During metastasis, cancer cells detach from the primary tumor and intrude apart into tissues in the bloodstream. To detect and isolate CTCs (circulating tumor cells), various techniques including centrifugation, magnetic separation, microchips, filtration, micro/nano substrates and biomarkers have been used. With the widespread adoption of microfluidic techniques, a large number of researchers have worked hard to develop more efficient and reliable CTC separation technologies ranging from immunomagnetic beads to size-based microfluidic devices. Currently, the major commercialised products for CTC separation techniques include the CellSearch system, which uses immunomagnetic beads, and the CelarCellFX1 system, which uses size-dependent isolation. The methods for isolating CTCs are mostly based on biological qualities of the tumor cells, such as specific antigen expression and receptor, or physical properties of the tumor cells, such as size and deformability. Inertial focusing, acoustics, microfluidic filters, optics and dielectrophoresis are some of the size and deformability-based approaches.
- circulating tumor cells (CTCs)
- microfluidic device
- Isolation
1. Introduction
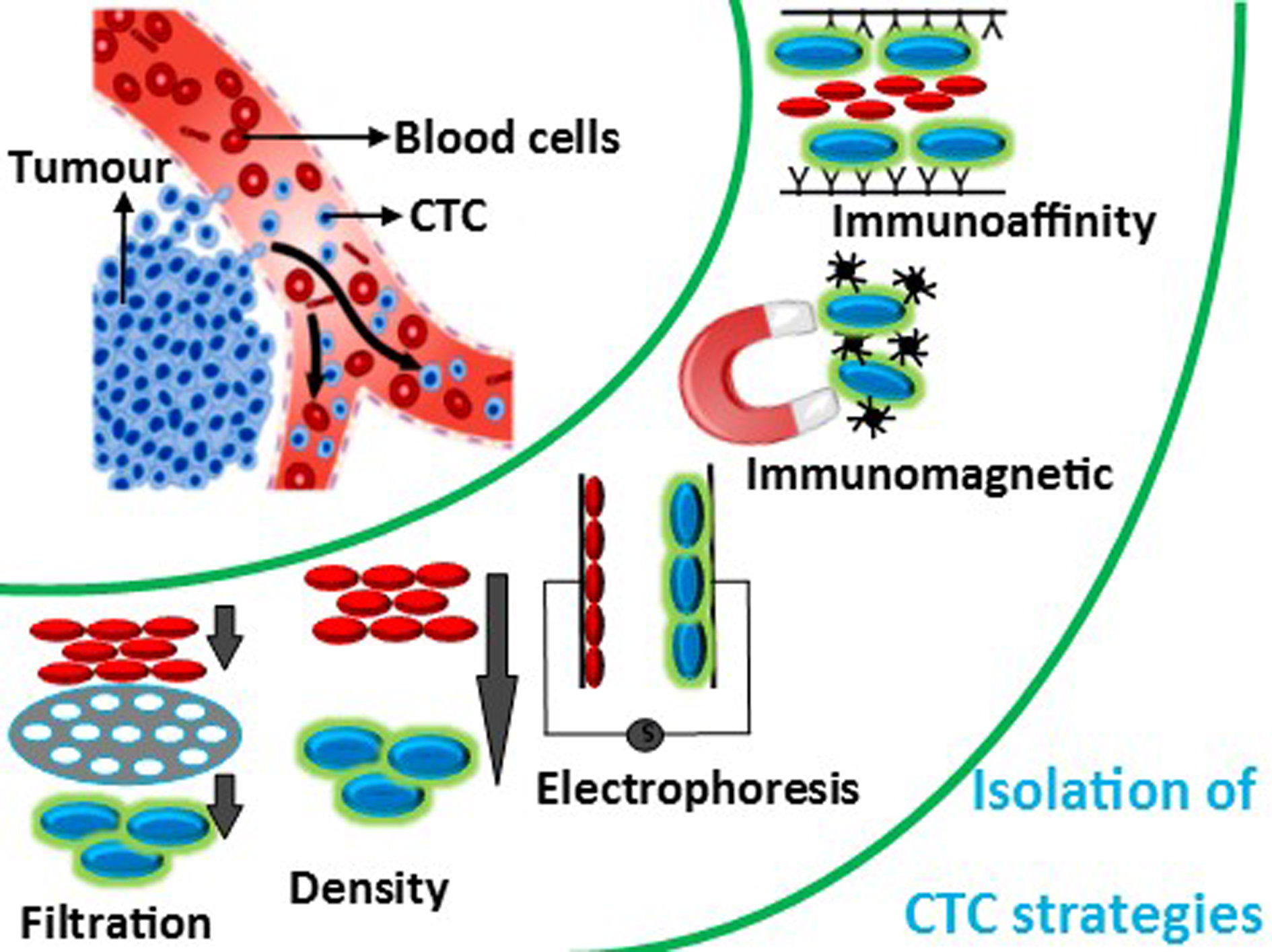
2. Size-Based Isolation
Due to its visibility and ease of management, CTC separation based on size and flexibility is one of the oldest approaches. The principle of separating cells from the main flow channel through filtration is, in fact, rather simple. Membrane devices are designed to act as a filter, allowing blood to flow while separating CTCs based on their size and deformability. When diluted blood travels through the main channel, cells greater than a certain size are captured by this membrane filtration set-up within the device, while smaller cells continue on their course and are separated. The risk of clogging, the requirement for frequent maintenance, cleaning, and the incapacity of cells to recover after filtration are all prevalent issues with these devices. This method’s most serious flaw is that it can’t separate more than one particle type in a single stage. Microfluidic systems capture tumor cells more efficiently via filtering because pore sizes and geometries are carefully controlled by microfabrication. Filters are divided into four types based on their structures: weir-type, pillars, crossflow, and membranes [118][28]. Size-based filtration using polymer membranes or microsieve membrane filter devices has been shown to extract CTCs from whole blood samples based on the morphological size differences between cancer cells (~15–40 μm in diameter) and leukocytes (~10 μm in diameter) [119][29]. The size, geometry, and density of the pores in the microfilters can be controlled uniformly and precisely. In addition, this technology can provide maximum sample processing capability via parallel arrays of multiple flow cells, which reduces processing time, cost, and filter clogging while facilitating mass production and high-throughput screening for large-scale clinical studies. Yoon et al. developed and reported a slanted weir microfluidic channel to reduce haemocyte contamination during CTC isolation [93][30]. With a flow rate of 2.5 mL/h and 3.8 mL/h for a breast cancer cell line (LM2 MDA-MB-231) at 0.8° weir to 0.5° weir, a high separation efficiency of ~97% was achieved. The viability of the collected tumor cells was also determined using the trypan blue assay, and it was found to be 97.1% for the 0.8° weir and 95.8% for the 0.5° weir. The viability of the 0.5° weir was slightly lower depending on the high flow rate and shear rate. This chip showed high separation efficiency with minimal contamination. However, the major drawback was its low throughput. Furthermore, Liu et al. developed a simple pyramid-shaped microchamber that is feasible, cost-effective, and highly efficient for CTC separation from breast carcinoma patients [82][31]. With an optimised flow rate of 200 μL/min, the capture efficiency of the device was assessed with a fresh blood sample in five sequence concentrations of 25–200 cells/mL using four different cancer cell lines (BGC823, H1975, PC-3, and SKBR3) spiked into DMEM medium. As a proof of concept, polystyrene beads with diameters of 10 μm (red beads) and 20 μm (blue beads) were allowed to pass through the pyramid-shaped channel at a flow rate of 200 μL/min. When the flow rate was increased to 300 μL/min, the capture efficiency increased to 92% and 89%, respectively, at different outlet heights of 6 μm and 8 μm. This method has advantages, including lower sample consumption, a simple experimental procedure, high capture efficiency, and ease of observation. Finally, from the DMEM medium, the SKBR3 cell line had a capture efficiency of 93%, while the healthy blood sample had a capture efficiency of 89%. Further, Fan et al. designed and developed a PDMS membrane filter-based technique for the isolation of CTCs [90][32]. At a flow rate of 10 mL/h, >90% cancer cell recovery was achieved from a blood sample spiked with lung cancer cells. Later, Zhang et al. created a label-free microfluidic device for isolating CTCs from breast cancer patients [105][33]. At a flow rate of 10 mL/h, the device demonstrated 73.6% capture efficiency and an 82% recovery rate. The main and side microchannels were 80 µm and 50 µm and 50 μm and 50 μm in width and height, respectively; the filter microchannel was 40 μm in width 10 μm in height. The device was used to isolate CTC cell strains such as SKBR3, MCF-7, and MDAMB231. Immunofluorescence staining was used to identify the cultured cells.3. Inertial Focusing Microchannel-Based Isolation
Inertial focusing is a phenomenon that occurs when suspended particles in a fluid stream migrate across flow lines and arrange themselves in equilibrium positions at specific cross-sectional positions. This behavior is caused by inertial forces within the channel and is controlled by channel geometry and flow conditions [122,123][34][35]. This phenomenon occurs in straight channels due to a balance of two dominating forces such as shear gradient inertial lift force (FSL), caused by the curvature of the fluid velocity profile and wall induced inertial lift force (FWL), caused by the particle’s interaction with the nearby wall. The particles are pushed toward the channel walls by FSL, while they are moved away from the walls and toward the channel center by FWL [124,125][36][37]. As a result, the particles tend to attain a state of equilibrium where these forces are equal. Zhou et al. designed a new multi-flow effect of a size-dependent inertial migration microfluidic (MFM) system for the precise detection and isolation of CTCs from spiked blood samples (H460 and HCC827) [62][38]. The separation efficiency and purity of CTCs were obtained to be >99% and >87%, respectively, from CTC-spiked blood samples. At a concentration of 10 cells per 5 mL, the device had an efficiency of >83%. The study showed that the average size of WBCs measured around 9 µm, and the average size of the detected CTCs was 30 µm. Additionally, the channel was examined for isolating CTCs from patient blood samples (stage IV lung cancer). The device has the advantage of having a high recovery rate even at very low concentrations, throughput and sensitivity; it had a disadvantage in terms of its performance and recovery rate due to the significant size overlap between target and non-target cells. Later, Gao et al. designed a label-free CTC isolation microfluidic device utilising the advantage of hydrodynamic forces [126][39]. The chip has a fishbone-shaped channel, rectangular reservoir and inertial focusing microchannel for CTC isolation. RBCs spiked with U87 cells were injected at a flow rate of 9 µL/min, showing 90% separation efficiency with 84.96% purity. Kulasinghe et al. designed a spiral microfluidic chip for the isolation of head and neck cancer cells (HNCs) [120][40]. The chip was tested with patients’ blood samples at a flow rate of 1.7 mL/min. The chip utilises inherent Dean vortex flow along with inertial lift force, which drives smaller hematologic cells towards the outer wall by facilitating the efficient separation of CTCs. The chip showed 54% detection efficiency. Furthermore, Warkiani et al. reported the label-free spiral microfluidic chip for the size-based separation of CTCs from the sample using hydrodynamic forces [127][41]. At a flow rate of 100 µL/min, the chip achieved ≥85% isolation efficiency. The chip could isolate CTCs from a 7.5 mL sample in less than 40 min. However, stacking three chips together yielded better results by isolating CTCs from a 7.5 mL samples in less than 10 min. Thus, the chip showed high throughput. Later, Ozbry et al. developed a microfluidic chip with a symmetrically curved channel for continuous and high-throughput isolation of cancer cells [128][42]. The cancer cell lines MDA-MB-231, Jurkat, K562, and HeLa were injected into the curvilinear channel at a curvature angle of 280°. The flow rate was increased constantly from 400 µL/min to 2700 µL/min at an interval of 90 s for each 100 µL increase in the injection volume. The study revealed cell size based on flow velocity. The chip exhibits high viability of >94%. Nam et al. fabricated a capillary inserted microfluidic device for the isolation tumor cells via viscoelastic flow [129][43]. The capillary tube facilitates 3D particle pre-alignment prior to separation. The presence of two outlets facilities the isolation of migrated particles with 5 and 10 µm diameter exhibiting ~99% isolation efficiency. At a flow rate of 200 µL/min, 94% of MCF-7 cells were isolated from leukocytes with 97% purity. Further, Abdulla et al. developed a self-amplified inertial focused (SAIF) microfluidic device for the size-based, high throughput isolation of CTCs [121][44]. The device demonstrated a narrow zigzag microchannel connected to expansion sites to enable size-based separation. The tested cancer cells such as lung cancer cells (A549), breast cancer cells (MCF-7), and cervical cancer cells (HeLa) isolation efficiency of ~80%. Che et al. developed label-free, size-based isolation of CTCs using vertex microfluidic chip [130][45]. At a flow rate of 8 mL/min (for diluted blood) and 800 µL/min (for whole blood); 83% capture efficiency was recorded. Thanormsridetchai et al. developed a spiral microfluidic device for capturing of CTCs [131][46]. The device with five spiral microchannels (500 µm height, 130 µm width, 5.5 mm length) was injected with samples at a flow rate of 1.0 mL/min. The device showed 90% capture efficiency.4. Dielectrophoresis-Based Isolation
Dielectrophoresis with external electric field sources is a quick, simple and well-known technique for manipulating a variety of biological particles within a microchannel [132][47]. It is also used to separate the movement of distinct cancer cells [133,134][48][49]. Cancerous cells could be separated from normal blood cells or the cell sample solution using the dielectrophoresis method based on cell properties such as size, morphology, deformability, mechanical, electrical and magnetic properties [122][34]. Chiu et al. investigated the size-dependent separation of cancer cell clusters using an optically induced dielectrophoresis (ODEP)-based microfluidic system [135][50]. The device was tested with a human prostate cancer cell line (PC-3) and leukocytes to evaluate its performance. The device could isolate as low as 15 cells/mL with a recovery rate of 41.5%. Overall, the proposed method could isolate CTCs with purity as high as 100% at a sample flow rate of 2.5 μL/min. Thus, the method was found to be promising in the isolation of CTCs with high sensitivity without interference from leukocytes. In another study, Li et al. demonstrated the dielectrophoresis technique using an array of wireless bipolar electrodes for the high-throughput isolations of CTCs [136][51]. The 32 parallel microchannels with 2950 µm, 200 µm, and 25 µm length, width, and height, respectively, were fabricated using the photolithography technique. The device could throughput 100 µL/h samples with a 39.6 mm2 device footprint. Further, Kim et al. developed a dielectrophoresis cell-trapping method for the trapping of cancer cells using a microfluidic device [137][52]. At a flow rate of 100 µL/min, 92 ± 9% of cells were isolated at the designated location. The technique enables the isolation of very low concentrations of cancer cells from large volumes of samples with high recovery. Liao et al. developed an optically induced dielectrophoresis (ODEP)-based microfluidic device for the isolation of high-purity CD45neg/EpCAMneg cells from the blood samples of cancer patients [85][53]. To recognize the EpCAM, surface marker-positive CTCs and CD45 surface marker-positive leucocytes were stained using fluorescent dyes. The diameters of PC-3 and SW620 cancerous cells were found to be 20.1 ± 1.5 and 1 µm, respectively. The device demonstrated 100% CTC capture purity in capturing live CD45neg/EpCAMneg cells. The device takes around 4 h for the analysis of 4 mL of sample suspension. The recovery rate of the microfluidic device was found to be 81.0 ± 0.7%.5. Magnetic Field-Based Isolation
Magnetic field-derived microfluidic chips are broadly classified as labelled methods and label-free methods of isolation. Positive and negative selection are the two most common methods of labelled magnetic isolation. CTCs can be actively isolated using functionalized magnetic nanoparticles (MNPs) when a magnetic field is applied. Specific antigen-coupled MNPs can bind to specific surface proteins expressions on CTCs, resulting in positive CTC selection [138][54]. Due to the diversity of cancer cells, CTCs shed from primitive tumors are highly heterogeneous, including epithelial cancer cells such as gastric cancer, mesenchymal cancer cells such as osteosarcoma and other cancer cells such as leukemia. This enables a wide range of antigens to be used to label different CTCs with antiepithelial cell adhesion molecule (EpCAM), which is the most commonly used antigen. On the other hand, negative enrichment of CTCs based on WBC depletion was achieved using anti-CD45 surface antigens because the antigens are particularly expressed on the surface of WBCs [139][55]. Due to inter-patient and intra-patient heterogeneity in tumor biology, particularly in the case of epithelial-mesenchymal transition (EMT), identifying CTC-specific markers becomes difficult. Meanwhile, label-free magnetic isolation isolates CTCs based on their size difference from hematological cells using magnetic fluids such as paramagnetic salt solutions or ferrofluids as media.5.1. Immunomagnetic (Label)-Based Isolation
Chang et al. developed a novel parallel flow micro-aperture chip system for CTC isolation in the spiked MCF-7 cell line at a flow rate of 2 mL/min [96][56]. CTCs with sizes ranging from 10 to 30 μm were found in the sample solution after it had been coated with antibody-mediated magnetic beads. The chip detected approximately 89% of the spiked MCF-7 breast cancer cell lines. The device has several advantages, including its ease of use, robustness, compatibility and versatility. The device was integrated with a PDMS microfiltration membrane for CTC capture and a parallel flow micro-aperture chip system for capturing CTCs. Furthermore, clinical samples revealed the possibility of isolating cancer cells (non-small-cell lung cancer cell line and pancreatic cancer cell line) that were bound on beads and captured on the chip’s surface. Furthermore, Kwak et al. investigated the selectivity and capture efficiency of the developed spiral-shaped channel device for two types of tumor cell lines, MDA-MB-231 and MCF-7, based on the level of EpCAM antigen expression [89][57]. The results showed that the capture efficiency of MDA-MB-231 and MCF-7 cells were 81.2 ± 3.5% and 96.3 ± 1.5%, respectively, at a flow rate of 150 µL/min. MDA-MB-231 cells had an average purity of 82.8%, while MCF-7 cells had an average purity of 85.9%. However, because of the low EpCAM expression in this reported device, several heterogeneous CTCs could not be detected and quantified. Recently, Kang et al. developed a positive and negative method for the isolation of CTCs (MDA-MB-231, PC-3, SKBR3, and MCF-7) by lateral magnetophoresis using magnetic nanobead-functionalized EpCAM and CD45/CD66b antibodies [140][58]. The lateral magnetophoresis technique was used to design a disposable chip with a microchannel on a multipurpose substrate fixed to ferromagnetic wires. The device works both on positive and negative methods for the isolation of CTCs using anti-EpCAM and anti-CD45/CD66b nanobeads. The ferromagnetic wires were inlaid at 5.7° towards the flow direction on the substrate. As the blood flowed through the lateral magnetophoretic microchannel, the residual magnetic nanobeads were bound to the ferromagnetic wires. The silicon-coated polymer film with a thickness of 12 µm was bonded to a microstructure PDMS replica to form a disposable microchannel substrate. The flow rate and suction rate for the sample and buffer were optimized in the positive method to 2 mL/h and 3.2 mL/h, respectively, resulting in the release of CTCs in the outlet at a flow rate of 0.8 mL/h. This device was evaluated for the isolation of the SKBR3 and MCF-7 cell lines, and the recovery rates were 93.9 ± 1.0% and 98.4 ± 1.5%, respectively. However, this method resulted in low EpCAM expression in MDA-MB-231 and PC-3 cells. Further, the flow rate for the sample and buffer was optimized to 2.8 mL/h for the negative method. The method yielded recovery rates of 85.2 ± 4.2 and 80.7 ± 7.6% for SKBR3 and MCF-7 cell lines, respectively, and 91.0 ± 2.0% and 75.7 ± 9.3% for MDA-MB-231 and PC-3 cells, respectively. A fluorescence microscope was then used to enumerate WBCs and CTCs from the outlet. The positive method produced more pure isolated CTCs than the negative method. Following this, Chen et al. developed a size-based microfluidic device with high capture efficiency for CTC isolation [97][59]. A few strong permanent magnets were fixed beneath the glass substrate to capture the magnetized CTCs. Three different cancerous cell lines (HCT116, SW480, and MCF-7) were tested with different EpCAM antibody expression levels to evaluate the device. Capture efficiency for MCF-7, HTC116, and SW480 was found to be up to 97.2 ± 6%, 85.7 ± 14.3%, and 91.5 ± 8.9%, respectively. Due to cell line accumulation, capture efficiency was decreased. The flow rate was optimised to 1.5 mL/h for the system operated without a magnet, which showed a capture efficiency of around 90%. The magnetic bead at a high processing rate of 3 mL/h showed a capture efficiency above 90% within 20 min. The live/dead assay revealed 96% cell viability. The reverse flushing process removed the majority of the CTCs from the channel. Despite the device’s high processing rate, there was a lack of capture efficiency. Furthermore, Shamloo et al. created a PDMS-based integrated microfluidic platform for CTC capture using an immunomagnetic technique [99][60]. The separation and mixing units, as mentioned in the fabrication section, use electric and magnetic forces for high throughput to increase the purity and capture efficiency in the microfluidic system. To evaluate the device’s capture efficiency, anti-EpCAM functionalized iron nanoparticles were tagged to different types of blood samples spiked with 100,000 cancerous cells, such as SKBR3 (human breast cancer cell line), PC-3 (prostate cancer cell line) and Colo205 (colon cancer cell line). The capture rate for SKBR3 and Colo205 cell lines was up to 97%, while the PC-3 cell line was 107%. As a result, this integrated microfluidic device has high compatibility and feasibility in cancer research. Later, Poudineh et al. developed magnetic raking cytometry to generate a phenotypic expression of captured CTCs [141][61]. The device consisted of circular nickel micromagnets with an array of X-shaped structures. The size of the micromagnets was increased along the channel to enhance the CTC capture efficiency. CTCs coated with anti-EpCAM-functionalised immunomagnetic beads were retained at the capture zone of the device. In addition, Poudineh et al. reported a microfluidic approach for profiling functional and biochemical phenotypes of CTCs [142][62]. The device consisted of four capture zones with an X-shaped morphology and a single-cell isolation area. The aptamer-coated CTCs functionalised with MNPs were captured at four capture zones by EpCAM expression. This was followed by releasing them to a single-cell isolation area using antisense DNA. The device showed 79 ± 4% recovery efficiency. Recently, Yin et al. constructed a dual-antibody (PSMA and EpCAM)-functionalised microfluidic device for the isolation of CTCs [143][63]. The dual-antibody-functionalised strategy showed a significant increase in the capture efficiency for LnCAP and LnCAP-EMP cancer cell lines. The device consists of antibody- and Fe3O4@microbead-functionalised Ni (nickel) micropillars under external magnetic conditions and a chaotic herringbone platform. The device could successfully identify CTCs from 20 out of 24 blood samples.5.2. Label-Free-Based Magnetic Isolation
Zhao et al. demonstrated size-based ferrohydrodynamic HeLa cell isolation using a microfluidic device [144][64]. Cell mixtures (HeLa cells, blood cells) and ferrofluids were mixed, then injected at a flow rate of 8 µL/min. The magnetic buoyancy force caused deflections of cells from their laminar flow patterns when the magnet was placed close to the channel. The force operating on cells inside ferrofluids is a body force proportional to cell volume, resulting in the spatial separation of cells of various sizes at the microchannel’s end. As a result, larger HeLa cells and smaller blood cells emerge through distinct pathways. The device exhibited >99% capture efficiency. The method was found to be cost-effective with high throughput. Furthermore, Zhao et al. used label-free size-based ferrohydrodynamic CTC isolation using a microfluidic device [145][65]. The device showed a high throughput of 6 mL/h with a recovery rate of 92.9%. The device could isolate CTCs as low as ~100 cells/mL. In addition, the device demonstrated recovery rates for cancer cells line such as H1299 (92.3%), A549 (88.3%), H3122 (93.7%), PC-3 (95.3%), MCF-7 (94.7%), and HCC1806 (12.2%). The device showed short-term cell viability, normal proliferation, and unaffected key biomarker expression. Later, Zhao et al. developed a label-free isolation method using ferrofluids to separate low-concentration cancer cells from cell culture lines in microfluidics [146][66]. The isolation depended on the variation in size of CTCs with WBCs in biocompatible ferrofluids. At a throughput of 1.2 mL/h, the device showed isolation efficiencies of 80 ± 3%, 81 ± 5%, 82 ± 5%, 82 ± 4%, and 86 ± 6% for A549 lung cancer, H1299 lung cancer, MCF-7 breast cancer, MDA-MB-231 breast cancer, and PC-3 prostate cancer cell lines, respectively.6. Acoustic-Based Isolation
An acoustic wave is a form of a mechanical wave that propagates across a longitudinal wave and is generated by mechanical stress from a piezoelectric transducer. Surface acoustic waves (SAWs) and bulk acoustic waves (BAWs) are the two forms of acoustic waves. Both have been widely employed in the field of microfluidics to manipulate micro-objects [147][67]. Travelling SAWs (TSAWs) and standing SAWs (SSAWs) are the two types of SAW-driven microfluidics. SAWs that propagate in one direction and radiate away from acoustic sources are known as travelling surface acoustic waves (TSAWs). Two opposing travelling SAWs interfering or a reflecting travelling SAW create stationary nodes and antinodes in an open or limited domain, resulting in standing surface acoustic waves (SSAWs). Alternatively, bulk acoustic waves (BAWs) are standing waves that propagate within the microchannel’s resonant chamber. To generate BAWs, a piezoelectric transducer is bonded to the microchannels and actuated by an AC power supply in BAW-based microfluidic devices. Unlike SAWs, which propagate along the material’s surface, bulk acoustic waves propagate within the material’s core. As a result, BAW-based microfluidic devices require more energy to create identical acoustic effects to SAW-based microfluidic devices [148,149][68][69]. Jiang et al. used the LCATs technique for the isolations of CTCs from breast cancer patients with different stages of cancer [103][70]. The advantage of LCATs was their ability to pump samples and trap CTCs without the use of a syringe pump. The device captured 230,000 cells with 200 pairs of dead-end side channels at 6 VP-P (peak-to-peak voltage) and 5.2 kHz, with an average of 1150 cells per pair of dead-end side channels. In less than 8 min, the device could process 7.5 mL of blood samples. However, the real CTC-spiked blood samples showed a capture efficiency of 92.8% with 90% viability. As a result, the technique must be improved in order to achieve higher capture efficiency in real-time applications. Wu et al. examined the acoustic separation of CTCs from leukocytes [150][71]. A piezoelectric substrate bound to a pair of interdigital transducers (IDTs) in a microfluidic channel generated two Rayleigh waves in opposite directions, resulting in periodic wave nodes and antinodes. In order to facilitate high throughput, a PDMS-glass hybrid channel was used to produce acoustic waves. At a throughput of 7.5 mL/h, 86% CTCs were recovered from the sample. Furthermore, Wang et al. developed a multi-stage device consisting of a pair of interdigital transducers (IDTs) and focused interdigital transducers (FIDTs) using microelectromechanical systems (MEMS) for the separation of CTCs by SAWs [151][72]. The acoustic waves generated by IDTs enabled the cells to be placed at pressure nodes, whereas acoustic waves generated by FIDTs push the RBCs from CTCs, resulting in isolation. At a flow rate of 0.3 µL/min, the device showed ~90% isolation efficiency for U87 glioma cells. Karthick et al. developed the acoustic impedance size-independent isolation of CTCs using a microfluidic device [152][73]. At an optimized flow rate, the device could recover 86% of HeLa cells and 88% of MDA-MA-231 CTCs. Later, Xue et al. presented an acoustic multifunctional micromanipulation (AMM) microstreaming device capable of patterning, tapping, isolating, and rotating microparticles with respect to size and shape [153][74]. A microcavity array with an inner micro vortex and outer micro vortex was generated by acoustic waves to achieve cell manipulation. The device showed ~90% isolation efficiency. Recently, Cushing et al. reported continuous-flow acoustophoretic negative selections of WBCs from CTCs with the help of negative acoustic contrast elastomeric particles (EPs) functionalised with CD45 antibodies [154][75]. EP-bound WBC aligned at the channel wall, enabling unbound CTCs to flow through the channel centre. The device facilitated the isolation of label-free CTCs from WBCs with a recovery rate of ~85–90%.7. Combined Method-Based Isolation
The combination of two or more modes of isolation techniques in a microfluidic device facilitates the highly efficient isolation of CTCs. Nasiri et al. fabricated a hybrid microfluidic system that uses inertial flow and magnetophoresis to isolate CTCs [107][76]. The MCF-7 cells were conjugated with EpCAM antibodies and MNPs to improve magnetic susceptibility. These surface-modified cells were mixed with blood cells and were injected into the hybrid device at a flow rate of 1000 µL/min. The device exhibited a separation efficiency of ~95% with a purity of ~93%. Furthermore, Raillon et al. combined a vortex chip and an impedance chip to create microfluidic devices for label-free, high-throughput CTC isolation and enumeration [95][77]. Firstly, a vortex chip was used to purify the cancer cells. Later, an impedance chip with a pair of electrodes measured the fluctuation of an applied electric field in the presence of CTCs. This device was subjected to beads and tumor cells as proof of concept. PEEK/Tefzel tubings were used to form connections along with the vortex chip, impedance chip and syringe-containing samples. In the vortex chip, the flow rate to capture CTCs was optimized to 100 µL/min. The channel was validated with 8, 15, and 20 µm fluorescent beads through which the vortex chip enriched beads with an amplitude ranging from 250 nA to 100–250 nA. By using an impedance chip, 1477 beads were detected, and 1294 beads were enumerated from the device. Finally, MCF-7 cells were assessed in the channel at an optimized flow rate of 100 μL/min. RBCs and PBMCs (peripheral blood mononuclear cells) were separated using Ficoll. Thus, it was observed that at 60 nA, 95% of MCF-7 cells were separated from RBCs and PBMCs by leaving 5% of MCF-7 as a false negative. Later, Shamloo et al. employed a passive and a hybrid centrifugal device design to isolate tumor cells with the help of MNPs [155][78]. In the passive design, a contraction–expansion array (CEA) microchannel with a bifurcation region was used to isolate tumor cells through inertial effects and bifurcation law. In the hybrid design, a CEA microchannel with stacks of magnets was used to isolate magnetically labelled tumor cells. The devices were utilised to isolate human breast cancer cells (MCF-7). The devices were performed with various centrifugal speeds, demonstrating a recovery rate of 76% at 2100 rpm for the passive design. On the other hand, the hybrid design showed an 85% recovery rate at 1200 rpm. Though the hybrid design showed a high recovery percentage, the passive design was less space-, cost-, and time-consuming. Chen developed a triplet microchannel spiral microfluidic chip that interconnected with many tilted slits based on inertial and deformability principles for the continuous isolation of CTCs [156][79]. Using inertial and viscous drag forces, cells of various sizes were made to achieve different equilibrium throughout the microchannel. The bigger CTCs were gathered at the central streamline. The chip showed a high isolation capacity of 90% at a flow rate of 80 mL/h. Later, Antfolk et al. fabricated a microfluidic device with two inlets and three outlets for the label-free, on-chip separation and enumeration of target tumor cells [157][80]. They bound together acoustic and dielectrophoresis chips through plasma treatment. The outlet of the acoustic chip was aligned to an inlet of the dielectrophoresis chip for the efficient isolation of target cells. Prostate tumor cells (DU145) were effectively isolated from peripheral blood mononuclear cells at a recovery rate of 76.2 ± 5.9%. Furthermore, Liu et al. designed a label-free inertial-ferrohydrodynamic CTC-capturing microfluidic device [158][81]. The technique enabled the high-throughput, high-resolution isolation of CTCs. The method could differentiate the ~1–2 μm diameter difference in cells for efficient separation. The developed method showed a recovery rate of 94% with high purity. In addition, Xu et al. created an integrated microfluidic device for CTC isolation [115][82]. The prefiltered CTCs were subjected to magnet-assisted isolation on a microfluidic chip comprised of anti-CD45 antibody-functionalized magnetic beads. For PC-9-spiked blood samples, the device demonstrated a capture efficiency of ~85% and a purity of 60.4%. Despite the fact that the method involved two steps of isolation with high throughput and minimal cell damage, the device lacked capture efficacy. Later, Garg et al. presented a multi-functional microfluidic microstreaming LCAT-based device for the size-based isolation, enrichment, and in situ biomarker-based sorting of cells from blood [159][83]. At a flow rate of 25 µL/min, targeted MCF-7 cells were trapped in microstreaming vortices at ~100% efficiency.8. Electrochemical-Based Isolation
Electrochemical detection relies on the transfer of electrons at the analyte-electrode interface, which is frequently accompanied by the process of analyte-receptor recognition. Electrochemical procedures have a fast response time, cheap cost, simplicity, clinically appropriate sensitivity, specificity and the potential to miniaturize when compared to other analytical methods [160][84]. Meanwhile, they are frequently used in conjunction with other technologies to achieve multimode detection with increased accuracy and sensitivity. Yan et al. fabricated a micropillar array electrochemical microchip for the isolation and analysis of CTCs [91][85]. The device surface was coated with a gold layer, followed by oligonucleotide modification via gold-thiol. Further, avidin and EpCAM antibodies were functionalised. In order to lyse the cells, the device was modified with two slices of gold to use as the working electrodes. By applying a voltage, the captured cells were lysed. The –OH ions generated during electrochemical lysis broke down the lipid bilayer of the captured cells. The device showed a capture efficiency of 85–100%. Furthermore, Gurudatt et al. developed an electrochemical microfluidic system that combines CTC separation, enrichment, and detection [116][86]. Whole blood cells flowing through a microchannel were initially functionalized with electroactive daunomycin (DM, an anticancer drug that can selectively interact with CTCs). The target species in the microfluidic channel exhibited a wave-like motion when an alternating current perpendicular to the hydrodynamic flow was applied and was segregated and enriched in a size-dependent manner. The CTCs were subsequently examined using a direct DM oxidation method with an electrochemical sensor at the channel end. With a separation efficiency of 92.0 ± 0.5% and a detection rate of 90.9%, this device is capable of successfully discriminating various cancer cells in patients’ blood samples.9. Biological Interaction-Based Isolation
Though CTCs are found in the bloodstream, they retain the characteristics of their original tumor cell from the metastatic sites. The expression of EpCAM is a pervasive biological property of CTCs. As a result, EpCAM was used as a specific biomarker for CTC isolation in positive selection. However, the EpCAM protein is present on CTCs but not on blood cells. Thus, other markers such as CD1513, CD6647, and CD45 are used as specific biomarkers for blood cells for negative selection. Stott et al. developed a herringbone microfluidic device by photolithography [162][87]. The microchannels were functionalised with EpCAM antibodies to facilitate CTC isolation. The presence of a herringbone pattern generates micro vortices, which results in thorough mixing of blood samples, facilitating the high interaction between the functionalised channel surface and CTCs. The device could isolate CTCs from patients’ blood with advanced prostate and lung cancer with a success rate of 93%. The device showed high throughput and promising results. Later, Song et al. developed an aptamer-tailed octopus chip (AP-Octopus-Chip) for capturing CTCs [161][88]. To improve capture efficiency, a deterministic lateral displacement (DLD)-patterned microfluidic chip was altered with multivalent aptamer-functionalized nanospheres. CTCs were forced to transverse streamlines and interact with AuNP-SYL3C modified micropillars. Blood cells that are smaller than CTCs stayed inside the initial flow streamline, and bigger CTCs interacted with them. The enriched CTCs were released after capture when the -AuS bond was broken by excess glutathione. Sheng et al. developed a geometrically enhanced mixing (GEM) chip for the capture and isolation of CTCs from pancreatic cancer cell lines [163][89]. Initially, anti-EpCAM was biotinylated and loaded to the surface of a microfluidic channel containing L3.6pl, BxPC-3, and MIAPaCa-2 cells in order to capture CTCs. Flow cytometry results show that L3.6pl cells bind strongly to anti-EpCAM, whereas MIAPaCa-2 cells do not. For capturing CTCs, the flow rate and velocity were optimised to 1 μL/s and 0.75 mm/s, respectively. The GEM chip detected ~23 CTCs from 7.5 mL of blood, with the capture efficiency of 90 ± 2% for the L3.6pl cells line and 92 ± 4% for the BxPC-3 cells. The device has the advantage of being able to isolate CTCs with sufficient throughput in 17 min. Overall, the device achieved >90% capture efficiency, >84% purity and a throughput of 3.6 mL of blood in 1 h. However, the device falls short in terms of CTC capture purity. Furthermore, Nieto et al. developed a soda-lime glass-based microfluidic device by using the laser-ablation direct writing method and laser-assisted thermal treatment for the isolation of CTCs [25]. With this treatment, the roughness, optical transparency and reshaping of the microstructures were improved. The surface-modified microchannel with EpCAM antibodies developed by this approach trapped the CTCs. The results showed a capture efficiency of ~76% for HEC-1A tumor cells. In addition, Jou et al. created the V-BioChip for isolating SKOV3 ovarian tumor cells from epithelial ovarian cancer patients’ blood samples [104][90]. Using anti-EpCAM antibody interactions on the device’s surface at a flow rate of 0.6 mL/h, the device demonstrated a capture efficiency of 48.3%. The combination of anti-EpCAM antibody and anti-N-cadherin antibody on the device surface resulted in a capture efficacy of 89.6%. Despite the functionalised surface, the obtained results showed a lower capture efficiency. Further, Wu et al. created a PLGA nanofiber, aptamer-functionalized microfluidic device for isolating ovarian cancer cells such as A2780 and OVCAR-3 cells [164][91]. The EpCAM-functionalised chip demonstrated a good capture efficiency of 89% for OVCAR-3 cells, while NC3S demonstrated high efficiency of 91% for A2780 with a release efficiency of 88% and 92%, respectively. Later, Reinholt et al. developed a PDMS microfluidic system to isolate HeLa (cervical cancer cell line) and CAOV-3 (ovarian cancer cell line) cancer cells [106][92]. For the capture of CTCs via a streptavidin–biotin conjugation, the microchannel surface was functionalized with a DNA aptamer. The capture efficiency was great when the CTCs were suspended in PBS buffer and flushed into the microchannel at a flow rate of 5 µL/min. The collected cells were also lysed using a DNA array channel. The cellular contents were allowed to flow out while the gDNA was isolated on the micropillar. The use of gDNA allows for the extraction of enormous amounts of data from a small number of cells without the need for genome amplification. In another study, Pulikkathodi et al. developed an AlGaN/GaN high-electron-mobility (HEMT) biosensor array for the detection and isolation of CTCs [165][93]. Furthermore, these chips are mounted on a thermos-curable polymeric substrate. The formed array has several aptamer-immobilized areas, which are sensitive to CTCs. The device showed high sensitivity and selectivity, making it a potential device for CTC isolation. Zhang et al. combined a size-based microfluidic device with surface-enhanced Raman spectroscopy (SERS) for the detection of tumor cells [166][94]. Three kinds of SERS aptamer nano vectors were utilised for the detection of breast cancer cell lines in accordance with surface protein expressions. Initially, at a flow rate of 1 µL/min, tumor cells were separated through filtration. Then, SERS receptors were used to analyse the captured CTCs. Recently, Chen et al. developed a 3D-printed microfluidic device for the isolation of CTC from a blood sample [81][95]. The channel surface was functionalised with EpCAM antibodies to capture EpCAM-positive cancer cell lines, such as MCF-7, SW480, PC-3, and EpCAM-negative 293T cells (Figure 8b). At a flow rate of 1 mL/h with a 2 cm channel length, the device showed a capture efficiency of up to ~92% for MCF-7, ~87.74 for SW480, and ~89.35 for PC-3. Cheng et al. designed and developed a 3D scaffold microfluidic device with a thermosensitive coating for the isolation and release of CTCs [167][96]. Gelatin hydrogel was coated on the surface of Ni (nickel) foam. In addition, the surface of the gelatin was functionalised with an anti-EpCAM monoclonal antibody to capture MCF-7 cells (Figure 8c). At an optimised flow rate of 50 µL/min, CTCs were captured. Further, the chip was transferred to an incubator at 37 °C in order to dissolve the gelatin hydrogel to facilitate the release of captured CTCs. The chip showed ~88% capture efficiency. The isolation of platelet-covered CTCs is extremely difficult due to the masking of surface epitopes. Furthermore, Jiang et al. designed a herringbone macromixing microfluidic platform using stealth CTCs as surface markers for the isolation of CTCs [168][97]. They used epithelial and mesenchymal phenotypes for the platelet-targeted isolation of CTCs. At first, the free platelets were isolated by hydrodynamic size-based isolation. Further, EpCAM/CD41 antibodies were employed for the isolation of platelet-covered CTCs. The device isolated 66% of lung, 60% of breast, and 80% of melanoma cancer cells. Zeinali et al. demonstrated the integrated immunoaffinity-based isolation of CTCs from pancreatic cancer patients [169][98]. The device could isolate epithelial and epithelial-to-mesenchymal transition CTCs simultaneously by using EpCAM and CD133 antibodies. At a flow rate of 1 mL/h, the device showed ≥97% CTC recovery with >76% purity. Yin et al. designed a micruifluidic device with a silicon filter with a pyramidal microcavity array (MCA) for the isolation of CTCs [170][99]. In order to improve the capture efficiency, the surface of the MCA filter was modified with an anti-EpCAM antibody. The device showed a capture efficiency of ~80% for MCF-7, SW620, and HeLa cell lines spiked in whole blood. The device could effectively filter various sizes of CTCs with high capture efficiency. Kermanshah et al. applied magnetic ranking cytometry (MagRC) to a biologically relevant study [171][100]. Nickel micromagnets of different sizes were developed to create isolation zones to capture magnetized CTCs. The blood samples of mice containing prostate cancer cells were mixed with EpCAM antibody-modified MNPs and were analysed using the MagRC device. Furthermore, Sun et al. developed a size-based separation where the microfluidic device has ~103 pores/mm2, exhibiting 68,000 effective pores with a pore diameter of 8 µm [172][101]. The capture efficiency for MCF-7 cells on the device was found to be 72 ± 10.6% when using the traditional ISET (isolation by size of epithelial tumor cell) technique at a flow rate of 1 mL/min, whereas the capture efficiency of M-ISET (microbeads assisting ISET) was found to be 93.3 ± 3%. As a result, the M-ISET method was found to be a powerful tool for improving the efficiency of CTC separation.10. Overview of Microfluidic Device Performance for the Isolation of Circulating Tumor Cells
Importantly, there are two types of CTC isolation methods: physical and biological. Physical approaches are typically based on physical properties, such as size, volume, deformability, density, dielectric properties, and viscosity, with benefits such as high capturing efficiency, simple sample preparation, and cost-effectiveness. On the other hand, biological approaches are based on antigen-antibody interactions. The main disadvantage, in this case, is that it is an expensive and time-consuming method. In addition, there are some challenges and drawbacks in identifying and separating CTCs. When dealing with microfluidic devices, five different technological criteria are to be considered: the detection limit, capture speed, biocompatibility, purity, and high throughput. There are various devices mentioned, such as spiral-shaped, slanted weir, T-shaped microchannel, and multi-flow microfluidic (MFM) systems, geometrically enhanced mixing (GEM) chips, PDMS-based integrated microfluidic platforms, pyramid-shaped microchambers, ODEP-based microfluidic devices, parallel flow micro-aperture chip systems, a label-free microfluidic device for the detection and separation of CTCs with different capture efficiency.References
- Wu, S.; Liu, S.; Liu, Z.; Huang, J.; Pu, X.; Li, J.; Yang, D.; Deng, H.; Yang, N.; Xu, J. Classification of Circulating Tumor Cells by Epithelial-Mesenchymal Transition Markers. PLoS ONE 2015, 10, e0123976.
- Cho, H.; Kim, J.; Song, H.; Sohn, K.Y.; Jeon, M.; Han, K.-H. Microfluidic technologies for circulating tumor cell isolation. Analyst 2018, 143, 2936–2970.
- Jackson, J.M.; Witek, M.A.; Kamande, J.W.; Soper, S.A. Materials and microfluidics: Enabling the efficient isolation and analysis of circulating tumour cells. Chem. Soc. Rev. 2017, 46, 4254–4280.
- Potdar, P.D.; Lotey, N.K. Role of circulating tumor cells in future diagnosis and therapy of cancer. J. Cancer Metastasis Treat. 2015, 1, 44–56.
- Garrido-Navas, C.; de Miguel-Pérez, D.; Exposito-Hernandez, J.; Bayarri, C.; Amezcua, V.; Ortigosa, A.; Valdivia, J.; Guerrero, R.; Garcia Puche, J.L.; Lorente, J.A. Cooperative and escaping mechanisms between circulating tumor cells and blood constituents. Cells 2019, 8, 1382.
- Kurkuri, M.D.; Al-Ejeh, F.; Shi, J.Y.; Palms, D.; Prestidge, C.; Griesser, H.J.; Brown, M.P.; Thierry, B. Plasma functionalized PDMS microfluidic chips: Towards point-of-care capture of circulating tumor cells. J. Mater. Chem. 2011, 21, 8841–8848.
- Liu, H.Y.; Hille, C.; Haller, A.; Kumar, R.; Pantel, K.; Hirtz, M. Highly efficient capture of circulating tumor cells by microarray in a microfluidic device. FASEB J. 2019, 33, lb230.
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801.
- Jie, X.-X.; Zhang, X.-Y.; Xu, C.-J. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications. Oncotarget 2017, 8, 81558.
- Cheng, S.-B.; Chen, M.-M.; Wang, Y.-K.; Sun, Z.-H.; Xie, M.; Huang, W.-H. Current techniques and future advance of microfluidic devices for circulating tumor cells. TrAC Trends Anal. Chem. 2019, 117, 116–127.
- Madhuprasad; Bhat, M.P.; Jung, H.-Y.; Losic, D.; Kurkuri, M.D. Anion Sensors as Logic Gates: A Close Encounter? Chem. Eur. J. 2016, 22, 6148–6178.
- Bhat, M.P.; Patil, P.; Nataraj, S.K.; Altalhi, T.; Jung, H.-Y.; Losic, D.; Kurkuri, M.D. Turmeric, naturally available colorimetric receptor for quantitative detection of fluoride and iron. Chem. Eng. J. 2016, 303, 14–21.
- Patil, P.; Bhat, M.P.; Gatti, M.G.; Kabiri, S.; Altalhi, T.; Jung, H.-Y.; Losic, D.; Kurkuri, M. Chemodosimeter functionalized diatomaceous earth particles for visual detection and removal of trace mercury ions from water. Chem. Eng. J 2017, 327, 725–733.
- Patil, P.; Ajeya, K.V.; Bhat, M.P.; Sriram, G.; Yu, J.; Jung, H.-Y.; Altalhi, T.; Kigga, M.; Kurkuri, M.D. Real-Time Probe for the Efficient Sensing of Inorganic Fluoride and Copper Ions in Aqueous Media. ChemistrySelect 2018, 3, 11593–11600.
- Bhat, M.P.; Kigga, M.; Govindappa, H.; Patil, P.; Jung, H.-Y.; Yu, J.; Kurkuri, M. A reversible fluoride chemosensor for the development of multi-input molecular logic gates. New J. Chem. 2019, 43, 12734–12743.
- Bhat, M.P.; Vinayak, S.; Yu, J.; Jung, H.-Y.; Kurkuri, M. Colorimetric Receptors for the Detection of Biologically Important Anions and Their Application in Designing Molecular Logic Gate. ChemistrySelect 2020, 5, 13135–13143.
- Pirzada, M.; Altintas, Z. Nanomaterials for healthcare biosensing applications. Sensors 2019, 19, 5311.
- Nolan, J.; Nedosekin, D.A.; Galanzha, E.I.; Zharov, V.P. Detection of apoptotic circulating tumor cells using in vivo fluorescence flow cytometry. Cytom. Part A 2019, 95, 664–671.
- Safaei, T.S.; Mohamadi, R.M.; Sargent, E.H.; Kelley, S.O. In situ electrochemical ELISA for specific identification of captured cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 14165–14169.
- Huaman, J.; Naidoo, M.; Zang, X.; Ogunwobi, O.O. Fibronectin regulation of integrin B1 and SLUG in circulating tumor cells. Cells 2019, 8, 618.
- Andergassen, U.; Zebisch, M.; Kölbl, A.C.; König, A.; Heublein, S.; Schröder, L.; Hutter, S.; Friese, K.; Jeschke, U. Real-time qPCR-based detection of circulating tumor cells from blood samples of adjuvant breast cancer patients: A preliminary study. Breast Care 2016, 11, 194–198.
- Wang, X.; Sun, L.; Zhang, H.; Wei, L.; Qu, W.; Zeng, Z.; Liu, Y.; Zhu, Z. Microfluidic chip combined with magnetic-activated cell sorting technology for tumor antigen-independent sorting of circulating hepatocellular carcinoma cells. PeerJ 2019, 7, e6681.
- Gossett, D.R.; Weaver, W.M.; Mach, A.J.; Hur, S.C.; Tse, H.T.K.; Lee, W.; Amini, H.; Di Carlo, D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 3249–3267.
- Xiao, Y.; Shen, M.; Shi, X. Design of functional electrospun nanofibers for cancer cell capture applications. J. Mater. Chem. B 2018, 6, 1420–1432.
- Nieto, D.; Couceiro, R.; Aymerich, M.; Lopez-Lopez, R.; Abal, M.; Flores-Arias, M.T. A laser-based technology for fabricating a soda-lime glass based microfluidic device for circulating tumour cell capture. Colloids Surf. B Biointerfaces 2015, 134, 363–369.
- Bhat, M.P.; Kurkuri, M.; Losic, D.; Kigga, M.; Altalhi, T. New optofluidic based lab-on-a-chip device for the real-time fluoride analysis. Anal. Chim. Acta 2021, 1159, 338439.
- Leung, C.-H.; Wu, K.-J.; Li, G.; Wu, C.; Ko, C.-N.; Ma, D.-L. Application of label-free techniques in microfluidic for biomolecules detection and circulating tumor cells analysis. TrAC Trends Anal. Chem. 2019, 117, 78–83.
- Autebert, J.; Coudert, B.; Bidard, F.-C.; Pierga, J.-Y.; Descroix, S.; Malaquin, L.; Viovy, J.-L. Microfluidic: An innovative tool for efficient cell sorting. Methods 2012, 57, 297–307.
- Lim, L.S.; Hu, M.; Huang, M.C.; Cheong, W.C.; Gan, A.T.L.; Looi, X.L.; Leong, S.M.; Koay, E.S.-C.; Li, M.-H. Microsieve lab-chip device for rapid enumeration and fluorescence in situ hybridization of circulating tumor cells. Lab Chip 2012, 12, 4388–4396.
- Yoon, Y.; Lee, J.; Ra, M.; Gwon, H.; Lee, S.; Kim, M.Y.; Yoo, K.-C.; Sul, O.; Kim, C.G.; Kim, W.-Y.; et al. Continuous Separation of Circulating Tumor Cells from Whole Blood Using a Slanted Weir Microfluidic Device. Cancers 2019, 11, 200.
- Liu, F.; Wang, S.; Lu, Z.; Sun, Y.; Yang, C.; Zhou, Q.; Hong, S.; Wang, S.; Xiong, B.; Liu, K.; et al. A simple pyramid-shaped microchamber towards highly efficient isolation of circulating tumor cells from breast cancer patients. Biomed. Microdevices 2018, 20, 83.
- Fan, X.; Jia, C.; Yang, J.; Li, G.; Mao, H.; Jin, Q.; Zhao, J. A microfluidic chip integrated with a high-density PDMS-based microfiltration membrane for rapid isolation and detection of circulating tumor cells. Biosens. Bioelectron. 2015, 71, 380–386.
- Zhang, X.; Lu, X.; Gao, W.; Wang, Y.; Jia, C.; Cong, H. A label-free microfluidic chip for the highly selective isolation of single and cluster CTCs from breast cancer patients. Transl. Oncol. 2021, 14, 100959.
- Zhou, Y.; Ma, Z.; Ai, Y. Sheathless inertial cell focusing and sorting with serial reverse wavy channel structures. Microsyst. Nanoeng. 2018, 4, 5.
- Martel, J.M.; Toner, M. Inertial Focusing in Microfluidics. Annu. Rev. Biomed. Eng. 2014, 16, 371–396.
- Wang, C.; Sun, S.; Chen, Y.; Cheng, Z.; Li, Y.; Jia, L.; Lin, P.; Yang, Z.; Shu, R. Inertial particle focusing and spacing control in microfluidic devices. Microfluid. Nanofluidics 2018, 22, 25.
- Ying, Y.; Lin, Y. Inertial Focusing and Separation of Particles in Similar Curved Channels. Sci. Rep. 2019, 9, 16575.
- Zhou, J.; Kulasinghe, A.; Bogseth, A.; O’Byrne, K.; Punyadeera, C.; Papautsky, I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst. Nanoeng. 2019, 5, 8.
- Gao, R.; Cheng, L.; Wang, S.; Bi, X.; Wang, X.; Wang, R.; Chen, X.; Zha, Z.; Wang, F.; Xu, X.; et al. Efficient separation of tumor cells from untreated whole blood using a novel multistage hydrodynamic focusing microfluidics. Talanta 2020, 207, 120261.
- Kulasinghe, A.; Tran, T.H.P.; Blick, T.; O’Byrne, K.; Thompson, E.W.; Warkiani, M.E.; Nelson, C.; Kenny, L.; Punyadeera, C. Enrichment of circulating head and neck tumour cells using spiral microfluidic technology. Sci. Rep. 2017, 7, 42517.
- Warkiani, M.E.; Khoo, B.L.; Wu, L.; Tay, A.K.P.; Bhagat, A.A.S.; Han, J.; Lim, C.T. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 2016, 11, 134–148.
- Ozbey, A.; Karimzadehkhouei, M.; Kocaturk, N.M.; Bilir, S.E.; Kutlu, O.; Gozuacik, D.; Kosar, A. Inertial focusing of cancer cell lines in curvilinear microchannels. Micro Nano Eng. 2019, 2, 53–63.
- Nam, J.; Tan, J.K.S.; Khoo, B.L.; Namgung, B.; Leo, H.L.; Lim, C.T.; Kim, S. Hybrid capillary-inserted microfluidic device for sheathless particle focusing and separation in viscoelastic flow. Biomicrofluidics 2015, 9, 064117.
- Abdulla, A.; Zhang, T.; Ahmad, K.Z.; Li, S.; Lou, J.; Ding, X. Label-free Separation of Circulating Tumor Cells Using a Self-Amplified Inertial Focusing (SAIF) Microfluidic Chip. Anal. Chem. 2020, 92, 16170–16179.
- Che, J.; Yu, V.; Dhar, M.; Renier, C.; Matsumoto, M.; Heirich, K.; Garon, E.B.; Goldman, J.; Rao, J.; Sledge, G.W.; et al. Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic Vortex technology. Oncotarget 2016, 7, 12748.
- Thanormsridetchai, A.; Ketpun, D.; Srituravanich, W.; Piyaviriyakul, P.; Sailasuta, A.; Jeamsaksiri, W.; Sripumkhai, W.; Pimpin, A. Focusing and sorting of multiple-sized beads and cells using low-aspect-ratio spiral microchannels. J. Mech. Sci. Technol. 2017, 31, 5397–5405.
- Khoshmanesh, K.; Nahavandi, S.; Baratchi, S.; Mitchell, A.; Kalantar-zadeh, K. Dielectrophoretic platforms for bio-microfluidic systems. Biosens. Bioelectron. 2011, 26, 1800–1814.
- Abd Rahman, N.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for Biomedical Sciences Applications: A Review. Sensors 2017, 17, 449.
- Chan, J.Y.; Kayani, A.B.A.; Ali, M.A.M.; Kok, C.K.; Majlis, B.Y.; Hoe, S.L.L.; Marzuki, M.; Khoo, A.S.-B.; Ostrikov, K.; Rahman, M.A.; et al. Dielectrophoresis-based microfluidic platforms for cancer diagnostics. Biomicrofluidics 2018, 12, 011503.
- Chiu, T.-K.; Chou, W.-P.; Huang, S.-B.; Wang, H.-M.; Lin, Y.-C.; Hsieh, C.-H.; Wu, M.-H. Application of optically-induced-dielectrophoresis in microfluidic system for purification of circulating tumour cells for gene expression analysis—Cancer cell line model. Sci. Rep. 2016, 6, 32851.
- Li, M.; Anand, R.K. High-Throughput Selective Capture of Single Circulating Tumor Cells by Dielectrophoresis at a Wireless Electrode Array. J. Am. Chem. Soc. 2017, 139, 8950–8959.
- Kim, S.H.; Ito, H.; Kozuka, M.; Hirai, M.; Fujii, T. Localization of low-abundant cancer cells in a sharply expanded microfluidic step-channel using dielectrophoresis. Biomicrofluidics 2017, 11, 054114.
- Liao, C.-J.; Hsieh, C.-H.; Chiu, T.-K.; Zhu, Y.-X.; Wang, H.-M.; Hung, F.-C.; Chou, W.-P.; Wu, M.-H. An Optically Induced Dielectrophoresis (ODEP)-Based Microfluidic System for the Isolation of High-Purity CD45(neg)/EpCAM(neg) Cells from the Blood Samples of Cancer Patients-Demonstration and Initial Exploration of the Clinical Significance of These Cells. Micromachines 2018, 9, 563.
- Chikaishi, Y.; Yoneda, K.; Ohnaga, T.; Tanaka, F. EpCAM-independent capture of circulating tumor cells with a ‘universal CTC-chip’. Oncol. Rep. 2017, 37, 77–82.
- Liu, Z.; Fusi, A.; Klopocki, E.; Schmittel, A.; Tinhofer, I.; Nonnenmacher, A.; Keilholz, U. Negative enrichment by immunomagnetic nanobeads for unbiased characterization of circulating tumor cells from peripheral blood of cancer patients. J. Med. 2011, 9, 70.
- Chang, C.-L.; Huang, W.; Jalal, S.I.; Chan, B.-D.; Mahmood, A.; Shahda, S.; O’Neil, B.H.; Matei, D.E.; Savran, C.A. Circulating tumor cell detection using a parallel flow micro-aperture chip system. Lab Chip 2015, 15, 1677–1688.
- Kwak, B.; Lee, J.; Lee, J.; Kim, H.S.; Kang, S.; Lee, Y. Spiral shape microfluidic channel for selective isolating of heterogenic circulating tumor cells. Biosens. Bioelectron. 2018, 101, 311–316.
- Kang, H.; Kim, J.; Cho, H.; Han, K.-H. Evaluation of Positive and Negative Methods for Isolation of Circulating Tumor Cells by Lateral Magnetophoresis. Micromachines 2019, 10, 386.
- Chen, H.; Zhang, Z.; Liu, H.; Zhang, Z.; Lin, C.; Wang, B. Hybrid magnetic and deformability based isolation of circulating tumor cells using microfluidics. AIP Adv. 2019, 9, 025023.
- Shamloo, A.; Ahmad, S.; Momeni, M. Design and Parameter Study of Integrated Microfluidic Platform for CTC Isolation and Enquiry; A Numerical Approach. Biosensors 2018, 8, 56.
- Poudineh, M.; Aldridge, P.M.; Ahmed, S.; Green, B.J.; Kermanshah, L.; Nguyen, V.; Tu, C.; Mohamadi, R.M.; Nam, R.K.; Hansen, A.; et al. Tracking the dynamics of circulating tumour cell phenotypes using nanoparticle-mediated magnetic ranking. Nat. Nanotechnol. 2017, 12, 274–281.
- Poudineh, M.; Labib, M.; Ahmed, S.; Nguyen, L.N.M.; Kermanshah, L.; Mohamadi, R.M.; Sargent, E.H.; Kelley, S.O. Profiling Functional and Biochemical Phenotypes of Circulating Tumor Cells Using a Two-Dimensional Sorting Device. Angew. Chem. Int. Ed. 2017, 56, 163–168.
- Yin, C.; Wang, Y.; Ji, J.; Cai, B.; Chen, H.; Yang, Z.; Wang, K.; Luo, C.; Zhang, W.; Yuan, C.; et al. Molecular Profiling of Pooled Circulating Tumor Cells from Prostate Cancer Patients Using a Dual-Antibody-Functionalized Microfluidic Device. Anal. Chem. 2018, 90, 3744–3751.
- Zhao, W.; Zhu, T.; Cheng, R.; Liu, Y.; He, J.; Qiu, H.; Wang, L.; Nagy, T.; Querec, T.D.; Unger, E.R.; et al. Label-Free and Continuous-Flow Ferrohydrodynamic Separation of HeLa Cells and Blood Cells in Biocompatible Ferrofluids. Adv. Funct. Mater. 2016, 26, 3990–3998.
- Zhao, W.; Cheng, R.; Jenkins, B.D.; Zhu, T.; Okonkwo, N.E.; Jones, C.E.; Davis, M.B.; Kavuri, S.K.; Hao, Z.; Schroeder, C.; et al. Label-free ferrohydrodynamic cell separation of circulating tumor cells. Lab Chip 2017, 17, 3097–3111.
- Zhao, W.; Cheng, R.; Lim, S.H.; Miller, J.R.; Zhang, W.; Tang, W.; Xie, J.; Mao, L. Biocompatible and label-free separation of cancer cells from cell culture lines from white blood cells in ferrofluids. Lab Chip 2017, 17, 2243–2255.
- Lenshof, A.; Evander, M.; Laurell, T.; Nilsson, J. Acoustofluidics 5: Building microfluidic acoustic resonators. Lab Chip 2012, 12, 684–695.
- Gao, Y.; Wu, M.; Lin, Y.; Xu, J. Acoustic Microfluidic Separation Techniques and Bioapplications: A Review. Micromachines 2020, 11, 921.
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F.; et al. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649.
- Jiang, R.; Agrawal, S.; Aghaamoo, M.; Parajuli, R.; Agrawal, A.; Lee, A.P. Rapid isolation of circulating cancer associated fibroblasts by acoustic microstreaming for assessing metastatic propensity of breast cancer patients. Lab Chip 2021, 21, 875–887.
- Wu, M.; Huang, P.-H.; Zhang, R.; Mao, Z.; Chen, C.; Kemeny, G.; Li, P.; Lee, A.V.; Gyanchandani, R.; Armstrong, A.J.; et al. Circulating Tumor Cell Phenotyping via High-Throughput Acoustic Separation. Small 2018, 14, 1801131.
- Wang, K.; Zhou, W.; Lin, Z.; Cai, F.; Li, F.; Wu, J.; Meng, L.; Niu, L.; Zheng, H. Sorting of tumour cells in a microfluidic device by multi-stage surface acoustic waves. Sens. Actuators B Chem. 2018, 258, 1174–1183.
- Karthick, S.; Pradeep, P.N.; Kanchana, P.; Sen, A.K. Acoustic impedance-based size-independent isolation of circulating tumour cells from blood using acoustophoresis. Lab Chip 2018, 18, 3802–3813.
- Bai, X.; Bin, S.; Yuguo, D.; Wei, Z.; Yanmin, F.; Yuanyuan, C.; Deyuan, Z.; Fumihito, A.; Lin, F. Parallel trapping, patterning, separating and rotating of micro-objects with various sizes and shapes using acoustic microstreaming. Sens. Actuators A Phys. 2020, 315, 112340.
- Cushing, K.; Undvall, E.; Ceder, Y.; Lilja, H.; Laurell, T. Reducing WBC background in cancer cell separation products by negative acoustic contrast particle immuno-acoustophoresis. Anal. Chim. Acta 2018, 1000, 256–264.
- Nasiri, R.; Shamloo, A.; Akbari, J. Design of a Hybrid Inertial and Magnetophoretic Microfluidic Device for CTCs Separation from Blood. Micromachines 2021, 12, 877.
- Raillon, C.; Che, J.; Thill, S.; Duchamp, M.; Desbiolles, B.X.E.; Millet, A.; Sollier, E.; Renaud, P. Toward Microfluidic Label-Free Isolation and Enumeration of Circulating Tumor Cells from Blood Samples. Cytom. Part A 2019, 95, 1085–1095.
- Shamloo, A.; Naghdloo, A.; Besanjideh, M. Cancer cell enrichment on a centrifugal microfluidic platform using hydrodynamic and magnetophoretic techniques. Sci. Rep. 2021, 11, 1939.
- Chen, H. A Triplet Parallelizing Spiral Microfluidic Chip for Continuous Separation of Tumor Cells. Sci. Rep. 2018, 8, 4042.
- Antfolk, M.; Kim, S.H.; Koizumi, S.; Fujii, T.; Laurell, T. Label-free single-cell separation and imaging of cancer cells using an integrated microfluidic system. Sci. Rep. 2017, 7, 46507.
- Liu, Y.; Zhao, W.; Cheng, R.; Puig, A.; Hodgson, J.; Egan, M.; Cooper Pope, C.N.; Nikolinakos, P.G.; Mao, L. Label-free inertial-ferrohydrodynamic cell separation with high throughput and resolution. Lab Chip 2021, 21, 2738–2750.
- Xu, M.; Liu, W.; Zou, K.; Wei, S.; Zhang, X.; Li, E.; Wang, Q. Design and Clinical Application of an Integrated Microfluidic Device for Circulating Tumor Cells Isolation and Single-Cell Analysis. Micromachines 2021, 12, 49.
- Garg, N.; Westerhof, T.M.; Liu, V.; Liu, R.; Nelson, E.L.; Lee, A.P. Whole-blood sorting, enrichment and in situ immunolabeling of cellular subsets using acoustic microstreaming. Microsyst. Nanoeng. 2018, 4, 17085.
- Li, X.-R.; Zhou, Y.-G. Electrochemical detection of circulating tumor cells: A mini review. Electrochem. Commun. 2021, 124, 106949.
- Yan, S.; Chen, P.; Zeng, X.; Zhang, X.; Li, Y.; Xia, Y.; Wang, J.; Dai, X.; Feng, X.; Du, W.; et al. Integrated Multifunctional Electrochemistry Microchip for Highly Efficient Capture, Release, Lysis, and Analysis of Circulating Tumor Cells. Anal. Chem. 2017, 89, 12039–12044.
- Gurudatt, N.G.; Chung, S.; Kim, J.-M.; Kim, M.-H.; Jung, D.-K.; Han, J.-Y.; Shim, Y.-B. Separation detection of different circulating tumor cells in the blood using an electrochemical microfluidic channel modified with a lipid-bonded conducting polymer. Biosens. Bioelectron. 2019, 146, 111746.
- Stott, S.L.; Hsu, C.-H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397.
- Song, Y.; Shi, Y.; Huang, M.; Wang, W.; Wang, Y.; Cheng, J.; Lei, Z.; Zhu, Z.; Yang, C. Bioinspired Engineering of a Multivalent Aptamer-Functionalized Nanointerface to Enhance the Capture and Release of Circulating Tumor Cells. Angew. Chem. Int. Ed. 2019, 58, 2236–2240.
- Sheng, W.; Ogunwobi, O.O.; Chen, T.; Zhang, J.; George, T.J.; Liu, C.; Fan, Z.H. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip 2014, 14, 89–98.
- Jou, H.-J.; Chou, L.-Y.; Chang, W.-C.; Ho, H.-C.; Zhang, W.-T.; Ling, P.-Y.; Tsai, K.-H.; Chen, S.-H.; Chen, T.-H.; Lo, P.-H.; et al. An Automatic Platform Based on Nanostructured Microfluidic Chip for Isolating and Identification of Circulating Tumor Cells. Micromachines 2021, 12, 473.
- Wu, Z.; Pan, Y.; Wang, Z.; Ding, P.; Gao, T.; Li, Q.; Hu, M.; Zhu, W.; Pei, R. A PLGA nanofiber microfluidic device for highly efficient isolation and release of different phenotypic circulating tumor cells based on dual aptamers. J. Mater. Chem. B 2021, 9, 2212–2220.
- Reinholt, S.J.; Craighead, H.G. Microfluidic Device for Aptamer-Based Cancer Cell Capture and Genetic Mutation Detection. Anal. Chem. 2018, 90, 2601–2608.
- Pulikkathodi, A.K.; Sarangadharan, I.; Hsu, C.-P.; Chen, Y.-H.; Hung, L.-Y.; Lee, G.-Y.; Chyi, J.-I.; Lee, G.-B.; Wang, Y.-L. Enumeration of circulating tumor cells and investigation of cellular responses using aptamer-immobilized AlGaN/GaN high electron mobility transistor sensor array. Sens. Actuators B Chem. 2018, 257, 96–104.
- Zhang, Y.; Wang, Z.; Wu, L.; Zong, S.; Yun, B.; Cui, Y. Combining Multiplex SERS Nanovectors and Multivariate Analysis for In Situ Profiling of Circulating Tumor Cell Phenotype Using a Microfluidic Chip. Small 2018, 14, 1704433.
- Chen, J.; Liu, C.-Y.; Wang, X.; Sweet, E.; Liu, N.; Gong, X.; Lin, L. 3D printed microfluidic devices for circulating tumor cells (CTCs) isolation. Biosens. Bioelectron. 2020, 150, 111900.
- Cheng, S.-B.; Xie, M.; Chen, Y.; Xiong, J.; Liu, Y.; Chen, Z.; Guo, S.; Shu, Y.; Wang, M.; Yuan, B.-F.; et al. Three-Dimensional Scaffold Chip with Thermosensitive Coating for Capture and Reversible Release of Individual and Cluster of Circulating Tumor Cells. Anal. Chem. 2017, 89, 7924–7932.
- Jiang, X.; Wong, K.H.K.; Khankhel, A.H.; Zeinali, M.; Reategui, E.; Phillips, M.J.; Luo, X.; Aceto, N.; Fachin, F.; Hoang, A.N.; et al. Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip 2017, 17, 3498–3503.
- Zeinali, M.; Murlidhar, V.; Fouladdel, S.; Shao, S.; Zhao, L.; Cameron, H.; Bankhead, A., III; Shi, J.; Cuneo, K.C.; Sahai, V.; et al. Profiling Heterogeneous Circulating Tumor Cells (CTC) Populations in Pancreatic Cancer Using a Serial Microfluidic CTC Carpet Chip. Adv. Biosyst. 2018, 2, 1800228.
- Yin, J.; Mou, L.; Yang, M.; Zou, W.; Du, C.; Zhang, W.; Jiang, X. Highly efficient capture of circulating tumor cells with low background signals by using pyramidal microcavity array. Anal. Chim. Acta 2019, 1060, 133–141.
- Kermanshah, L.; Poudineh, M.; Ahmed, S.; Nguyen, L.N.M.; Srikant, S.; Makonnen, R.; Pena Cantu, F.; Corrigan, M.; Kelley, S.O. Dynamic CTC phenotypes in metastatic prostate cancer models visualized using magnetic ranking cytometry. Lab Chip 2018, 18, 2055–2064.
- Sun, N.; Li, X.; Wang, Z.; Li, Y.; Pei, R. High-purity capture of CTCs based on micro-beads enhanced isolation by size of epithelial tumor cells (ISET) method. Biosens. Bioelectron. 2018, 102, 157–163.
