Wang, F.; Xu, J.; Jiang, J.; Liu, P.; Li, F.; Ye, J.; Zhou, M. Hydrotreatment of vegetable oil for green diesel over activated carbon supported molybdenum carbide catalyst. Fuel 2018, 216, 738–746.
- Li, Shanshan; Li, Ning; Li, Guangyi; Li, Lin; Wang, Aiqin; Cong, Yu; Wang, Xiaodong; Xu, Guoliang; Zhang, Tao; Protonated titanate nanotubes as a highly active catalyst for the synthesis of renewable diesel and jet fuel range alkanes. Applied Catalysis B: Environmental 2015, 170 , 124-134, doi.org/10.1016/j.apcatb.2015.01.022.Malins, K. Synthesis of renewable hydrocarbons from vegetable oil feedstock by hydrotreatment over selective sulfur-free SiO2-Al2O3 supported monometallic Pd, Pt, Ru, Ni, Mo and bimetallic NiMo catalysts. Fuel 2021, 285, 119129.
- I. Nur Azreena; H.L.N. Lau; N. Asikin-Mijan; M.A. Hassan; Saiman Mohd Izham; E. Kennedy; M. Stockenhuber; M.S. Mastuli; Fahad A. Alharthi; Abdulaziz Ali Alghamdi; et al.Y.H. Taufiq-Yap A promoter effect on hydrodeoxygenation reactions of oleic acid by zeolite beta catalysts. Journal of Analytical and Applied Pyrolysis 2021, 155, 105044, 10.1016/j.jaap.2021.105044.Alsultan, G.A.; Asikin-Mijan, N.; Lee, H.V.; Albazzaz, A.S.; Taufiq-Yap, Y.H. Deoxygenation of waste cooking to renewable diesel over walnut shell-derived nanorode activated carbon supported CaO-La2O3 catalyst. Energy Convers. Manag. 2017, 151, 311–323.
- Malins, K. Synthesis of renewable hydrocarbons from vegetable oil feedstock by hydrotreatment over selective sulfur-free SiO2-Al2O3 supported monometallic Pd, Pt, Ru, Ni, Mo and bimetallic NiMo catalysts. Fuel 2021, 285, 119129. Asikin-Mijan, N.; Rosman, N.A.; Abdulkareem-Alsultan, G.; Mastuli, M.S.; Lee, H.V.; Nabihah-Fauzi, N.; Lokman, I.M.; Alharthi, F.A.; Alghamdi, A.A.; Aisyahi, A.A.; et al. Production of renewable diesel from Jatropha curcas oil via pyrolytic-deoxygenation over various multi-wall carbon nanotube-based catalysts. Process Saf. Environ. Prot. 2020, 142, 336–349.
- Alsultan, G.A.; Asikin-Mijan, N.; Lee, H.V.; Albazzaz, A.S.; Taufiq-Yap, Y.H. Deoxygenation of waste cooking to renewable diesel over walnut shell-derived nanorode activated carbon supported CaO-La2O3 catalyst. Energy Convers. Manag. 2017, 151, 311–323. Nur Azreena, I.; Lau, H.L.N.; Asikin-Mijan, N.; Hassan, M.A.; Izham, S.M.; Safa Gamal, M.; Nor Adira Wan Khalit, W.; Arumugam, M.; Kennedy, E.; Stockenhuber, M.; et al. Hydrodeoxygenation of fatty acid over La-modified HZSM5 for premium quality renewable diesel production. J. Anal. Appl. Pyrolysis 2022, 161, 105406.
- Asikin-Mijan, N.; Rosman, N.A.; Abdulkareem-Alsultan, G.; Mastuli, M.S.; Lee, H.V.; Nabihah-Fauzi, N.; Lokman, I.M.; Alharthi, F.A.; Alghamdi, A.A.; Aisyahi, A.A.; et al. Production of renewable diesel from Jatropha curcas oil via pyrolytic-deoxygenation over various multi-wall carbon nanotube-based catalysts. Process Saf. Environ. Prot. 2020, 142, 336–349. Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, S.; Polychronopoulou, K.; Goula, M.A. Effect of operating parameters on the selective catalytic deoxygenation of palm oil to produce renewable diesel over Ni supported on Al2O3, ZrO2 and SiO2 catalysts. Fuel Process. Technol. 2020, 209, 106547.
- Nur Azreena, I.; Lau, H.L.N.; Asikin-Mijan, N.; Hassan, M.A.; Izham, S.M.; Safa Gamal, M.; Nor Adira Wan Khalit, W.; Arumugam, M.; Kennedy, E.; Stockenhuber, M.; et al. Hydrodeoxygenation of fatty acid over La-modified HZSM5 for premium quality renewable diesel production. J. Anal. Appl. Pyrolysis 2022, 161, 105406. Li, G.; Li, N.; Yang, J.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of renewable diesel with the 2-methylfuran, butanal and acetone derived from lignocellulose. Bioresour. Technol. 2013, 134, 66–72.
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, S.; Polychronopoulou, K.; Goula, M.A. Effect of operating parameters on the selective catalytic deoxygenation of palm oil to produce renewable diesel over Ni supported on Al2O3, ZrO2 and SiO2 catalysts. Fuel Process. Technol. 2020, 209, 106547. Pérez, W.; Marín, J.; del Río, J.; Peña, J.; Rios, L. Upgrading of palm oil renewable diesel through hydroisomerization and formulation of an optimal blend. Fuel 2017, 209, 442–448.
- Li, G.; Li, N.; Yang, J.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of renewable diesel with the 2-methylfuran, butanal and acetone derived from lignocellulose. Bioresour. Technol. 2013, 134, 66–72. Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, A.A.; AlKhoori, S.I.; Polychronopoulou, K.; Goula, M.A. Continuous selective deoxygenation of palm oil for renewable diesel production over Ni catalysts supported on Al2O3 and La2O3–Al2O3. RSC Adv. 2021, 11, 8569–8584.
- Pérez, W.; Marín, J.; del Río, J.; Peña, J.; Rios, L. Upgrading of palm oil renewable diesel through hydroisomerization and formulation of an optimal blend. Fuel 2017, 209, 442–448.
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, A.A.; AlKhoori, S.I.; Polychronopoulou, K.; Goula, M.A. Continuous selective deoxygenation of palm oil for renewable diesel production over Ni catalysts supported on Al2O3 and La2O3–Al2O3. RSC Adv. 2021, 11, 8569–8584.
