The continued focus on improving the quality of human life has encouraged the development of increasingly efficient, durable, and cost-effective products in healthcare. Over the last decade, there has been substantial development in the field of technical and interactive textiles that combine expertise in electronics, biology, chemistry, and physics. Most recently, the creation of biosensors capable of quantifying biometric data in biological fluids to detect a specific disease or the physical condition of an individual is being studied. The ultimate goal is to provide access to medical diagnosis anytime and anywhere. Presently, alcohol is considered the most commonly used addictive substance worldwide, being one of the main causes of death in road accidents. Thus, it is important to think of solutions capable of minimizing this public health problem such as the case of alcohol biosensors using sweat as biological fluid of detection, which is reviewed in this study.
- road accidents
- alcohol
- biomarkers
- biosensor
- biomimetic
1. Sweat Composition
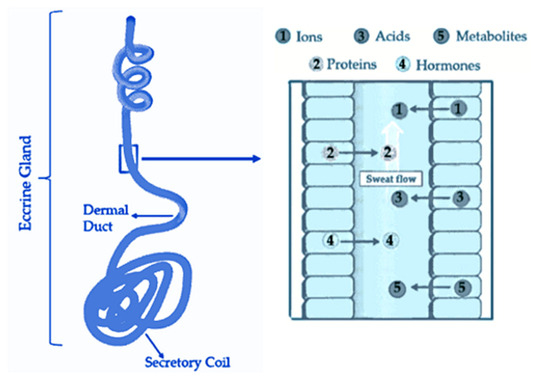
-
Sweat Released Rate: According to a study, the average sweat rate during physical activity is approximately 0.5 µL/min/cm2 with a range of 0.17 to 1.21 µL/min/cm2 [1615]. When considering all areas of the skin, the resting sweat rate should be less than the sweat rate during exercise by 40% [1615]. Thus, here can say that, on average, about 1.2 µL/min/cm2 corresponds to the amount released by an individual at rest. However, it’s important to note that the palm region has a skin with higher density of sweat glands, adding ease of collection [1716]. Furthermore, in addition to liquid phase sweat detection (sensible sweat), there are some devices capable of measuring volatile organic compounds (VOCs) released through skin (insensible sweat), that have shown high correlation with blood alcohol levels [1817][1918].
-
Contamination: Chemicals absorbed by the skin through different cosmetics can be released through sweat and interfere with detection capability. Thus, sweat should be quickly absorbed by the detection platform to avoid contamination from the skin. A way to prevent different components from interfering with the intended analyte reading is, for example, the use of a semipermeable membrane, responsible for allowing only certain substances to pass through it by diffusion [9].
-
Sample Evaporation: it is necessary to have a fast detection in order to obtain reliable results, as evaporation acts quickly on small volumes of exposed sweat, which may change the concentration of its constituents [9].
2. Alcohol in Sweat: Correlation between Sweat and Blood
3. Flexible Biosensors to Detect Alcohol in Sweat
A wearable electrochemical biosensor capable of monitoring alcohol consumption by detecting and quantifying Ethyl Glucuronide (EtG), was developed in 2016 [2928]. To this end, in this study, two coplanar sensors were developed with gold (Au) and zinc oxide (ZnO) integrated into polyimide (PI) by bonding. Up until then, it was possible to detect EtG in sweat using complex techniques such as gas chromatography associated with mass spectrophotometry, however they were deemed and inadequate processes for on-site diagnosis, not allowing real-time feedback from the person [2019][3029]. Thus, this project allowed to monitor alcohol consumption by detecting EtG in sweat through wearable biosensors, reacting with colour change through a LED in the presence of EtG in human sweat. Regarding to the results obtained, the ZnO sensor showed a detection capability in the concentration range of 0.001–100 μg/L up to 4 h. On the other hand, the Au sensor demonstrated an ability to detect EtG in the concentration range of 1–10,000 μg/L up to 9 h [2928]. The authors concluded that the biosensor could detect ingestion of alcohol up to 11 standard drinks in the United States for a period of 4 to 9 h [2928]. In 2019, the chemist Jan Halamék and his team at the University of Albany in New York aimed at developing a new non-invasive method to assess the level of alcohol content in the blood of an individual based on the presence of ethanol in sweat. To do so, 26 volunteers of different ages, genres and eating habits participated [1615]. This detection system uses a polyethylene strip composed of two enzymes, alcohol oxidase, and horseradish peroxidase in order to relate blood ethanol concentrations with sweat ethanol concentrations from a series of biochemical reactions. As soon as the strip meets the skin, the chemical reaction of the two enzymes with the ethanol in sweat produces a colour change, resulting in a blue-green tone, increasing its intensity with the increasing concentration of ethanol in the analysis sample. The study allowed the quantification of ethanol in the human sweat of 26 volunteers as how to prove that as the individual ingests alcoholic drinks, the concentration of ethanol in sweat increases linearly with the concentration of blood alcohol levels [1615]. Some research groups have integrated the pilocarpine iontophoresis process into the biosensors to stimulate sweating and monitor alcohol concentration in induced sweat. This is a transdermal administration of pilocarpine followed by amperometry detection of ethanol. In the study developed by Jan Halámek the same process was only used to obtain the amount of sweat needed for the initial proof of concept corresponding to 8μL, where concentrations were added millimolar ethanol (Mm) of 0; 10.85 and 17.35 corresponding to 0% BAC; 0.05% BAC and 0.08% BAC, respectively [1615]. Kim et al. developed a flexible portable biosensor consisting of a temporary tattoo, which adhered to the skin in order to accurately measure the level of blood alcohol through ethanol concentrations in human sweat [2625]. This biosensor integrates a substance capable of inducing sweat through iontophoresis, pilocarpine, enabling the amperometry detection of ethanol in sweat by the enzyme AOx. Regarding the results, the biosensor exhibited a highly selective and sensitive response to ethanol. The bodily effects in humans have shown significant differences in the current response before and after alcohol consumption, reflecting increased levels of ethanol in sweat after alcohol consumption. The device was considered more effective in relation to the Breathalyzer method, since it avoids possible inaccuracies caused by changes in temperature, humidity, environmental factors such as alcoholic vapors or such as foods/drugs taken orally, resulting in a lower risk of tampering [2827]. A summary of the flexible biosensors discussed above is given in Table 1.2.
| Platform | Target Analyte/Bioreceptor | Measurement Technique | Linear Range | Ref. |
|---|---|---|---|---|
| Electrochemical biosensor: flexible co-planar Au or ZnO integrated in PI from bonding | EtG/EtG antibody | Electrochemical Impedance Spectroscopy (EIS) | 2 × 10−6–2.17 mM | [24] |
| Optical biosensor: polyethylene strip composed of two enzymes | Ethanol/Alcohol Oxidase (AOx) and Horseradish peroxidase (HRP) | Chronoamperometry | 0–54.23 mM | [31] |
| Electrochemical biosensor: hydrogel adhesive with screen printed electrodes | Ethanol/AOx | Chronoamperometry | 3.0–36.0 mM | [32] |

References
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. Teymourian, H.; Moonla, C.; Tehrani, F.; Vargas, E.; Aghavali, R.; Barfidokht, A.; Tangkuaram, T.; Mercier, P.P.; Dassau, E.; Wang, J. Microneedle-Based Detection of Ketone Bodies along with Glucose and Lactate: Toward Real-Time Continuous Interstitial Fluid Monitoring of Diabetic Ketosis and Ketoacidosis. Anal. Chem. 2020, 92, 2291–2300.
- Selvam, A.P.; Muthukumar, S.; Kamakoti, V.; Prasad, S. A wearable biochemical sensor for monitoring alcohol consumption lifestyle through Ethyl glucuronide (EtG) detection in human sweat. Sci. Rep. 2016, 6, 23111. Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal Microfluidic Electrochemical Detection System: Enhanced Sweat Sampling and Metabolite Detection. ACS Sens. 2017, 2, 1860–1868.
- Hair, M.E.; Gerkman, R.; Mathis, A.I.; Halámková, L.; Halámek, J. Noninvasive Concept for Optical Ethanol Sensing on the Skin Surface with Camera-Based Quantification. Anal. Chem. 2019, 91, 15860–15865. Sekar, M.; Pandiaraj, M.; Bhansali, S.; Ponpandian, N.; Viswanathan, C. Carbon fiber based electrochemical sensor for sweat cortisol measurement. Sci. Rep. 2019, 9, 403.
- Christie, G.; Gual, A.; Nicola, M.; Davis-Martin, R.E.; Alessi, S.M.; Boudreaux, E.D. Alcohol Use Disorder in the Age of Technology: A Review of Wearable Biosensors in Alcohol Use Disorder Treatment. Front. Psychiatry 2021, 12, 642813. Rahman, M.M. Selective capturing of phenolic derivative by a binary metal oxide microcubes for its detection. Sci. Rep. 2019, 9, 19234.
- Rahman, M.M.; Karim, M.R.; Alam, M.M.; Zaman, M.B.; Alharthi, N.; Alharbi, H.; Asiri, A.M. Facile and efficient 3-chlorophenol sensor development based on photolumenescent core-shell CdSe/ZnS quantum dots. Sci. Rep. 2020, 10, 557.
- Rahman, M.M.; Khan, S.B.; Gruner, G.; Al-Ghamdi, M.S.; Daous, M.A.; Asiri, A.M. Chloride ion sensors based on low-dimensional α-MnO2–Co3O4 nanoparticles fabricated glassy carbon electrodes by simple I–V technique. Electrochim. Acta 2013, 103, 143–150.
- Sekine, Y.; Kim, S.B.; Zhang, Y.; Bandodkar, A.J.; Xu, S.; Choi, J.; Irie, M.; Ray, T.R.; Kohli, P.; Kozai, N.; et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry. Lab Chip 2018, 18, 2178–2186.
- Sempionatto, J.R.; Martin, A.; García-Carmona, L.; Barfidokht, A.; Kurniawan, J.F.; Moreto, J.R.; Tang, G.; Shin, A.; Liu, X.; Escarpa, A.; et al. Skin-worn Soft Microfluidic Potentiometric Detection System. Electroanalysis 2019, 31, 239–245.
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171.
- Legner, C.; Kalwa, U.; Patel, V.; Chesmore, A.; Pandey, S. Sweat sensing in the smart wearables era: Towards integrative, multifunctional and body-compliant perspiration analysis. Sens. Actuators A Phys. 2019, 296, 200–221.
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 2012, 14, 398–402.
- Sakharov, D.A.; Shkurnikov, M.; Vagin, M.; Yashina, E.I.; Karyakin, A.; Tonevitsky, A. Relationship between Lactate Concentrations in Active Muscle Sweat and Whole Blood. Bull. Exp. Biol. Med. 2010, 150, 83–85.
- Buono, M.J. Sweat Ethanol Concentrations are Highly Correlated with Co-Existing Blood Values in Humans. Exp. Physiol. 1999, 84, 401–404.
- Alvear-Ordenes, I.; García-López, D.; de Paz, J.A.; González-Gallego, J. Sweat lactate, ammonia, and urea in rugby players. Int. J. Sport. Med. 2005, 26, 632–637.
- Hair, M.E.; Gerkman, R.; Mathis, A.I.; Halámková, L.; Halámek, J. Noninvasive Concept for Optical Ethanol Sensing on the Skin Surface with Camera-Based Quantification. Anal. Chem. 2019, 91, 15860–15865.
- Toma, K.; Suzuki, S.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. External ears for non-invasive and stable monitoring of volatile organic compounds in human blood. Sci. Rep. 2021, 11, 10415.
- Høiseth, G.; Morini, L.; Polettini, A.; Christophersen, A.; Mørland, J. Ethyl Glucuronide in Hair Compared with Traditional Alcohol Biomarkers—A Pilot Study of Heavy Drinkers Referred to an Alcohol Detoxification Unit. Alcohol. Clin. Exp. Res. 2009, 33, 812–816.
- Junghanns, K.; Graf, I.; Pflüger, J.; Wetterling, G.; Ziems, C.; Ehrenthal, D.; Zöllner, M.; Dibbelt, L.; Backhaus, J.; Weinmann, W.; et al. Urinary ethyl glucuronide (EtG) and ethyl sulphate (EtS) assessment: Valuable tools to improve verification of abstention in alcohol-dependent patients during in-patient treatment and at follow-ups. Addiction 2009, 104, 921–926.
- Jenkins, A.J. Drug Testing in Alternate Biological Specimens; Humana Press: Totowa, NJ, USA, 2008.
- Webster, G.D.; Gabler, H.C. Feasibility of transdermal ethanol sensing for the detection of intoxicated drivers. Annu. Proc. Assoc. Adv. Automot. Med. 2007, 51, 449–464.
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768.
- Kamei, T.; Tsuda, T.; Mibu, Y.; Kitagawa, S.; Wada, H.; Naitoh, K.; Nakashima, K. Novel instrumentation for determination of ethanol concentrations in human perspiration by gas chromatography and a good interrelationship between ethanol concentrations in sweat and blood. Anal. Chim. Acta 1998, 365, 259–266.
- Nyman, E.; Palmlöv, A. The Elimination of Ethyl Alcohol in Sweat1. Skand. Arch. Physiol. 1936, 74, 155–159.
- Barnett, N.P.; Tidey, J.; Murphy, J.G.; Swift, R.; Colby, S.M. Contingency management for alcohol use reduction: A pilot study using a transdermal alcohol sensor. Drug Alcohol Depend. 2011, 118, 391–399.
- Marques, P. Evaluating TransdErmal Alcohol mEasuring dEvicEs Final Report. 2007. Available online: www.nhtsa.dot.gov (accessed on 13 December 2021).
- (PDF) In Vivo Evaluation of BACtrack® Skyn: A Discrete Wrist-Worn Transdermal Alcohol Monitoring Device Marketed to the Public. Available online: https://www.researchgate.net/publication/356696470_In_vivo_evaluation_of_BACtrackR_Skyn_a_discrete_wrist-worn_transdermal_alcohol_monitoring_device_marketed_to_the_public (accessed on 1 April 2022).
- Christie, G.; Gual, A.; Nicola, M.; Davis-Martin, R.E.; Alessi, S.M.; Boudreaux, E.D. Alcohol Use Disorder in the Age of Technology: A Review of Wearable Biosensors in Alcohol Use Disorder Treatment. Front. Psychiatry 2021, 12, 642813.
- Selvam, A.P.; Muthukumar, S.; Kamakoti, V.; Prasad, S. A wearable biochemical sensor for monitoring alcohol consumption lifestyle through Ethyl glucuronide (EtG) detection in human sweat. Sci. Rep. 2016, 6, 23111.
- Caravati, E.M.; Anderson, K.T. Breath Alcohol Analyzer Mistakes Methanol Poisoning for Alcohol Intoxication. Ann. Emerg. Med. 2010, 55, 198–200.
