Conducting polymDers are an important class of functional materials that has been widely applied to fabricate electrochemical biosensors, because of their interesting and tunable chemical, electrical, and structural properties. scription of Conducting pPolymers can also be designed through chemical grafting of functional groups, nanostructured, or associated with other functional materials such as nanoparticles to provide tremendous improvements in sensitivity, selectivity, stability and reproducibility of the biosensor’s response to a variety of bioanalytes. Such biosensors are expected to play a growing and significant role in delivering the diagnoand their Use to immobilize Biomoleculestic information and therapy monitoring since they have advantages including their low cost and low detection limit.
- Conducting Polymers
- Thin Films
- Biomolecules
- Biosensors
1. Definition of Conducting Polymers
Conducting polymers are an important class of functional materials that has been widely applied to fabricate electrochemical biosensors, because of their interesting and tunable chemical, electrical, and structural properties. Conducting polymers can also be designed through chemical grafting of functional groups, nanostructured, or associated with other functional materials such as nanoparticles to provide tremendous improvements in sensitivity, selectivity, stability and reproducibility of the biosensor’s response to a variety of bioanalytes. Such biosensors are expected to play a growing and significant role in delivering the diagnostic information and therapy monitoring since they have advantages including their low cost and low detection limit.
2. Description of Conducting Polymers
Conducting polymers have attracted much interest since Shirakawa et al. demonstrated in 1977 that halogen doping of polyacetylene strongly increased its conductivity [1] [1]. Thanks to this revolutionary research, Shirakawa, MacDiarmid, and Heeger were awarded the Nobel Prize in Chemistry in 2000, and opened the way to the development of other conducting polymers combining properties of organic polymers and electronic properties of semiconductors. Another major breakthrough in this field was achieved by Diaz et al., who reported the electrodeposition of highly conductive, stable and processable polypyrrole films [2][3][4][2,3,4]. Following these pioneering studies, numerous conducting polymers have been prepared and used in various applications, such as polyacetylene, polypyrrole (PPy), polyaniline (PANI), polycarbazole, polythiophene (PTh), poly(3,4-ethylenedioxythiophene) (PEDOT), polyphenylene, poly(phenylene vinylene), and polyfluorene. All these organic polymers are characterized by alternating single (σ) and double (π) bonds and by the presence of π electrons delocalized across their entire conjugated structure, thus resulting in polymers which can be easily oxidized or reduced [5]. This doping, that can be performed upon oxidation (p-doping) or reduction (n-doping), increases significantly the conductivity of the polymers since this conductivity can vary from less than 10−6 S/cm in the neutral state [5] [5] to more than 105 S/cm in the doped state [6][7][6,7]. The conductivity of the polymers is also dependent on a number of factors including the nature and concentration of the dopant [8][9][10][8,9,10], temperature [11][12][13][11,12,13], swelling/deswelling [14], polymer morphology [8], pH and applied potential [15], and polymer chain length [16]. For most heterocyclic polymers, such as PPy [17] [17] or PTh [18], the mechanism of conduction corresponds to a p-doping and starts with the removal of one electron from the initial monomer leading to the formation of an unstable radical cation (named polaron). Then, a second electron is removed from another monomer or from an oligomer, leading to the formation of a dication (named bipolaron) [19]. Under an applied electric field, these polarons and bipolarons serve as charge carriers which are delocalized over the polymer chains and their movement along polymer chains produces electronic conductivity [20].
Conducting polymers have become an important class of materials since they combine some useful properties of organic polymers (such as strength, plasticity, flexibility, toughness or elasticity) with unusual electronic [5], optical [21][22][21,22] and thermoelectric [23][24] [23,24] properties due to the charge mobility along the π electron polymer chains. These unique properties explain the use of conducting polymers in a wide variety of applications including energy storage with rechargeable batteries [25][26][25,26] and supercapacitors [27][28][27,28], photovoltaics with solar cells [29][30][31][32][29,30,31,32], light-emitting diodes [33][34][33,34], electrocatalysis [35], anti-corrosion [36][37][36,37] or electrochromic applications such as electrochromic displays [38][39][38,39] or rearview mirrors and smart windows [40][41][40,41].
23. Preparation of Conducting Polymers
Although it is possible to prepare conducting polymers using gas phase techniques such as CVD [42] [42] or plasma polymerization [43][44][43,44], conducting polymers are mostly prepared via chemical or electrochemical oxidative polymerization even if it is sometimes possible to use non-oxidative chemical polymerization methods such as Grignard metathesis [45] [45] or dehydrobrominative polycondensation [46]. In traditional chemical oxidative polymerization [47], the synthesis of polymers can be done under harsh oxidative conditions with the use of oxidants such as K2Cr2O7, KMnO4, K2S2O8, KIO3 and FeCl3 [48], or under mild conditions by using, for example, the catalytic action of redox enzymes to produce hydrogen peroxide that initiates the polymerization [49], or less frequently at the liquid/air interface [50]. However, the electrochemical oxidative polymerization is the most frequently used method, mainly because it allows a better control of the polymer deposition [51]. Electrochemical polymerization is carried out with a classical three-electrode set-up in an electrochemical cell containing a monomer, a solvent and a supporting salt. The electropolymerization can be achieved either with a potentiodynamic technique such as cyclic voltammetry where the current response to a linearly cycled potential sweep between two or more set values is measured, with a potentiostatic technique where a constant potential is applied to initiate the polymerization, or with a galvanostatic technique where a constant current is applied to initiate polymerization. The potentiostatic technique allows easy control of the film thickness through Faraday’s law, whereas potentiodynamic techniques lead to more homogeneous and adherent films on the electrode. Additionally, the galvanostatic technique is generally considered as the best approach since it allows to follow the growth of the conducting polymer film by monitoring the potential changes with time which reflects the conductivity.
Conducting polymers have been widely used in the area of bioanalytical and biomedical science [52][53] [52,53], drug delivery [54][55][56][54,55,56], tissue engineering [57][58][59][57,58,59], and cell culture [60][61][62][60,61,62] due to their intrinsic properties and biocompatibility [63][64][65][66][63,64,65,66]. In addition, conducting polymers represent an attractive sensitive material for biosensors due to their electrical properties that allow to convert biochemical information into electrical signals. Additionally, conducting polymers can be easily modified by grafting of functional groups which offers the possibility to enhance their abilities to detect and quantify bioanalytes or to maximize the interactions between the biomolecules and the functionalized polymer. Therefore, after a short description of the electrochemical techniques used in conducting polymer-based biosensors, a series of examples of such biosensors will be described to highlight the recent advances in the field of conducting polymer-based electrochemical biosensors.
34. Immobilization of Biomolecules by Conducting Polymers
Biological sensing element immobilization plays a fundamental role in the performance characteristics of biosensors since biomolecules must be directly attached to the surface of the biosensor to obtain a good sensitivity and a long operational life. The most commonly used methods to immobilize biomolecules to polymers are physical adsorption, covalent attachment and entrapment (Figure 1). The choice of immobilization strategy mainly depends on the type of biological element. Indeed, antibodies and ssDNA are preferentially immobilized by adsorption or covalent binding onto the surface of the conducting polymer films to facilitate the access of the analyte to these biorecognition molecules when entrapment is generally used to immobilize oxidoreductases within the polymer film to facilitate the electron transfer from the enzyme’s redox center to the analyte solution surrounding the conducting polymer and the rapid redox reaction of electroactive species such as hydrogen peroxide generated by enzymatic catalysis.
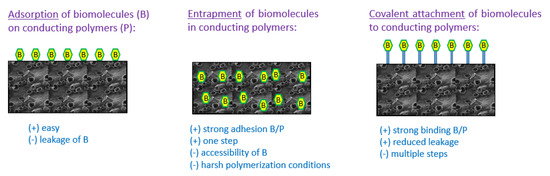
Figure 1. Strategies of immobilization of biomolecules in/on conducting polymers: advantages and drawbacks.
The method of covalent immobilization uses the functional groups of biomolecules (such as –COOH, -NH2, or -SH) for binding with a conducting polymer. Thus, a biomolecule containing amino groups has the capacity to form amide bonds with a conducting polymer bearing carboxylic groups. For example, Kim et al. have developed a glucose biosensor with a conducting electrosynthesized poly(terthiophene benzoic acid) bearing benzoic acid groups which allow the immobilization of glucose oxidase (GOx) through amide bond formation [67]. Similarly, Tuncagil et al. electrosynthesized the conducting polymer 4-(2,5-di(thiophen-2-yl)-1H-pyrrol-1-yl) benzenamine to immobilize GOx through amide bonds [68]. Moreover, covalent attachment of biomolecules is frequently achieved by initial synthesis of functionalized monomers with an amino side group, followed by electrochemical polymerization of these functionalized monomers leading to conducting polymer films with interfacial attachable side groups that can be covalently bound to biomolecules containing the corresponding groups. To facilitate the formation of covalent bonds between biomolecules and polymers, crosslinking agents such as glutaraldehyde [69][70] [69,70] or 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) [71][72] [71,72] are commonly used. The covalent immobilization method has the benefit of providing low diffusional resistance, giving strong binding force between biomolecule and polymer, thus reducing loss of biomolecule. Therefore, these electrodes are more stable in time even if it may be difficult in some cases to retain the biomolecule activity.
The adsorption method is very simple and only consists in the physical adsorption of the biomolecule on the polymer surface. Sometimes, the presence of opposite charges into the conducting polymer and the biomolecule facilitates the immobilization of the biomolecule. Thus, negatively charged glucose oxidase was successfully adsorbed onto positively charged polyaniline-polyisoprene films at pH 4.5 to provide a material sensitive to glucose concentration changes [73]. This method has the benefit of providing small perturbation of the biomolecule native structure and function and so generally leads to very sensitive responses. However, a strong drawback is that direct physical adsorption of biomolecule on a surface generally leads to poor long-term stability of the sensor because of biomolecule leakage from the surface when changes in the environment arise (pH, ionic strength) even if the modification of the surface by a polymer film can slow this leakage [74][75][74,75].
Entrapment is another method widely used for the immobilization of enzymes [76][77][76,77], antibodies [78] [78] or DNA [79]. It involves the preparation of an electrolyte solution containing both monomer and biomolecule, followed by the electropolymerization of the whole solution. Thus, a polymer film containing biomolecules is formed at the electrode surface. Entrapment is an interesting technique since it leads to a strong adhesion between biomolecule and polymer film in a single step. Additionally, this strategy includes the possibility of controlling the amount of entrapped biomolecules simply by controlling the thickness of the electrodeposited polymer film. Entrapment generally leads to biosensors with a good sensitivity and a long lifetime. On the contrary, entrapment can generate problems associated with inaccessibility of the embedded biomolecule. Additionally, some conducting polymers require very acidic conditions or high oxidation potential during the electropolymerization process to be prepared but these conditions are not favorable to biomolecules [80]. It is also important to note that supporting electrolytes are usually used during the electropolymerization process to increase the conductivity of the monomer solution. Besides, the electrolytes tend to compete with the biomolecules for the polymer doping sites, and so reduce the amount of biomolecule entrapped which is a problem especially for costly biomolecules. A solution to this problem is the use of biomolecules as counter-ions during the growth of the conducting polymer film to allow a more efficient entrapment as previously done with polypyrrole and GOx enzyme [81]. To enhance the incorporation of enzymes into polymers during their electropolymerization, it is also possible to use sinusoidal voltages as evidenced by Lupu et al. who developed dopamine biosensors based on tyrosinase entrapped into PEDOT film [82].
