Metabolic diseases, such as obesity, Type II diabetes and hepatic steatosis, are a significant public health concern affecting more than half a billion people worldwide. The prevalence of these diseases is constantly increasing in developed countries, affecting all age groups. The pathogenesis of metabolic diseases is complex and multifactorial. Inducer factors can either be genetic or linked to a sedentary lifestyle and/or consumption of high-fat and sugar diets. In 2002, a new concept of “environmental obesogens” emerged, suggesting that environmental chemicals could play an active role in the etiology of obesity. Bisphenol A (BPA), a xenoestrogen widely used in the plastic food packaging industry has been shown to affect many physiological functions and has been linked to reproductive, endocrine and metabolic disorders and cancer. BPA was banned in baby bottles in Canada in 2008 and in all food-oriented packaging in France from 1 January 2015. Since the BPA ban, substitutes with a similar structure and properties have been used by industrials even though their toxic potential is unknown. Bisphenol S has mainly replaced BPA in consumer products as reflected by the almost ubiquitous human exposure to this contaminant.
- BPA substitutes
- metabolic disorders
- endocrine disruptors
- bisphenol A
1. Introduction
2. A Strong Link between BPA and Metabolic Disorders
A very large number of studies are devoted to the effect of BPA on the development of metabolic disorders. A recent meta-analysis conducted on 133 studies carried out in humans and selected according to exposure relevance revealed an association between exposure to BPA and a higher risk of developing Type II diabetes [8]. Urinary and plasmatic levels of BPA are positively associated with a higher risk of developing Type II diabetes. A study based on data from the National Health and Nutrition Examination survey (NHANES), including 1521 participants, also revealed higher BPA concentrations in obese participants, therefore suggesting an association between BPA and obesity [9]. An association also exists between exposure to BPA and acute insulin resistance, general obesity, abdominal obesity and the prevalence of diabetes [10]. Numerous epidemiological studies strengthen these observations [11,12,13][11][12][13]. These epidemiological data are supported by experimental data mainly in rodents showing an effect of BPA on organs involved in energy metabolism such as the liver, skeletal muscle, adipose tissue, pancreas and central nervous system (Figure 1). These studies have highlighted a number of characteristics of BPA, such as low-dose effects on adipocyte differentiation and on insulin production by β-pancreatic cells [14]. The effects are observed during adult exposure as well as after the perinatal period, which represents a more sensitive window of exposure [15,16,17][15][16][17].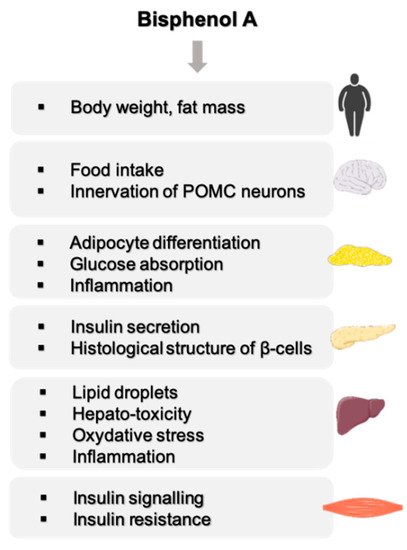
2.1. Effect of BPA on Body Weight
In rodents, maternal exposure to BPA was shown to increase postnatal body weights [16,18,19,20][16][18][19][20]. The dose–response relationship between BPA exposure and body weight gain often follows a non-monotonic inverted-U shape effect with an increase in body and fat mass in response to low doses (below the NOAEL) that were not always observed at high doses [16,19,20][16][19][20]. These non-monotonic effects are not always seen in both sexes. In females exposed in utero, adipose tissue mass is increased at low doses of BPA (0.26 mg/kg/j) but not at higher doses (2.72 mg/kg/j). In males, adipose tissue mass is increased proportionally to BPA exposure dose [15]. Body weight increase is often more pronounced and persistent in female offspring. This sexual dimorphism is not seen in all experimental conditions. In the study by Wei et al., an increased body weight of rats exposed in utero to BPA is observed, independently of sex, in standard feeding conditions and under a high-fat diet (HFD) or carbohydrate diet [21]. Studies conducted on adults revealed that the exposure of gestating mice to BPA (100 µg/kg bw/d) leads to an increased body weight [22]. Some studies revealed a decreased weight following perinatal exposure to BPA and others revealed no effects on body weight [23,24,25][23][24][25]. The differences obtained by the different studies mentioned above could be explained by the strains used, differing from one study to another; some strains were more sensitive to estrogenomimetic processes that mediate at least in part the effect of BPA on energy hoemostasis [26]. In addition, the exposure window, duration and mode of administration of BPA [26], and the type of feed [27] are key factors to take into consideration. Therefore, the impact of BPA on body weight gain can differ according to experiments. However, observations on key organs of energetic metabolism (liver, skeletal muscle, adipose tissue and pancreas) support the fact that BPA is not only an endocrine disruptor but also a metabolic disruptor. Since the route of exposure to BPA is mainly through food and beverage containers, the more an individual consumes processed foods stored in plastic containers, the more they will be exposed to BPA.2.2. Effect of BPA on the Central Nervous Functions Related to Energy Homeostasis
Proopiomelanocortin (POMC) neurons in the hypothalamic arcuate nucleus (ARC) are anorexigenic neurons that inhibit food intake and increase metabolic rate, while agouti-related peptide (AgRP) and neuropeptide Y (NPY) are orexigenic neuropeptides that stimulate appetite and reduce metabolic rate. These two sets of neurons form the hypothalamic melanocortin system, the physiological system that regulates feeding and energy balance. The activity of the melanocortin system is controlled by hormones, such as estradiol, leptin, ghrelin, and is sexually dimorphic. MacKay et al. analyzed whether in utero BPA exposure could alter the development of the melanocortin system and be linked to the obesogenic effect of BPA [28]. Thise study revealed impaired glucose tolerance in males exposed to BPA associated with reduced POMC neuron innervation. This effect was associated with increased NPY and AgRP expression in ARC when mice were fed with HFD. In females, BPA exposure induced increased body weight gain, food intake, adiposity and leptin concentrations, associated with reduced POMC mRNA expression in the ARC when fed an HFD diet. In BPA-exposed females, estrogen receptor α presents similar patterns of expression than in males, suggesting a masculinizing effect of BPA. Thise study demonstrates that in utero exposure to BPA alters the structure of the hypothalamic energy balance system and increases vulnerability to developing metabolic disorders. In 2017, the same authors extended their study to determine whether their prior observations were simply consequences of obesity or a phenotype produced by BPA exposure [29]. Therefore, they studied leptin sensitivity and hypothalamic structures in BPA-exposed animals before the onset of obesity or metabolic phenotypes. BPA-exposed animals were resistant to leptin-induced suppression of food intake, body weight loss, and hypothalamic POMC upregulation. Both males and females had a reduced density of POMC projections in the paraventricular nucleus of the hypothalamus. These results suggest that BPA may exert its effects through developmental programming of the melanocortin system, permanently altering the neurobiology of metabolic homeostasis. Salehi et al. explored whether the effect of BPA on POMC neurons was direct by using different cell lines, including POMC-expressing cell models [30]. Thise study demonstrated that exposure to BPA significantly induced the mRNA levels of POMC in primary cultures and cell lines. Furthermore, cell treatments with anti-inflammatory compounds, or with a PPARγ antagonist, abolished BPA-mediated POMC induction, indicating that BPA may have direct effects on hypothalamic POMC neurons through neuro-inflammatory mechanisms and PPARγ receptor.2.3. BPA, a Disruptor of Carbohydrate Homeostasis
Many studies, mainly conducted by Angel Nadal’s team, showed that exposure to BPA leads to the dysregulation of carbohydrate metabolism by a mechanism involving estrogen receptors in Langherans islets [22,31,32,33,34,35][22][31][32][33][34][35]. In adult male mice, a few days of subcutaneaous exposure (1 and 4 days) to low doses of BPA (10 and 100 µg/kg/day) induces an alteration of glucose tolerance, hyper insulinemia, and increased the content of insulin in β-cells [33,36][33][36]. The same effect was observed in vitro in the presence of BPA at concentrations of 1 nM and 10 nM [36]. Langherans islets of adult mice orally exposed to 100 µg/kg/day of BPA present increased insulin secretion in response to glucose (Figure 2) [37].

References
- Heindel, J.J. History of the Obesogen Field: Looking Back to Look Forward. Front. Endocrinol. 2019, 10, 14.
- Shahnazaryan, U.; Wójcik, M.; Bednarczuk, T.; Kuryłowicz, A. Role of Obesogens in the Pathogenesis of Obesity. Medicina 2019, 55, 515.
- Fénichel, P.; Brucker-Davis, F.; Chevalier, N. The history of Distilbène® (Diethylstilbestrol) told to grandchildren—The transgenerational effect. In Annales d’Endocrinologie; Elsevier Masson: Paris, France, 2015; Volume 76, pp. 253–259.
- Rudel, R.A.; Gray, J.M.; Engel, C.L.; Rawsthorne, T.W.; Dodson, R.E.; Ackerman, J.M.; Rizzo, J.; Nudelman, J.L.; Brody, J.G. Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ. Health Perspect. 2011, 119, 914–920.
- Vom Saal, F.S.; Cooke, P.S.; Buchanan, D.L.; Palanza, P.; Thayer, K.A.; Nagel, S.C.; Parmigiani, S.; Welshons, W.V. A physiologically based approach to the study of bisphenol A and other estro-genic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol. Ind. Health 1998, 14, 239–260.
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155.
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 89–106.
- Hwang, S.; Lim, J.-E.; Choi, Y.; Jee, S.H. Bisphenol A exposure and type 2 diabetes mellitus risk: A meta-analysis. BMC Endocr. Disord. 2018, 18, 81.
- Liu, B.; Lehmler, H.-J.; Sun, Y.; Xu, G.; Liu, Y.; Zong, G.; Sun, Q.; Hu, F.B.; Wallace, R.B.; Bao, W. Bisphenol A substitutes and obesity in US adults: Analysis of a population-based, cross-sectional study. Lancet Planet. Health 2017, 1, e114–e122.
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of Urinary Bisphenol A Concentration with Medical Disorders and Laboratory Abnormalities in Adults. JAMA 2008, 300, 1303.
- Carwile, J.L.; Michels, K.B. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res. 2011, 111, 825–830.
- Melzer, D.; Rice, N.E.; Lewis, C.; Henley, W.E.; Galloway, T.S. Association of Urinary Bisphenol A Concentration with Heart Disease: Evidence from NHANES 2003/06. PLoS ONE 2010, 5, e8673.
- Shankar, A.; Teppala, S. Relationship between Urinary Bisphenol A Levels and Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2011, 96, 3822–3826.
- Vandenberg, L.N. Low-Dose Effects of Hormones and Endocrine Disruptors. Vitam. Horm. 2014, 94, 129–165.
- Miyawaki, J.; Sakayama, K.; Kato, H.; Yamamoto, H.; Masuno, H. Perinatal and Postnatal Exposure to Bisphenol A Increases Adipose Tissue Mass and Serum Cholesterol Level in Mice. J. Atheroscler. Thromb. 2007, 14, 245–252.
- Rubin, B.S.; Murray, M.K.; Damassa, D.A.; King, J.C.; Soto, A.M. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ. Health Perspect. 2001, 109, 675–680.
- Ryan, K.; Haller, A.M.; Sorrell, J.E.; Woods, S.C.; Jandacek, R.J.; Seeley, R.J. Perinatal Exposure to Bisphenol-A and the Development of Metabolic Syndrome in CD-1 Mice. Endocrinology 2010, 151, 2603–2612.
- Susiarjo, M.; Xin, F.; Bansal, A.; Stefaniak, M.; Li, C.; Simmons, R.A.; Bartolomei, M. Bisphenol A Exposure Disrupts Metabolic Health across Multiple Generations in the Mouse. Endocrinology 2015, 156, 2049–2058.
- Angle, B.M.; Do, R.P.; Ponzi, D.; Stahlhut, R.W.; Drury, B.E.; Nagel, S.C.; Welshons, W.V.; Besch-Williford, C.L.; Palanza, P.; Parmigiani, S.; et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. 2013, 42, 256–268.
- Somm, E.; Schwitzgebel, V.M.; Toulotte, A.; Cederroth, C.R.; Combescure, C.; Nef, S.; Aubert, M.L.; Hüppi, P.S. Perinatal Exposure to Bisphenol A Alters Early Adipogenesis in the Rat. Environ. Health Perspect. 2009, 117, 1549–1555.
- Wei, J.; Lin, Y.; Li, Y.; Ying, C.; Chen, J.; Song, L.; Zhou, Z.; Lv, Z.; Xia, W.; Chen, X. Perinatal Exposure to Bisphenol A at Reference Dose Predisposes Offspring to Metabol-ic Syndrome in Adult Rats on a High-Fat Diet. Endocrinology 2011, 152, 3049–3061.
- Magdalena, P.A.; Vieira, E.; Soriano, S.; Menes, L.; Burks, D.; Quesada, I.; Nadal, A. Bisphenol A Exposure during Pregnancy Disrupts Glucose Homeostasis in Mothers and Adult Male Offspring. Environ. Health Perspect. 2010, 118, 1243–1250.
- Ishido, M.; Masuo, Y.; Kunimoto, M.; Oka, S.; Morita, M. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J. Neurosci. Res. 2004, 76, 423–433.
- Kabuto, H.; Amakawa, M.; Shishibori, T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004, 74, 2931–2940.
- Ryan, B.C.; Vandenbergh, J.G. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm. Behav. 2006, 50, 85–93.
- Richter, C.A.; Birnbaum, L.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; Saal, F.S.V. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224.
- Vom Saal, F.S.; Richter, C.A.; Ruhlen, R.R.; Nagel, S.C.; Timms, B.G.; Welshons, W.V. The importance of appropriate controls, animal feed, and animal models in inter-preting results from low-dose studies of bisphenol A. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 140–145.
- Mackay, H.; Patterson, Z.R.; Khazall, R.; Patel, S.; Tsirlin, D.; Abizaid, A. Organizational Effects of Perinatal Exposure to Bisphenol-A and Diethylstilbestrol on Arcuate Nucleus Circuitry Controlling Food Intake and Energy Expenditure in Male and Female CD-1 Mice. Endocrinology 2013, 154, 1465–1475.
- Mackay, H.; Patterson, Z.R.; Abizaid, A. Perinatal Exposure to Low-Dose Bisphenol-A Disrupts the Structural and Functional Development of the Hypothalamic Feeding Circuitry. Endocrinology 2017, 158, 768–777.
- Salehi, A.; Loganathan, N.; Belsham, D.D. Bisphenol A induces Pomc gene expression through neuroinflammatory and PPARγ nuclear receptor-mediated mechanisms in POMC-expressing hypo-thalamic neuronal models. Mol. Cell. Endocrinol. 2019, 479, 12–19.
- Martinez-Pinna, J.; Marroqui, L.; Hmadcha, A.; Lopez-Beas, J.; Soriano, S.; Villar-Pazos, S.; Alonso-Magdalena, P.; Dos Santos, R.S.; Quesada, I.; Martin, F.; et al. Oestrogen receptor β mediates the actions of bisphenol-A onion channel expression in mouse pancreatic beta cells. Diabetologia 2019, 62, 1667–1680.
- Villar-Pazos, S.; Martinez-Pinna, J.; Castellano-Muñoz, M.; Magdalena, P.A.; Marroqui, L.; Quesada, I.; Gustafsson, J.-A.; Nadal, A. Molecular mechanisms involved in the non-monotonic effect of bisphenol-a on Ca2+ entry in mouse pancreatic β-cells. Sci. Rep. 2017, 7, 11770.
- Alonso-Magdalena, P.; Morimoto, S.; Ripoll, C.; Fuentes, E.; Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006, 114, 106–112.
- Nadal, A.; Magdalena, P.A.; Soriano, S.; Quesada, I.; Ropero, A.B. The pancreatic β-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol. Cell. Endocrinol. 2009, 304, 63–68.
- Ropero, A.B.; Pang, Y.; Alonso-Magdalena, P.; Thomas, P.; Nadal, Á. Role of ERβ and GPR30 in the endocrine pancreas: A matter of estrogen dose. Steroids 2012, 77, 951–958.
- Alonso-Magdalena, P.; Ropero, A.B.; Carrera, M.P.; Cederroth, C.R.; Baquie, M.; Gauthier, B.R.; Nef, S.; Stefani, E.; Nadal, A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE 2008, 3, e2069.
- Soriano, S.; Alonso-Magdalena, P.; García-Arévalo, M.; Novials, A.; Muhammed, S.J.; Salehi, A.; Gustafsson, J.-A.; Quesada, I.; Nadal, A. Rapid Insulinotropic Action of Low Doses of Bisphenol-A on Mouse and Human Islets of Langerhans: Role of Estrogen Receptor β. PLoS ONE 2012, 7, e31109.
- Makaji, E.; Raha, S.; Wade, M.G.; Holloway, A.C. Effect of Environmental Contaminants on Beta Cell Function. Int. J. Toxicol. 2011, 30, 410–418.
- Dahlman-Wright, K.; Cavailles, V.; Fuqua, S.A.; Jordan, V.C.; Katzenellenbogen, J.A.; Korach, K.; Maggi, A.; Muramatsu, M.; Parker, M.G.; Gustafsson, J. International Union of Pharmacology. LXIV. Estrogen Receptors. Pharmacol. Rev. 2006, 58, 773–781.
- Thomas, P.; Dong, J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179.
- Sharma, G.; Prossnitz, E.R. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic β-cells. Endocrinology 2011, 152, 3030–3039.
- Alonso-Magdalena, P.; Laribi, O.; Ropero, A.B.; Fuentes, E.; Ripoll, C.; Soria, B.; Nadal, A. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic α-cells through a nonclassical membrane estrogen receptor within intact islets of Langer-hans. Environ. Health Perspect. 2005, 113, 969–977.
