Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Khair Alhareth.
Pregnancy-associated disorders affect around 20% of pregnancies each year around the world. The risk associated with pregnancy therapeutic management categorizes pregnant women as “drug orphan” patients. In the last few decades, nanocarriers have demonstrated relevant properties for controlled drug delivery, which have been studied for pregnancy-associated disorders. To develop new drug dosage forms it is mandatory to have access to the right evaluation models to ensure their usage safety and efficacy.
- placenta
- experimental models
- nanocarriers
- pregnancy
1. Nanocarriers for Pregnancy-Associated Disorders
It is not possible to translate drug clinical data from a non-pregnant woman to an expecting mother since women’s bodies experience various changes during pregnancy [12][1]. Almost all physiological functions in a woman’s body is adapted to allow for the fetus’ development [13][2], which will affect the drug disposition. Absorption of the drug is modified for pregnant women because of the delayed gastric emptying, decrease of gastric pH, and intestinal motility. The distribution of hydrophilic drugs is changed through the increase in the total body water, and the distribution of hydrophobic drugs is increased with a higher fat compartment. The modification of CYP450 and UGT activities affects the metabolization of xenobiotics. Finally, the renal clearance of most of the drugs is increased thanks to the increase in cardiac output [14,15][3][4].
All these body modifications in pregnant women can change the benefit/risk ratio of a drug for the future mother. However, they can also influence the risk for the fetus by increasing the concentration of drugs in contact with the placenta and favor its transplacental passage. During pregnancy, the principal route of communication between the mother and the baby is governed by the placenta. Therefore, fetal exposure to any substance depends on the transplacental passage.
The placenta can be described as a utero–fetal structure since it comes from maternal and embryonic cells. The human placenta is a hemochorial transient organ, which means that the mother’s blood is in direct contact with the placental cells. As presented Figure 1, the placenta is attached to the mother’s uterine wall, and the blood is discharged into what is called the intervillous space from the spiral arteries. The fetus is linked to the placenta by the umbilical cord, which groups two fetal arteries and one vein. The blood circulation is then divided into arborescent structures called the chorionic villi. These villous structures are composed of three different cell layers: the syncytiotrophoblast, the villous cytotrophoblast, and endothelial cells. The syncytiotrophoblast is derived from the fusion of cytotrophoblasts to form large syncytia called the syncytiotrophoblast. This cell layer forms the outer part of the villi and is in direct contact with the maternal blood, ensuring the transplacental passage of nutrients and gases. The placenta provides all the needed substances to ensure the healthy development of the baby.
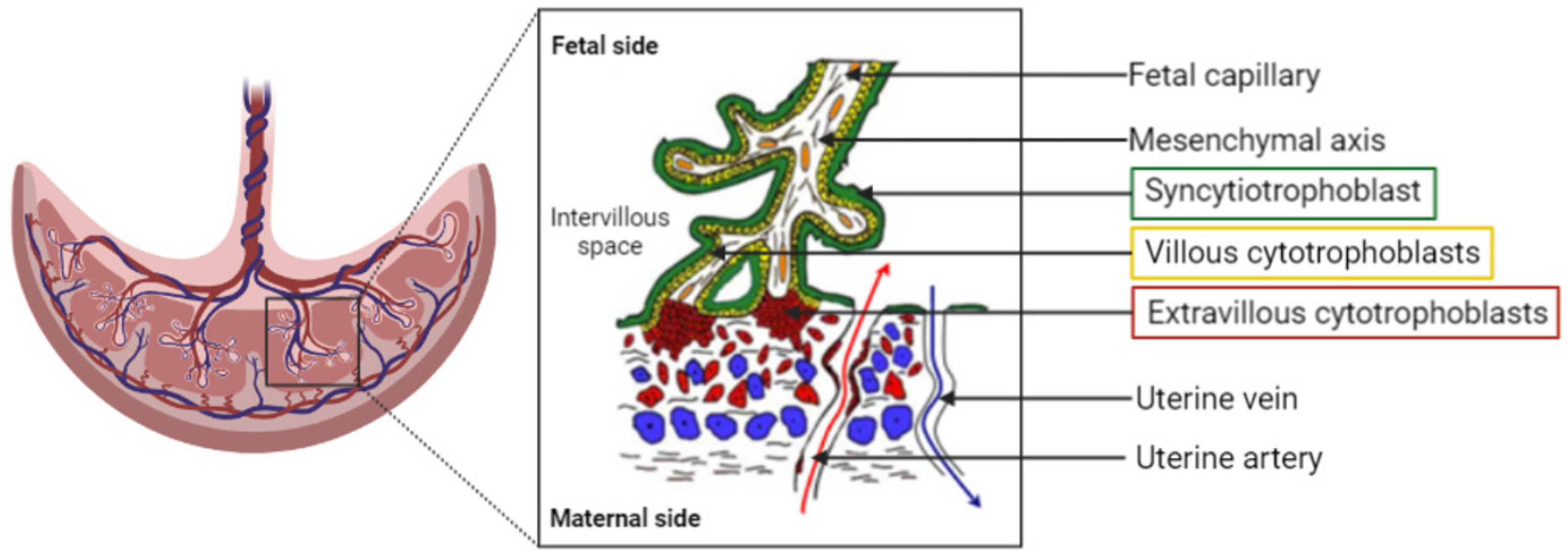
Figure 1. Representation of the organization of the human placenta, maternal–fetal blood circulation, and the villous, the placenta functional and structural unit. A villous is composed of a mesenchymal axis (white) comprising fetal capillaries (orange) grouped together to form the umbilical cord. The mesenchymal axis is covered by a specific cell type: the villous cytotrophoblasts (yellow), which fuse to renew the syncytiotrophoblast (green). The syncytiotrophoblast is in direct contact with the maternal blood within the intervillous space/chamber. The oxygenated blood is flooding the placenta from the uterine arteries (red arrow), whereas deoxygenated blood exits the intervillous space through the uterine veins (blue arrow).
Even if the placenta is designated as a barrier, it cannot prevent the passage of all exogenous components that are administered to the mother. Nowadays, many common medications are small molecules, which can cross from the maternal side to the fetal side. Today, gathering as much knowledge as possible about drug safety, teratogenicity, and efficacy in the context of pregnancy is a necessity for healthcare professionals to deliver the best medical care [16,17][5][6]. Modulating the transplacental passage of drugs can be achieved thanks to the advancements in pharmaceutical technology and drug delivery. Therefore, nanocarriers as drug delivery systems could address this specific challenge for women’s treatment during pregnancy.
Nanocarriers are defined as nanoparticles with a size from 1 to 100 nm [18][7]. With specific drug delivery systems, it is possible to enhance pharmacokinetic properties of the drug by increasing the residence time and avoiding the rapid clearance of the compound. Coating the surface of nanocarriers with polyethylene glycol (PEG) chains of various lengths, called PEGylation, is a common technique used to improve systemic circulation time and decrease immunogenicity. This confers stealth properties to the administered nanocarriers [19][8]. The administered PEGylated nanocarriers will circulate long enough to deliver the active pharmaceutical ingredient (API) to the desired target by passive targeting.
This passive targeting relies on the carrier characteristics (size, surface charge, stealthiness) and the biological environment, but does not allow it to enter a specific organ or specific cell type. A specific ligand can be grafted to the nanocarrier and therefore bind the desired target by active targeting or vectorization [20][9].
Choosing the physicochemical and surface properties of nanocarriers allows the biodistribution of the drug to be controlled and modified [21,22][10][11] and the physiological changes during pregnancy to be coped with. The use of nanomedicine is a promising approach to lowering toxicity, increasing therapeutic efficiency, and having a controlled and sustained released of the API [23,24,25,26][12][13][14][15] and thus for pregnant women’s treatment [27][16].
The rising interest in the development of nanocarrier-based medicine for pregnant women begs the question of their evaluation and safety considerations. Clinical trials including pregnant women are restricted and therefore imply proper preclinical evaluation models. Human placenta is donated from hospitals and has several characteristics, and it is defined with high inter-species differences compared to other mammals. The use of human cells and tissues is key to avoid variation between species to ensure complete and trustworthy results [28,29][17][18].
2. In Vivo Models
Animal models are live organisms and therefore have the advantage of exhibiting several properties useful to studying the future of administered medicines and their possible toxic effects on the body. They indeed give useful information specifically about learning about the biodistribution of molecules in a living organism. This knowledge has a special importance in the development of nanomedicines. Drug loading into nanocarriers aims to modify its distribution or to achieve its targeting to a specific organ. In vivo experiments are usually performed to demonstrate the proof of concept for the strategy of nanomedicines, i.e., decreased toxicity and increased efficacy compared to the conventional dosage form. Many experiments on pregnant animal models have been performed to study and understand the interaction with the placental barrier of standardized inorganic nanoparticles, e.g., gold [30[19][20][21][22],31,32,33], polystyrene [34[23][24],35], silica nanoparticles [36[25][26][27][28],37,38,39], quantum dots [40,41][29][30], and carbon nanotubes [42][31] with calibrated size and shape. These studies commonly suggest that the transplacental passage in vivo in murine animals depends highly on the size, the nature of the coating, and the gestational maturity of the placenta. Nanoparticles and nanocarriers under 100 nm exhibit a higher transplacental passage than other NPs at every stage of the placenta’s development. It seems that feto/placental accumulation of NPs is gestational-stage dependent, i.e., the transplacental passage of NPs can be facilitated at an early gestational age for numerous NPs of diverse sizes compared to a late pregnancy stage [32,33,39,43][21][22][28][32]. A specific coating could prevent transplacental passage depending on the nature of the NP, such as, for example, PEG-coated QDs [40][29]. Not all NPs able to cross the placenta and reach fetuses always have harmful effects on the offspring and the mother, and the toxic effect could be attributed to certain physicochemical characteristics as a small size and positive surface charge [36,38][25][27]. Other teams used healthy pregnant animals to evaluate nanocarriers as drug delivery systems, such as liposomes to deliver higher concentrations of drugs to the placenta such as indomethacin for the treatment of preterm labor management [44,45][33][34] or vasodilators to treat fetal growth restriction induced by impaired uteroplacental blood flow [46][35] or to ensure that gadolinium, a contrasting agent for medical imaging of the mother, does not cross the placenta [47][36]. These studies concluded that liposomes are suitable drug delivery systems to control biodistribution compared to the free drug because they modify the physicochemical properties of the liposomes. Changes in biodistribution can be improved by modifying the surface of nanocarriers with PEG chains or by adding a specific peptide or antibody to target a specific receptor present at the surface of the placenta [48][37] and encouraging results have arisen from these studies. King et al. designed a tumor-homing peptide and RGD-coated nanocarriers, which exhibited efficient targeting towards the placenta in gravid mice compared to non-functionalized nanocarriers and fetal transfer. Then they tested insulin-like growth factor-loaded nanocarriers in a pregnant mouse model of fetal growth restriction and showed an improvement in fetal and placental weight compared to the free drug [49][38]. Specific placental targeting was performed by using functionalized liposomes with a peptide derived from VAR2CSA. This protein is known to be produced by plasmodium falciparum-infected erythrocytes in a context of pregnancy malaria. VAR2CSA specifically recognized the chondroitin sulfate A at the surface of the placenta and facilitated the penetration of the parasite into the placenta. Zhang et al. based the functionalization of the liposomes on this mechanism and demonstrated a significant translocation of liposomes inside the placenta compared to the other organs. In the context of choriocarcinoma, the targeted delivery of methotrexate to the trophoblast cells showed high therapeutic efficiency compared to free methotrexate and lower toxicity [50][39]. Certain studies were performed on pathological animal models to evaluate the therapeutic efficiency of nanocarriers in the context of pregnancy-associated disorders such as uterine inflammation [51,52][40][41] or preeclampsia [53,54][42][43]. Some nanoparticles/nanocarriers were experimented with in a gravid mice pathological model of intrauterine inflammation. Tian et al. demonstrated that the intrauterine inflammation could enhance maternofetal transfer of NPs during a late stage of gestation. Injection of gold nanoparticles of 3, 13, and 32 nm in a pathological model of pregnant mice showed an increased accumulation of NPs of 3 and 13 nm inside fetal tissues compared to healthy mice. The 32 nm NPs could not cross the placental barrier in either model [51][40]. Another study focused on testing the therapeutic efficiency of dendrimer NPs containing N-acetyl-L-cysteine (DNAC) [52][41]. The NPs were administered via intraperitoneal injection to the pregnant mouse with intrauterine inflammation. The results demonstrated that DNAC significantly reduced the preterm birth rate and altered the placental immune profile with a decrease of CD8+ cell infiltration, an improvement in neurobehavior, and a reduction in the neuroinflammation of fetuses, reflecting the therapeutic efficiency of the administered drug. One of the physiopathological mechanisms of preeclampsia (PE) is characterized by an overexpression of an antiangiogenic factor, sFlt1, provoking placental ischemia and the secretion of inflammatory and oxidant factors. This induces vascular endothelium damage in the mother (systemic vasculopathy) with vital organ impairment, leading to potential eclampsia. Direct consequences are the termination of pregnancy and placenta expulsion, endangering the mother’s and the baby’s life. Many potential therapeutics developed are based on the downregulation of sFlt-1. Yu et al. used a pregnant rat model of preeclampsia by injection of TNF-α to test PAMAM nanocarriers loaded with an anti-sFlt1 siRNA. In vivo results showed a decrease in circulating levels of sFlt-1, mean arterial pressure, and a significant increase in fetuses’ weight and placentae compared to the control groups. This suggests that siRNA-sFlt1-PAMAM has a positive effect on preeclampsia symptoms in the PE gravid rat model [53][42]. In another study, a new preeclampsia pregnant mouse model was developed based on placental RGD-targeting liposomes to deliver a specific siRNA to induce preeclampsia-like symptoms. Formulations were tested on an in vivo model of healthy pregnant mice, and a biodistribution study showed a higher uptake of RGD liposomes than regular PEG liposomes. After collecting the mice’s placenta and pups, results showed an increase in sFLT-1 mRNA expression by the trophoblasts. [54][43]. Few studies testing nanocarriers on pathological in vivo models have been reported. One hypothesis to explain this underuse of pathological animal models could be the lack of knowledge of the pathophysiology mechanisms of diseases in humans. It is certainly easy to reproduce symptoms but difficult to reproduce the physiological mechanisms, and therefore difficult to obtain a reliable animal model. For example, pathological models used for preeclampsia can arise from genetic modifications as the STOX-1 model [55][44], from surgical procedures as the reduced uterine perfusion pressure (RUPP) model [56[45][46][47],57,58], or from drug induction with TNF-α [53][42]. Such models can recreate the symptoms but are missing the accurate mechanisms of the disease and therefore are not completely trustworthy to test therapeutic efficiency and cannot be uniquely considered to screen future treatments [59][48]. They appeared to be the ideal in vivo model to use for biomedical studies because of their small size, ease of maintenance, and short life cycle [63][49]. However, the most important part is to understand that an in vivo model must be chosen regarding the pathology and the API studied. Regarding the study of nanocarriers as drug delivery systems, mice and rats have proven to be useful to evaluate their biodistribution, placental targeting, transplacental passage, fetotoxicity, and therapeutic efficiency on specific diseases. Despite these studies and even with the similarities in placental structure and physiology between rodents and humans, there are still some differences between those species [64][50]. Other characteristics should also be taken into account when choosing an in vivo model to evaluate nanomedicine in the context of fetal medicine and obstetric studies, such as the placentation mechanism, the number of fetuses per pregnancy, and the gestation length [65][51]. Therefore, other species could be encountered in this type of trial, like rabbits or sheep [66,67][52][53]. To study human medical matter will require, at some point, human material such as cells to overcome species differences. Therefore, in vitro models can bring useful answers to nanocarrier evaluation.References
- Costantine, M.M. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 2014, 5, 65.
- Tan, E.K.; Tan, E.L. Alterations in physiology and anatomy during pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 791–802.
- Hodge, L.S.; Tracy, T.S. Alterations in drug disposition during pregnancy. Expert Opin. Drug Metab. Toxicol. 2007, 3, 557–571.
- Feghali, M.; Venkataramanan, R.; Caritis, S. Pharmacokinetics of drugs in pregnancy. Semin. Perinatol. 2015, 39, 512–519.
- Mitchell, A.A.; Gilboa, S.M.; Werler, M.M.; Kelley, K.E.; Louik, C.; Hernández-Díaz, S. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am. J. Obstet. Gynecol. 2011, 205, 51.e1–51.e8.
- Shields, K.E.; Lyerly, A.D. Exclusion of pregnant women from industry-sponsored clinical trials. Obstet. Gynecol. 2013, 122, 1077–1081.
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037.
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51.
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198.
- Moros, M.; Mitchell, S.G.; Grazú, V.; de la Fuente, J.M. The fate of nanocarriers as nanomedicines in vivo: Important considerations and biological barriers to overcome. Curr. Med. Chem. 2013, 20, 2759–2778.
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21.
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent design to treat complex disease. Pharm. Res. 2006, 23, 1417–1450.
- Muoth, C.; Aengenheister, L.; Kucki, M.; Wick, P.; Buerki-Thurnherr, T. Nanoparticle transport across the placental barrier: Pushing the field forward! Nanomedicine 2016, 11, 941–957.
- Refuerzo, J.S.; Longo, M.; Godin, B. Targeted nanoparticles in pregnancy: A new frontier in perinatal therapeutics. Am. J. Obstet. Gynecol. 2017, 216, 204–205.
- Alhareth, K. How should we plan the future of nanomedicine for cancer diagnosis and therapy? Int. J. Pharm. 2017, 532, 657–659.
- Valero, L.; Alhareth, K.; Gil, S.; Lecarpentier, E.; Tsatsaris, V.; Mignet, N.; Fournier, T.; Andrieux, K. Nanomedicine as a potential approach to empower the new strategies for the treatment of preeclampsia. Drug Discov. Today 2018, 23, 1099–1107.
- Nikitina, L.; Dohr, G.; Juch, H. Studying nanoparticle interaction with human placenta: Festina lente! Nanotoxicology 2015, 9, 133–134.
- Harris, L.K. Nanomedicine; Future Medicine Ltd.: London, UK, 2016; pp. 2235–2238.
- Semmler-Behnke, M.; Lipka, J.; Wenk, A.; Hirn, S.; Schäffler, M.; Tian, F.; Schmid, G.; Oberdörster, G.; Kreyling, W.G. Size dependent translocation and fetal accumulation of gold nanoparticles from maternal blood in the rat. Part. Fibre Toxicol. 2014, 11, 33.
- Tsyganova, N.A.; Khairullin, R.M.; Terentyuk, G.S.; Khlebtsov, B.N.; Bogatyrev, V.A.; Dykman, L.A.; Erykov, S.N.; Khlebtsov, N.G. Penetration of pegylated gold nanoparticles through rat placental barrier. Bull. Exp. Biol. Med. 2014, 157, 383–385.
- Yang, H.; Sun, C.; Fan, Z.; Tian, X.; Yan, L.; Du, L.; Liu, Y.; Chen, C.; Liang, X.J.; Anderson, G.J.; et al. Effects of gestational age and surface modification on materno-fetal transfer of nanoparticles in murine pregnancy. Sci. Rep. 2012, 2, 847.
- Rattanapinyopituk, K.; Shimada, A.; Morita, T.; Sakurai, M.; Asano, A.; Hasegawa, T.; Inoue, K.; Takano, H. Demonstration of the clathrin- and caveolin-mediated endocytosis at the maternal-fetal barrier in mouse placenta after intravenous administration of gold nanoparticles. J. Vet. Med. Sci. 2014, 76, 377–387.
- Kenesei, K.; Murali, K.; Czéh, Á.; Piella, J.; Puntes, V.; Madarász, E. Enhanced detection with spectral imaging fluorescence microscopy reveals tissue- and cell-type-specific compartmentalization of surface-modified polystyrene nanoparticles. J. Nanobiotechnol. 2016, 14, 55.
- Huang, Q.T.; Chen, J.H.; Hang, L.L.; Liu, S.S.; Zhong, M. Activation of PAR-1/NADPH Oxidase/ROS signaling pathways is crucial for the thrombin-induced sFlt-1 production in extravillous trophoblasts: Possible involvement in the pathogenesis of preeclampsia. Cell. Physiol. Biochem. 2015, 35, 1654–1662.
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M.; Yoshida, T.; Ogura, T.; Nabeshi, H.; Nagano, K.; et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 2011, 6, 321–328.
- Pinto, S.R.; Helal-Neto, E.; Paumgartten, F.; Felzenswalb, I.; Araujo-Lima, C.F.; Martínez-Máñez, R.; Santos-Oliveira, R. Cytotoxicity, genotoxicity, transplacental transfer and tissue disposition in pregnant rats mediated by nanoparticles: The case of magnetic core mesoporous silica nanoparticles. Artif. Cell. Nanomed. Biotechnol. 2018, 46, 527–538.
- Pietroiusti, A.; Vecchione, L.; Malvindi, M.A.; Aru, C.; Massimiani, M.; Camaioni, A.; Magrini, A.; Bernardini, R.; Sabella, S.; Pompa, P.P.; et al. Relevance to investigate different stages of pregnancy to highlight toxic effects of nanoparticles: The example of silica. Toxicol. Appl. Pharmacol. 2018, 342, 60–68.
- Sweeney, S.; Adamcakova-Dodd, A.; Thorne, P.S.; Assouline, J.G. Multifunctional nanoparticles for real-time evaluation of toxicity during fetal development. PLoS ONE 2018, 13, e0192474.
- Chu, M.; Wu, Q.; Yang, H.; Yuan, R.; Hou, S.; Yang, Y.; Zou, Y.; Xu, S.; Xu, K.; Ji, A.; et al. Transfer of Quantum Dots from Pregnant Mice to Pups Across the Placental Barrier. Small 2010, 6, 670–678.
- Wang, Z.; Zhang, S.; Qu, G.; Liu, S. The capability of quantum dots in crossing the placental barrier and the potential influence on erythrocytes. J. Nanosci. Nanotechnol. 2013, 13, 6529–6532.
- Huang, X.; Zhang, F.; Sun, X.; Choi, K.Y.; Niu, G.; Zhang, G.; Guo, J.; Lee, S.; Chen, X. The genotype-dependent influence of functionalized multiwalled carbon nanotubes on fetal development. Biomaterials 2014, 35, 856–865.
- Huang, J.P.; Hsieh, P.C.H.; Chen, C.Y.; Wang, T.Y.; Chen, P.C.; Liu, C.C.; Chen, C.C.; Chen, C.P. Nanoparticles can cross mouse placenta and induce trophoblast apoptosis. Placenta 2015, 36, 1433–1441.
- Refuerzo, J.S.; Alexander, J.F.; Leonard, F.; Leon, M.; Longo, M.; Godin, B. Liposomes: A nanoscale drug carrying system to prevent indomethacin passage to the fetus in a pregnant mouse model. Am. J. Obstet. Gynecol. 2015, 212, 508.e1–508.e7.
- Refuerzo, J.S.; Leonard, F.; Bulayeva, N.; Gorenstein, D.; Chiossi, G.; Ontiveros, A.; Longo, M.; Godin, B. Uterus-targeted liposomes for preterm labor management: Studies in pregnant mice. Sci. Rep. 2016, 6, 34710.
- Cureton, N.; Korotkova, I.; Baker, B.; Greenwood, S.; Wareing, M.; Kotamraju, V.R.; Teesalu, T.; Cellesi, F.; Tirelli, N.; Ruoslahti, E.; et al. Selective Targeting of a Novel Vasodilator to the Uterine Vasculature to Treat Impaired Uteroplacental Perfusion in Pregnancy. Theranostics 2017, 7, 3715–3731.
- Shetty, A.N.; Pautler, R.; Ghagahda, K.; Rendon, D.; Gao, H.; Starosolski, Z.; Bhavane, R.; Patel, C.; Annapragada, A.; Yallampalli, C.; et al. A liposomal Gd contrast agent does not cross the mouse placental barrier. Sci. Rep. 2016, 6, 27863.
- Zhang, B.; Liang, R.; Zheng, M.; Cai, L.; Fan, X. Surface-functionalized nanoparticles as efficient tools in targeted therapy of pregnancy complications. Int. J. Mol. Sci. 2019, 20, 3642.
- King, A.; Ndifon, C.; Lui, S.; Widdows, K.; Kotamraju, V.R.; Agemy, L.; Teesalu, T.; Glazier, J.D.; Cellesi, F.; Tirelli, N.; et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2016, 2, e1600349.
- Zhang, B.; Tan, L.; Yu, Y.; Wang, B.; Chen, Z.; Han, J.; Li, M.; Chen, J.; Xiao, T.; Ambati, B.K.; et al. Placenta-specific drug delivery by trophoblast-targeted nanoparticles in mice. Theranostics 2018, 8, 2765–2781.
- Tian, X.; Zhu, M.; Du, L.; Wang, J.; Fan, Z.; Liu, J.; Zhao, Y.; Nie, G. Intrauterine inflammation increases materno-fetal transfer of gold nanoparticles in a size-dependent manner in murine pregnancy. Small 2013, 9, 2432–2439.
- Lei, J.; Rosenzweig, J.M.; Mishra, M.K.; Alshehri, W.; Brancusi, F.; McLane, M.; Almalki, A.; Bahabry, R.; Arif, H.; Rozzah, R.; et al. Maternal dendrimer-based therapy for inflammation-induced preterm birth and perinatal brain injury. Sci. Rep. 2017, 7, 6106.
- Yu, J.; Jia, J.; Guo, X.; Chen, R.; Feng, L. Modulating circulating sFlt1 in an animal model of preeclampsia using PAMAM nanoparticles for siRNA delivery. Placenta 2017, 58, 1–8.
- Yu, Q.; Qiu, Y.; Wang, X.; Tang, J.; Liu, Y.; Mei, L.; Li, M.; Yang, M.; Tang, L.; Gao, H.; et al. Efficient siRNA transfer to knockdown a placenta specific lncRNA using RGD-modified nano-liposome: A new preeclampsia-like mouse model. Int. J. Pharm. 2018, 546, 115–124.
- Doridot, L.; Passet, B.; Méhats, C.; Rigourd, V.; Barbaux, S.; Ducat, A.; Mondon, F.; Vilotte, M.; Castille, J.; Breuiller-Fouché, M.; et al. Preeclampsia-like symptoms induced in mice by fetoplacental expression of STOX1 are reversed by aspirin treatment. Hypertension 2013, 61, 662–668.
- Li, J.; LaMarca, B.; Reckelhoff, J.F. A model of preeclampsia in rats: The reduced uterine perfusion pressure (RUPP) model. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1–H8.
- Fushima, T.; Sekimoto, A.; Minato, T.; Ito, T.; Oe, Y.; Kisu, K.; Sato, E.; Funamoto, K.; Hayase, T.; Kimura, Y.; et al. Reduced uterine perfusion pressure (RUPP) model of preeclampsia in Mice. PLoS ONE 2016, 11, e0155426.
- Morton, J.S.; Levasseur, J.; Ganguly, E.; Quon, A.; Kirschenman, R.; Dyck, J.R.B.; Fraser, G.M.; Davidge, S.T. Characterisation of the Selective Reduced Uteroplacental Perfusion (sRUPP) Model of Preeclampsia. Sci. Rep. 2019, 9, 9565.
- Erlandsson, L.; Nääv, Å.; Hennessy, A.; Vaiman, D.; Gram, M.; Åkerström, B.; Hansson, S.R. Inventory of Novel Animal Models Addressing Etiology of Preeclampsia in the Development of New Therapeutic/Intervention Opportunities. Am. J. Reprod. Immunol. 2016, 75, 402–410.
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211.
- Carter, A.M. Animal Models of Human Placentation—A Review. Placenta 2007, 28, S41–S47.
- Andersen, M.D.; Alstrup, A.K.O.; Duvald, C.S.; Mikkelsen, E.F.R.; Vendelbo, M.H.; Ovesen, P.G.; Pedersen, M. Animal models of fetal medicine and obstetrics. In Experimental Animal Models of Human Diseases—An Effective Therapeutic Strategy; InTech: London, UK, 2018.
- Swanson, A.M.; David, A.L. Animal models of fetal growth restriction: Considerations for translational medicine. Placenta 2015, 36, 623–630.
- Grigsby, P.L. Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy. Semin. Reprod. Med. 2016, 34, 11–16.
More
