Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Irfana Kabir Ahmad.
Poor waste management has adverse impacts on the environment and human health. The recent years have seen increasing interest in using black soldier fly (BSF), Hermetia illucens, as an organic waste converter. Black soldier fly larvae (BSFL) feed voraciously on various types of organic waste, including food wastes, agro-industrial by-products, and chicken and dairy manure, and reduce the initial weight of the organic waste by about 50% in a shorter period than conventional composting. The main components of the BSFL system are the larvero, where the larvae feed and grow, and the fly house, where the adults BSF live and reproduce.
- bioconversion
- black soldier fly
- composting
1. Organic Waste Treatment via Black Soldier Fly Farming
The unique characteristic of BSFL is that they can consume large amounts of organic wastes for growth until they reach the prepupae stage [33][1]. Compared to conventional composting, BSFL are more effective in reducing 50% of the organic waste in a shorter period. Because of this, researchers have focused on using BSF as a sustainable alternative for treating organic waste. Table 1 lists the different types of organic waste used in BSF waste treatment. Organic waste treatment with BSF is cost-effective and emits less pollution [34][2]. According to Diener et al. [35][3], large-scale facilities use BSFL to produce protein and treat up to 200 tons of waste each day. However, the details of the current design and operating procedures for effective large-scale BSF treatment is commercially sensitive information and not shared [36][4]. This section presents a brief review of the systems and designs of the BSF waste treatment used by previous researchers.
Table 1. BSF waste treatment studies using different types of organic waste.
| Substrate | Subject | Reference |
|---|---|---|
| Food waste | Carbon and nitrogen conversion in food wastes by BSFL. | [37][5] |
| Effects of moisture/water content on larval growth and composting efficiency. | [38,39][6][7] | |
| Fruit and vegetable waste | The potential of fruit and vegetable waste as rearing media for BSFL. | [40][8] |
| Poultry feed | The BSFL nutrition composition changes throughout its life cycle. | [15][9] |
| Human manure | The efficiency of BSFL bioconversion in human fecal waste treatment. | [41][10] |
| The reduction of pathogenic microorganisms in human feces by BSFL. | [42][11] | |
| Animal manure | Use of BSFL in chicken manure management. | [43,44][12][13] |
| Effects of companion bacteria when BSFL converts chicken manure (CHM) into insect biomass. | [15,43][9][12] | |
| Comparison of the suitability of different manures as a feeding substrate for BSFL. | [45,46][14][15] | |
| Bioconversion of dairy manure by BSFL. | [14,47,48][16][17][18] |
2. Black Soldier Fly Organic Waste Processing System
The BSF organic waste processing facility consists of waste pre-processing (e.g., particle size reduction, dewatering, and inorganic waste removal), BSFL biowaste treatment, separation of BSFL from the process residue, and larvae and residue refinement into marketable products [49][19]. Figure 21 shows the flow of a basic BSF treatment process. The system comprises the larvero, which is a place where the larvae grow, while the fly house is where the adults live and reproduce [21][20]. A rearing facility that maintains healthy adult and larval BSF is essential to ensure a sufficient and continuous supply of offspring for organic waste treatment [49][19]. Larvae and frass are the by-products produced in biomass by the end of the BSF treatment process, which can be converted into animal feedstuff and organic fertilizer. Overall, BSFs are particularly compelling, since they offer a natural and cost-effective potential alternative for recycling biological waste.
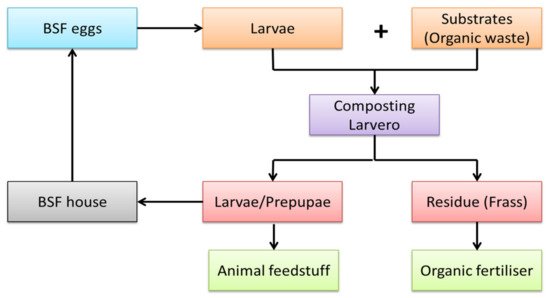
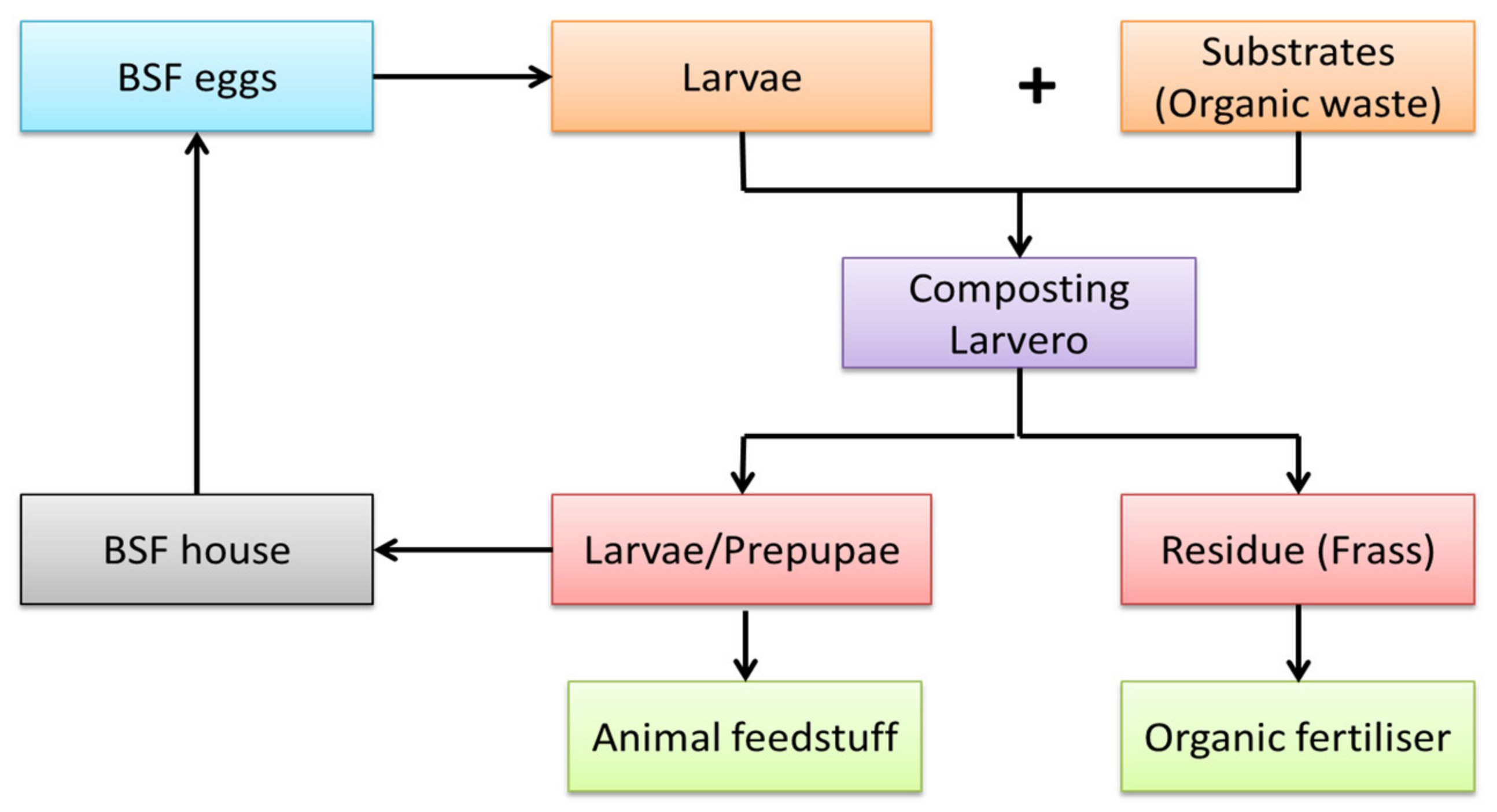
Figure 21. A flow of a basic BSF treatment process.
3. Black Soldier Fly Egg-Trapping
The initial step of BSF treatment is to purchase the BSF eggs from the market or capture them in the wild. The major components in the egg-trapping are baiting and trapping materials. The egg-trap set-up to acquire BSF eggs in the wild consists of a rearing box, the substrate as a food source (any organic material, such as fruits, vegetables, or animal manure), a nylon net, and corrugated cardboard pieces. Booth and Sheppard [50][21] used chicken manure as bait and placed the corrugated cardboard on chicken manure as a trap for the female BSF to lay the eggs in the flute of the corrugated cardboard. The nylon net allows the odor to escape while preventing parasitic and predatory insects from entering and disrupting the system [51][22]. The rearing box containing the substrates is placed in the open to attract female BSFs.
Previous studies showed that female BSFs laid eggs on various organic matters. Sripontan et al. [51][22] investigated the use of several substrates to attract females and found more egg clutches near the fruit waste traps than animal manure. Ewusie et al. [52][23] found that piggery dump waste is a more suitable site to trap BSF eggs than poultry and sheep waste microhabitats. ThIt is study found that, even though female BSFs are attracted to various types of decaying organic waste, they tend to choose those with higher nutrition content for their offspring. In addition to the baiting materials, environmental factors influence the efficiency of BSF egg-trapping. The vegetation growing around the site, for example, is an ideal trapping site because it provides a resting area for the adults and protects them from heat and rain [53][24]. Placing the rearing box at a desirable site will increase the egg-trapping efficiency. The corrugated cardboard where the females deposited their eggs is placed in the larvero system [54][25]. The hatched larvae are reared until they are 4–6 days old (DOL), and are then placed in the BSFL treatment unit. The organic waste for BSFL feeding substrates is pre-treated to optimize BSFL conversion performance [14][16].
4. Pre-Treatment of the Black Soldier Fly System
The pre-treatment of the substrate is critical to improving substrate biodegradability/digestibility, making it easier for the BFSL to digest the substrate. It also promotes the growth of the BFSL. The capacity of BSFL to degrade waste in a specific time can be evaluated by using the waste reduction index (WRI, Equation (1)). Higher WRI values indicate a good reduction efficiency [9][26].
WRI = [(W−R)/W]/t × 100
where W is the total quantity of feeding substrate used during the time t, and R is the residue left at harvesting time t.
Dortmans et al. [49][19] recommended shredding or grinding the substrate after separating the inorganic materials from the hazardous materials because the larvae take longer to break down large chunks of the substrate due to their small mouthparts. The shredding or grinding also increases the surface area of the substrate, which promotes the growth of beneficial microbes and homogenizes the substrates, which improves the consistency of nutrient availability in the mixtures [55][27].
Several studies have investigated the pre-treatment methods for improving substrate biodegradability and nutrient viability [14][16]. Gao et al. [56][28] used shredded maize straw as a substrate; the optimal fermented straw was obtained by mixing Aspergillus oryzae in an inoculation ratio of 4000:1 and fermenting it for 24 h in a 32.3% water content. Wong and Lim [57][29] investigated the self-fermentation of waste coconut endosperm and discovered that the optimum duration to increase the nutrition content in waste coconut endosperm is four weeks. These studies added microorganisms to the substrate to increase the hydrolysis rate of lignocellulosic materials [33][1]. Researchers also recommended pre-treating by mixing the waste with other substrates to obtain a balanced nutrient content that ensures larval growth. For example, researchers have added rice straw with glucose to restaurant waste [58][30] and dairy manure to soybean curd residue [14][16]. Xiao et al. [16][31] found that mixing sewage sludge with chicken manure shortened the larval development time from 39 days to 12–13 days, compared to using only a sewage sludge mixture. This It istudy shows showed that blending two or more substrates could enhance the nutritional value, which ensures larval growth and, thus, helps the BSF complete its life cycle in a shorter time. In summary, pre-treatment is one of the factors determining the effectiveness of a BSF treatment system.
5. Black Soldier Fly Treatment System
A particular concern with BSFL organic waste treatment is its varying reliability and efficiency [59][32]. Researchers used various types of organic waste as a substrate in BSF treatment, including kitchen waste [12][33], poultry waste [60][34], dairy manure [48][18], and human feces [42][11]. Tinder et al. [61][35] contended that the macronutrients in organic wastes, such as protein, carbohydrates, fibers, and lipids, have a considerable influence on the process performance. Protein is an essential nutrient in larval feeding substrates because of its significant positive effect on larval development [62][36]. Nguyen et al. [55][27] and Oonincx et al. [45][14] found that BSFL reared in organic wastes with higher protein contents have higher larval weights, larval proteins, bioconversion rates, feed conversion rates, and shorter development times and lower lipid contents. Jalil et al. [63][37] have proven that larvae reared in protein food sources are larger than those feeding on a carbohydrate food source. Jucker et al. [40][8] found that larvae feeding on substrates with low protein contents have a longer development time, are smaller, and have higher lipid contents if the carbohydrate content is high [40][8]. The different nutrient contents of the organic wastes determine the BSFL’s performance. For example, municipal organic solid wastes have the highest lipid contents; municipal organic solid waste and animal manure have higher protein contents than fruits and vegetables, which have higher carbohydrate contents. Animal manures and fruit and vegetable wastes have a higher median fiber and ash content [59][32]. Therefore, enhancing and balancing the nutritional value of the BSFL feeding substrates by blending several nutrient-rich substrates during the pre-treatment step can improve the process performance and quality of BSFL biomass. The efficiency of BSFL treatment is dependent on the type, quantity, and quality of feed, and also various environmental factors.
Previous studies have determined the optimum range for the operating parameters for process performance. BSFL can live in aggressive environmental conditions, such as drought, oxygen scarcity, and food shortages, to a certain degree. Nonetheless, the ideal temperature range for BSF adults’ mating and oviposition is between 27–37 °C, 40–60% humidity, and 60–70% relative humidity [64][38]. Lalander et al. [38][6] studied the BSF composting process efficiency by providing ventilation and found that 80–90% water content is adequate for BSFL feeding substrate. Cheng et al. [39][7] investigated the trade-off between larval growth rate and residue sieving efficiency. They found that even though a higher moisture content of the food waste results in a higher larval growth rate, it is difficult to separate the residue from the BSFL biomass. They recommended an optimum range of 70–75% for efficient residue sieving because sieving is no longer feasible at a moisture content of more than 80%. The studies on substrate pH have determined the impact of pH on larval development. Ma et al. [18][39] found that both acidic (pH = 2, pH = 4, and pH = 6) and basic (pH = 8 and pH = 10) conditions contribute to high BSFL survival. However, a pH range of 6 to 10 is more conducive for optimal larval growth and higher larval weight relative to a pH range of 2 to 4 because the alkalization of the substrates by larval activity stimulates protease activity, which increases the amount of protein available for larval growth [65][40]. The BSFL bioconversion process increases the pH of the substrate and leaves an alkaline residue, making the substrate suitable for use as fertilizer; the ideal pH range of 7 to 8 stimulates plant growth and provides a favorable environment for nurturing beneficial bacterial populations in the residue [25,66][41][42].
The substrates and neonate larvae (4–6 DOL) were placed in the larvero of the BSFL treatment unit to begin the composting process. The young larvae feed on the substrate and grow while processing and reducing the waste. The number of BSFL added to the substrate is dependent on the amount of waste in a specified volume and surface area [49][19]. Lalander et al. [19][43] have shown that the efficiency of the operation is influenced by larval density, feeding rate, and feeding mechanism. The ideal condition for an experimental setting is a larval density of 1.2 larvae/cm2 and a feeding rate of 163 mg/larva/day (dry base), which yielded up to 1.1 kg/m2/day of larval compost and 59 g/m2/day of larval biomass on a dry basis [67][44]. The BSFL can be fed by a batch (TFS) or daily (DFS) feeding system. Meneguz et al. [13][45] found that TFS larvae have a shorter development time, but DFS larvae have a higher final weight. The thickness of the substrate layer in the larvero is limited to no more than 5 cm. The larvae cannot process the entire layer if the substrate is more than 5 cm thick, and the unprocessed substrate will rot and produce an odor that attracts other filth flies. The composting process in the larvero continues until the larvae grow large enough for harvest after 14–18 days of feeding. The prepupae crawled out on their own and had the advantage of being already separated from the residue. A high portion of the prepupae remained in the substrate, which resulted in an unwanted fly population and a loss of harvest [49][19]. Therefore, it is essential to sieve the substrate to separate the BSFL biomass from residue before refining the marketable products.
6. Post-Treatment of the Black Soldier Fly System
The main by-products of the BSFL organic waste treatment are the larvae and frass that need to be separated to obtain valuable products, such as animal feedstock and fertilizer. BSFL accumulates adequate nutrients, such as proteins and lipids, during the larval stage, since this is the only feeding duration in the BSF lifecycle. The larvae then undergo pupation and emerge as adult flies, leaving a residue called frass. The main advantage of the BSF system is that the larvae can consume a variety of organic materials for growth, including decomposable by-products and wastes, until they reach the prepupae stage [33][1]. Diener et al. [27][46] recommended adding a food supply to the larvero daily, providing a drainage system to drain the excess leachate, and a ramp for the prepupae to crawl out from the larvero, which also ensures the easy separation of the pupae from the frass [27][46].
The sieving is conducted in the final stage to refine the residue quality since many prepupae are still present in the material [49][19]. In the conventional approach of BSFL bioconversion, the organic waste is fed directly to the larvae without any moisture adjustment [27][46]. This method is straightforward and saves time; however, it is difficult to separate the residue from BSFL biomass when the residue is excessively wet with a moisture content of 82–86%, making it too viscous for sieving [30][47]. Proper moisture control can overcome this problem. Cheng et al. [39][7] conducted an experiment to determine the effect of food waste moisture contents of 70%, 75%, and 80% on residue separation. The ideal moisture contents for sieving food waste are 70 and 75%. Residue with 80% initial moisture content is not suitable for sieving because the adhesive property of the water molecules promotes particle aggregation, which increases the particle size. Dortmans et al. [49][19] suggested harvesting, which is the process of separating the larvae from the residue using a manual or automated shaking sieve after 12 days of BSFL waste treatment, when the larvae have reached their maximum weight and nutritional value but have not developed into prepupae. The larvae or prepupae can be processed to produce animal feed, and the residue is refined to produce organic fertilizer.
References
- Singh, A.; Kumari, K. An inclusive approach for organic waste treatment and valorisation using Black Soldier Fly larvae: A review. J. Environ. Manag. 2019, 251, 109–569.
- Raksasat, R.; Lim, J.W.; Kiatkittipong, W.; Kiatkittipong, K.; Ho, Y.C.; Lam, M.K.; Font-Palma, C.; Zaid, H.F.M.; Cheng, C.K. A review of organic waste enrichment for inducing palatability of black soldier fly larvae: Wastes to valuable resources. Environ. Pollut. 2020, 267, 1–17.
- Wang, Y.S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91.
- Diener, S.; Zurbrügg, C.; Tockner, K. Bioaccumulation of heavy metals in the black soldier fly, Hermetia illucens and effects on its life cycle. J. Insects Food Feed. 2015, 1, 261–270.
- Lohri, C.R.; Diener, S.; Zabaleta, I.; Mertenat, A.; Zurbrügg, C. Treatment technologies for urban solid biowaste to create value products: A review with focus on low- and middle-income settings. Rev. Environ. Sci. Biotechnol. 2017, 16, 81–130.
- Pang, W.; Hou, D.; Chen, J.; Nowar, E.E.; Li, Z.; Hu, R.; Tomberlin, J.K.; Yu, Z.; Li, Q.; Wang, S. Reducing greenhouse gas emissions and enhancing carbon and nitrogen conversion in food wastes by the black soldier fly. J. Environ. Manag. 2020, 260, 1–8.
- Lalander, C.; Ermolaev, E.; Wiklicky, V.; Vinnerås, B. Process efficiency and ventilation requirement in black soldier fly larvae composting of substrates with high water content. Sci. Total Environ. 2020, 729, 138–968.
- Cheng, J.Y.K.; Chiu, S.L.H.; Lo, I.M.C. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323.
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, 1–21.
- Jucker, C.; Erba, D.; Leonardi, M.G.; Lupi, D.; Savoldelli, S. Assessment of Vegetable and Fruit Substrates as Potential Rearing Media for Hermetia illucens (Diptera: Stratiomyidae) Larvae. Environ. Entomol. 2017, 46, 1415–1423.
- Banks, I.J.; Gibson, W.T.; Cameron, M.M. Growth rates of black soldier fly larvae fed on fresh human faeces and their implication for improving sanitation. Trop. Med. Int. Health 2014, 19, 14–22.
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbrügg, C.; Lindström, A.; Vinnerås, B. Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—From a hygiene aspect. Sci. Total Environ. 2013, 458–469, 312–318.
- Bortolini, S.; Macavei, L.I.; Saadoun, J.H.; Foca, G.; Ulrici, A.; Bernini, F.; Malferrari, D.; Setti, L.; Ronga, D.; Maistrello, L. Hermetia illucens (L.) larvae as chicken manure management tool for circular economy. J. Clean. Prod. 2020, 262, 1–10.
- Mazza, L.; Ur Rehman, K.; Salvatore, F.; Tomberlin, J.K. Management of chicken manure using black soldier fly (Diptera: Stratiomyidae) larvae assisted by companion bacteria. Waste Manag. 2020, 102, 312–318.
- Oonincx, D.G.A.B.; Van Huis, A.; Van Loon, J.J.A. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects Food Feed. 2015, 1, 131–139.
- Ur Rehman, K.; Rehman, A.; Cai, M.; Zheng, L.; Xiao, X.; Somroo, A.A.; Wang, H.; Li, W.; Yu, Z.; Zhang, J. Conversion of mixtures of dairy manure and soybean curd residue by black soldier fly larvae (Hermetia illucens L.). J. Clean. Prod. 2017, 154, 366–373.
- Miranda, C.D.; Cammack, J.A.; Tomberlin, K. Life-History Traits of the Black Soldier Fly, Hermetia illucens (L.) (Diptera: Stratiomyidae). Animals 2019, 9, 281.
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag. 2011, 31, 1316–1320.
- Myers, H.M.; Tomberlin, J.K.; Lambert, B.D.; Kattes, D. Development of black soldier fly (Diptera: Stratiomyidae) larvae fed dairy manure. Environ. Entomol. 2008, 37, 11–15.
- Da Silva, G.D.P.; Hesselberg, T. A Review of the Use of Black Soldier Fly Larvae, Hermetia illucens (Diptera: Stratiomyidae), to Compost Organic Waste in Tropical Regions. Neotrop. Entomol. 2020, 49, 151–162.
- Dortmans, B.M.A.; Diener, S.; Verstappen, B.M.; Zurbrügg, C. Black Soldier Fly Biowaste Processing—A Step-by-Step Guide Eawag; Swiss Federal Institute of Aquatic Science and Technology: Dübendorf, Switzerland, 2017; pp. 6–54.
- Booth, D.C.; Sheppard, C. Oviposition of the Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae): Eggs, Masses, Timing, and Site Characteristics. Environ. Entomol. 1984, 14, 421–423.
- Sripontan, Y.; Juntavimon, T.; Songin, S.; Chiu, C.I. Egg-trapping of black soldier fly, Hermetia illucens (L.) (Diptera: Stratiomyidae) with various wastes and the effects of environmental factors on egg-laying. Khon Kaen Agric. J. 2017, 45, 179–184.
- Ewusiea, E.A.; Kwapong, P.K.; Ofosu-Buduc, G.; Sandrock, C.; Akumahc, A.M.; Narteyc, E.K.; Tetegaga, C.; Agyakwah, S.K. The black soldier fly, Hermetia illucens (Diptera: Stratiomyidae): Trapping and culturing of wild colonies in Ghana. Sci. Afr. 2019, 5, e00134.
- Caruso, D.; Devic, E.; Subamia, I.W.; Talamond, P.; Baras, E. Technical Handbook of Domestication and Production of Diptera Black Soldier Fly (BSF) Hermetia illucens, Stratiomyidae; PT Penerbit IPB Press: Bogor, Indonesia, 2013; p. 141.
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610.
- Boaru, A.; Vig, A.; Ladoşi, D.; Păpuc, T.; Struţi, D.; Georgescu, B. The use of various oviposition structures for the black soldier fly, Hermetia illucens L. (Diptera: Stratiomydae) in improving the reproductive process in captivity. Adv. Agric. Bot. 2019, 11, 12–20.
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Influence of resources on Hermetia illucens. (Diptera: Stratiomyidae) larval development. J. Med. Entomol. 2013, 50, 898–906.
- Gao, Z.; Wanga, W.; Lu, X.; Zhu, F.; Liu, W.; Wang, X.; Lei, C. Bioconversion performance and life table of black soldier fly (Hermetia illucens) on fermented maize straw. J. Clean. Prod. 2019, 230, 974–980.
- Wong, C.; Lim, J. Optimization of self-fermented period of waste coconut endosperm destined to feed black soldier fly larvae in enhancing the lipid and protein yields. Renew. Energy 2017, 111, 646–654.
- Xiao, X.; Mazza, L.; Yu, Y.; Cai, M.; Zheng, L.; Tomberlin, J.K.; Yu, J.; Van Huis, A.; Yu, Z.; Fasulo, S.; et al. Ef fi cient co-conversion process of chicken manure into protein feed and organic fertilizer by Hermetia illucens L. (Diptera: Stratiomyidae) larvae and functional bacteria. J. Environ. Manag. 2018, 217, 668–676.
- Li, W.; Li, M.; Zheng, L.; Liu, Y.; Zhang, Y.; Yu, Z.; Ma, Z.; Li, Q. Simultaneous utilization of glucose and xylose for lipid accumulation in black soldier fly. Biotechnol. Biofuels 2015, 8, 4–9.
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of Black Soldier Fly (Diptera: Stratiomyidae) Larvae to Recycle Food Waste. Environ. Entomol. 2015, 44, 406–410.
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrügg, C.; Mathys, A. Decomposition of biowaste macronutrients, microbes, and chemicals in black soldier fly larval treatment: A review. Waste Manag. 2018, 82, 302–318.
- Zhou, Z.; Karlsen, Ø.; He, S.; Olsen, R.E.; Yao, B.; Ringø, E. The effect of dietary chitin on the autochthonous gut bacteria of Atlantic cod (Gadus morhua L.). Aquac. Res. 2013, 44, 1889–1900.
- Tinder, A.C.; Puckett, R.T.; Turner, N.D.; Cammack, J.A.; Tomberlin, J.K. Bioconversion of sorghum and cowpea by black soldier fly (Hermetia illucens (L.)) larvae for alternative protein production. J. Insects Food Feed 2017, 3, 121–130.
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag. 2020, 102, 319–329.
- Jalil, N.A.A.; Abdullah, S.H.; Ahmad, I.K.; Basri, N.E.A.; Mohamed, Z.S. Decomposition of food waste from protein and carbohydrate sources by black soldier fly larvae, Hermetia illucens L. J. Environ. Biol. Environ. Biol. 2021, 42, 756–761.
- Ma, J.; Lei, Y.; ur Rehman, K.; Yu, Z.; Zhang, J.; Li, W.; Li, Q.; Tomberlin, J.K.; Zheng, L. Dynamic Effects of Initial pH of Substrate on Biological Growth and Metamorphosis of Black Soldier Fly (Diptera: Stratiomyidae). Environ. Entomol. 2018, 47, 159–165.
- Meneguz, M.; Gasco, L.; Tomberlin, J.K. Impact of pH and feeding system on black soldier fly (Hermetia illucens, L.; Diptera: Stratiomyidae) larval development. PLoS ONE 2018, 13, 1–15.
- Surendra, K.C.; Tomberlin, J.K.; van Huis, A.; Cammack, J.A.; Heckmann, L.H.L.; Khanal, S.K. Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Manag. 2020, 117, 58–80.
- Choi, S.; Hassanzadeh, N. BSFL Frass: A Novel Biofertilizer for Improving Plant Health While Minimizing Environmental Impact. Candian Sci. Fair J. 2019, 2, 41–46.
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219.
- Parra Paz, A.S.; Carrejo, N.S.; Gómez Rodríguez, C.H. Effects of Larval Density and Feeding Rates on the Bioconversion of Vegetable Waste Using Black Soldier Fly Larvae Hermetia illucens (L.), (Diptera: Stratiomyidae). Waste Biomass Valoriz. 2015, 6, 1059–1065.
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gascoac, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 9, 5776–5784.
- Diener, S.; Studt Solano, N.M.; Roa Gutiérrez, F.; Zurbrügg, C.; Tockner, K. Biological treatment of municipal organic waste using black soldier fly larvae. Waste Biomass Valorizat. 2011, 2, 357–363.
- Diener, S.; Zurbrugg, C.; Gutierrez, F.R.; Nguyen, D.H.; Morel, A.; Koottatep, T.; Tockner, K. Black soldier fly larvae for organic waste treatment—Prospects and constraints. In Proceedings of the WaterSafe 2011 2nd WasteSafe 2011, 2nd International Conference on Solid Waste Management in Developing Countries, Khulna, Bangladesh, 13–15 February 2011; pp. 978–984.
More
