Layered metal nitride halides MNX (M = Ti, Zr, Hf; X = Cl, Br, I) have two polymorphs, including α- and β-forms, which have the FeOCl and SmSI structures, respectively. These compounds are band insulators and become metals and show superconductivity after electron doping by intercalating alkali metals between the layers. The superconductivity of β-form had been extensively characterized from decades ago, but it is not easy to consistently interpret all experimental results using conventional phonon-mediated Bardeen–Cooper–Schriefer mechanisms. The titanium compound TiNCl crystallizes only in the α-form structure. TiNCl also exhibits superconductivity as high as ~16 K after electron doping by intercalating metals and/or organic basis. It is important to compare the superconductivity of different M–N networks. However, α-form compounds are vulnerable to moisture, unlike β-form ones. The intercalation compounds are even more sensitive to humid air.
- α-form layered nitride halides
- electron doping
- Synthesis
1. Introduction
2. Conventional Preparation
3. Preparation Using Sodium Amide
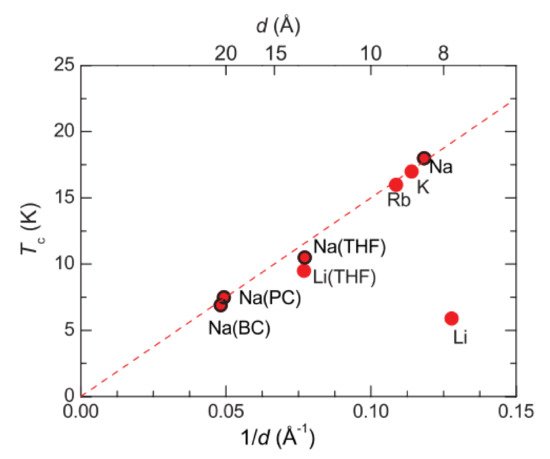
4. Electron Doping by Intercalation
References
- Yamanaka, S.; Kawaji, H.; Hotehama, K.; Ohashi, M. A new layer-structured nitride superconductor. Lithium-intercalated β-zirconium nitride chloride, LixZrNCl. Adv. Mater. 1996, 8, 771–774.
- Yamanaka, S.; Hotehama, K.I.; Kawaji, H. Superconductivity at 25.5K in electron-doped layered hafnium nitride. Nature 1998, 392, 580–582.
- Yamanaka, S.; Yasunaga, T.; Yamaguchi, K.; Tagawa, M. Structure and superconductivity of the intercalation compounds of TiNCl with pyridine and alkali metals as intercalants. J. Mater. Chem. 2009, 19, 2573.
- Yamanaka, S. Intercalation and superconductivity in ternary layer structured metal nitride halides (MNX: M = Ti, Zr, Hf; X = Cl, Br, I). J. Mater. Chem. 2010, 20, 2922–2933.
- Juza, R.; Heners, J. Über Nitridhalogenide des Titans und Zirkons. Z. Für Anorg. Und Allg. Chem. 1964, 332, 159–172.
- Zhang, S.; Tanaka, M.; Zhu, H.; Yamanaka, S. Superconductivity of layered β-HfNCl with varying electron-doping concentrations and interlayer spacings. Supercond. Sci. Technol. 2013, 26, 085015.
- Ueno, K.; Shimotani, H.; Yuan, H.; Ye, J.; Kawasaki, M.; Iwasa, Y. Field-induced superconductivity in electric double layer transistors. J. Phys. Soc. Jpn. 2014, 83, 032001.
- Saito, Y.; Kasahara, Y.; Ye, J.; Iwasa, Y.; Nojima, T. Metallic ground state in an ion-gated two-dimensional superconductor. Science 2015, 350, 409–413.
- Zhang, S.; Gao, M.-R.; Fu, H.-Y.; Wang, X.-M.; Ren, Z.-A.; Chen, G.-F. Electric Field Induced Permanent Superconductivity in Layered Metal Nitride Chlorides HfNCl and ZrNCl. Chin. Phys. Lett. 2018, 35, 097401.
- Wang, X.; Zhang, S.; Fu, H.; Gao, M.; Ren, Z.; Chen, G. Dominant role of processing temperature in electric field induced superconductivity in layered ZrNBr. N. J. Phys. 2019, 21, 023002.
- Nakagawa, Y.; Kasahara, Y.; Nomoto, T.; Arita, R.; Nojima, T.; Iwasa, Y. Gate-controlled BCS-BEC crossover in a two-dimensional superconductor. Science 2021, 372, 190–195.
- Nakagawa, Y.; Saito, Y.; Nojima, T.; Inumaru, K.; Yamanaka, S.; Kasahara, Y.; Iwasa, Y. Gate-controlled low carrier density superconductors: Toward the two-dimensional BCS-BEC crossover. Phys. Rev. B 2018, 98, 064512.
- Taguchi, Y.; Kitora, A.; Iwasa, Y. Increase in Tc upon reduction of doping in LixZrNCl superconductors. Phys. Rev. Lett. 2006, 97, 107001.
- Peng, J.; Zhang, S. Synthesis and Superconductivity of Electron-Doped β-ZrNCl with Partial Substitution of Ti on Zr Site. J. Supercond. Nov. Magn. 2018, 31, 61–65.
- Tou, H.; Maniwa, Y.; Koiwasaki, T.; Yamanaka, S. Unconventional superconductivity in electron-doped layered Li0.48(THF)yHfNCl. Phys. Rev. Lett. 2001, 86, 5775–5778.
- Taguchi, Y.; Hisakabe, M.; Iwasa, Y. Specific heat measurement of the layered nitride superconductor LixZrNCl. Phys. Rev. Lett. 2005, 94, 2–5.
- Yokoya, T.; Ishiwata, Y.; Shin, S.; Shamoto, S.; Iizawa, K.; Kajitani, T.; Hase, I.; Takahashi, T. Changes of electronic structure across the insulator-to-metal transition of quasi-two-dimensional Na-intercalated β-HfNCl studied by photoemission and X-ray absorption. Phys. Rev. B 2001, 64, 153107.
- Takeuchi, T.; Tsuda, S.; Yokoya, T.; Tsukamoto, T.; Shin, S.; Hirai, A.; Shamoto, S.; Kajitani, T. Soft X-ray emission and high-resolution photoemission study of quasi-two-dimensional superconductor NaxHfNCl. Physica C 2003, 392–396, 127–129.
- Yokoya, T.; Takeuchi, T.; Tsuda, S.; Kiss, T.; Higuchi, T.; Shin, S.; Iizawa, K.; Shamoto, S.; Kajitani, T.; Takahashi, T. Valence-band photoemission study of β-ZrNCl and the quasi-two-dimensional superconductor NaxZrNCl. Phys. Rev. B 2004, 70, 193103.
- Tou, H.; Maniwa, Y.; Yamanaka, S. Superconducting characteristics in electron-doped layered hafnium nitride: 15N isotope effect studies. Phys. Rev. B 2003, 67, 100509.
- Weht, R.; Filippetti, A.; Pickett, W.E. Electron doping in the honeycomb bilayer superconductors (Zr, Hf)NCl. Europhys. Lett. 1999, 48, 320–325.
- Sugimoto, A.; Sakai, Y.; Ekino, T.; Zhang, S.; Tanaka, M.; Yamanaka, S.; Gabovich, A.M. Scanning Tunnelling Microscopy and Spectroscopy of the Layered Nitride Superconductor α-NaxTiNCl. Phys. Procedia 2016, 81, 73–76.
- Ekino, T.; Takasaki, T.; Muranaka, T.; Fujii, H.; Akimitsu, J.; Yamanaka, S. Tunneling spectroscopy of MgB2 and Li0.5(THF)yHfNCl. Phys. B Condens. Matter 2003, 328, 23–25.
- Kawaji, H.; Hotehama, K.I.; Yamanaka, S. Superconductivity of Alkali Metal Intercalated β-Zirconium Nitride Chloride, AxZrNCl (A = Li, Na, K). Chem. Mater. 1997, 9, 2127–2130.
- Takano, T.; Kishiume, T.; Taguchi, Y.; Iwasa, Y. Interlayer-spacing dependence of Tc in LixMyHfNCl (M: Molecule) superconductors. Phys. Rev. Lett. 2008, 100, 247005.
- Kasahara, Y.; Kishiume, T.; Kobayashi, K.; Taguchi, Y.; Iwasa, Y. Superconductivity in molecule-intercalated LixZrNCl with variable interlayer spacing. Phys. Rev. B 2010, 82, 054504.
- Kasahara, Y.; Kishiume, T.; Takano, T.; Kobayashi, K.; Matsuoka, E.; Onodera, H.; Kuroki, K.; Taguchi, Y.; Iwasa, Y. Enhancement of Pairing Interaction and Magnetic Fluctuations toward a Band Insulator in an Electron-Doped LixZrNCl Superconductor. Phys. Rev. Lett. 2009, 103, 077004.
- Kotegawa, H.; Oshiro, S.; Shimizu, Y.; Tou, H.; Kasahara, Y.; Kishiume, T.; Taguchi, Y.; Iwasa, Y. Strong suppression of coherence effect and appearance of pseudogap in the layered nitride superconductor LixZrNCl: 91Zr- and 15N-NMR studies. Phys. Rev. B 2014, 90, 020503.
- Kasahara, Y.; Kuroki, K.; Yamanaka, S.; Taguchi, Y. Unconventional superconductivity in electron-doped layered metal nitride halides MNX (M = Ti, Zr, Hf; X = Cl, Br, I). Physica C 2015, 514, 354–367.
- Cross, J.B.; Schlegel, H.B. Molecular orbital studies of titanium nitride chemical vapor deposition: Gas phase β -elimination. Chem. Phys. Lett. 2001, 340, 343–347.
- Umanskii, S.Y.; Novoselov, K.P.; Minushev, A.K.; Siodmiak, M.; Frenking, G.; Korkin, A.A. Thermodynamics and kinetics of initial gas phase reactions in chemical vapor deposition of titanium nitride. Theoretical study of TiCl4 ammonolysis. J. Comput. Chem. 2001, 22, 1366–1376.
- Saeki, Y.; Matsuzaki, R.; Yajima, A.; Akiyama, M. Reaction Process of Titanium Tetrachloride with Ammonia in the Vapor Phase and Properties of the Titanium Nitride Formed. Bull. Chem. Soc. Jpn. 1982, 55, 3193–3196.
- Fowles, G.W.A.; Pollard, F.H. Studies on the behaviour of halides of the transition metals with ammonia. Part II. The reaction of titanium tetrachloride with ammonia. J. Chem. Soc. 1953, 22, 2588.
- Kurtz, S.R.; Gordon, R.G. Chemical vapor deposition of titanium nitride at low temperatures. Thin Solid Films 1986, 140, 277–290.
- Ohashi, M.; Yamanaka, S.; Hattori, M. Chemical vapor transport of layer structured crystal β-ZrNCl. J. Solid State Chem. 1988, 77, 342–347.
- Yajima, A.; Segawa, Y.; Matsuzaki, R.; Saeki, Y. Reaction Process of Zirconium Tetrachloride with Ammonia in the Vapor Phase and Properties of the Zirconium Nitride Formed. Bull. Chem. Soc. Jpn. 1983, 56, 2638–2642.
- Sosnov, E.A.; Malkov, A.A.; Malygin, A.A. Chemical transformations at the silica surface upon sequential interactions with titanium tetrachloride and ammonia vapors. Russ. J. Gen. Chem. 2015, 85, 2533–2540.
- Ohashi, M.; Yamanaka, S.; Hattori, M. Synthesis of β-ZrClN by Thermal Decomposition of Zirconium(IV) Amide Trichloride. Bull. Chem. Soc. Jpn. 1986, 59, 2627–2628.
- Odahara, J.; Sun, W.; Miura, A.; Rosero-Navarro, N.C.; Nagao, M.; Tanaka, I.; Ceder, G.; Tadanaga, K. Self-Combustion Synthesis of Novel Metastable Ternary Molybdenum Nitrides. ACS Mater. Lett. 2019, 1, 64–70.
- Miura, A. Low-temperature synthesis and rational design of nitrides and oxynitrides for novel functional material development. J. Ceram. Soc. Jpn. 2017, 125, 552–558.
- Tanaka, M.; Kataoka, N.; Matsumoto, R.; Inumaru, K.; Takano, Y.; Yokoya, T. Synthetic Route of Layered Titanium Nitride Chloride TiNCl Using Sodium Amide. ACS Omega 2022, 7, 6375–6380.
- Kuhn, A.; Hoppe, H.; Strähle, J.; Garcia-Alvarado, F. Electrochemical Lithium Intercalation in Titanium Nitride Chloride. J. Electrochem. Soc. 2004, 151, A843.
- Ohashi, M.; Uyeoka, K.; Yamanaka, S.; Hattori, M. Co-Intercalation of Tetrahydrofuran and Propylene Carbonate with Alkali Metals in β-ZrNCl Layer Structured Crystal. Bull. Chem. Soc. Jpn. 1991, 64, 2814–2818.
- Zhang, S.; Tanaka, M.; Yamanaka, S. Superconductivity in electron-doped layered TiNCl with variable interlayer coupling. Phys. Rev. B 2012, 86, 024516.
- Zhang, S.; Tanaka, M.; Watanabe, E.; Zhu, H.; Inumaru, K.; Yamanaka, S. Superconductivity of alkali metal intercalated TiNBr with alpha-type nitride layers. Supercond. Sci. Technol. 2013, 26, 122001.
- Yamanaka, S.; Okumura, H.; Zhu, L. Alkali metal intercalation in layer structured α-HfNBr. J. Phys. Chem. Solids 2004, 65, 565–569.
- Hotehama, K.; Koiwasaki, T.; Umemoto, K.; Yamanaka, S.; Tou, H. Effect of Swelling on the Superconducting Characteristics in Electron-Doped β-ZrNCl and HfNCl. J. Phys. Soc. Jpn. 2010, 79, 014707.
- Harshman, D.R.; Fiory, A.T. Modeling Intercalated Group-4-Metal Nitride Halide Superconductivity with Interlayer Coulomb Coupling. J. Supercond. Nov. Magn. 2015, 28, 2967–2978.
